Abstract
Effect of addition of multigrain premix (MGP) prepared using a combination of cereals, pulses and oilseeds at 40% level, on nutritional properties of multigrain biscuit, its in-vitro and in-vivo protein digestibility and protein profiling were studied. The incorporation of MGP significantly increased the protein content (from 7.37 to 16.61%), insoluble dietary fiber (from 1.71 to 6.67%), soluble dietary fiber (from 0.46 to 2.42%). The significant increase in the levels of isoleucine (ND-34.79%), methionine (0.04 to 7.65%), tryptophan (0.22 to 5.95%) valine (0.38 to 16.58%), lysine (0.36 to 7.32%), and threonine (0.51 to 7.2%) was observed, whereas fatty acid profile of MGP incorporated biscuits showed increased polyunsaturated fatty acids and decreased saturated fatty acids. The vitamin–mineral profile of MGP incorporated biscuits showed increased the thiamin (0.07–0.21 mg/100 g), riboflavin (0.09–0.28 mg/100 g), calcium (12.89–45.28 mg/100 g) and iron (1.13–3.47 mg/100 g) contents. The in-vitro protein digesibility of multigrain and control biscuits indicated that the proteins present in multigrain biscuits had high digestibility (71.73%) as compared to control biscuit (38.13%). The in-vivo studies indicated that, the protein quality of multigrain biscuits was comparable with casein protein with high protein efficiency ratio of 3.02. The electrophoretic pattern of multigrain biscuits showed subunit molecular weight distribution of different protein units and aggregation of protein bands at high molecular weight region of 85 to 166 kD. The outcome of the study indicated the possibility of utilising MGP to improve the overall nutritional quality of biscuits.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bakery products have become one of the important parts of modern-day lifestyle, and bakery industry is now visualizing a rapid change to satisfy the consumer demand for ready-to-eat bakery products with health benefits (Nayak 2014; Kotasianins et al. 2002). Among the different bakery products, biscuits form an important and highly consumable snack item. Owing to this there is a vast scope of improving the nutritional quality of the biscuit to cater the huge consumer demand for biscuits with healthier ingredients and also to make it more nutritious and tasty (Kumar et al. 2016).
There are several reports available on the development of nutritious biscuits by using composite flours containing wheat flour and other nutritious ingredients (Mridula et al. 2007). Research work on the use of different healthy ingredient, such as protein-rich flours like defatted soya flour (Zaker et al. 2012), chickpea flour (Yadav et al. 2012a), green gram flour (Rajiv et al. 2012); oilseeds like virgin coconut meal (Srivastava et al. 2010), defatted peanut meal (Yadav et al. 2012b); industrial by-products like wheat germ (Bansal and Sudha 2011); dairy proteins (Gallagher et al. 2005) etc. to improve the nutritional quality of biscuits were reported. However, the success of this effort was only intended to the limited improvement of the nutritional quality. None of the composite flour biscuits reported so far contain all the nutrients in balanced amounts. One of the most promising approaches is by incorporating multigrain mix with a combination of cereals, pulses, millets and oilseeds into biscuits to improve the overall nutritional quality (Kumar et al. 2015b). In recent years, few studies were reported on the use of multigrain mix to improve the overall nutritional quality of bread and traditional products like parotta (Indrani et al. 2010, 2011), halwa mix (Banu et al. 2013), porridge mix (Mandge et al. 2011) supplementary food (Khanam et al. 2013) etc. These studies reported that incorporation of multigrain mix at 10–25% level significantly improved the overall nutritional quality of the products, such as protein quality, dietary fiber and vitamin–mineral profile.
The literature survey indicated that there was no systematic study reported so far on the effect of multigrain mix on the nutritional quality of biscuits. Therefore, it was thought appropriate to incorporate multigrain premix (MGP) in biscuits and follow the improvement on the nutritional properties of biscuits in terms of proximate composition, soluble and insoluble dietary fiber, vitamin, mineral profile, fatty acid profile, amino acid profile, in vitro, in vivo protein digestibility studies, and SDS PAGE analysis.
Materials and methods
Raw materials
The different flours used in MGP were barley (Hordeum vulgare L.), sorghum (Sorghum vulgare), chickpea (Cicer arietinum), whole dry pea (Pisum sativum), wheat flour and the ingredients for biscuit preparation like sugar, Marvo brand bakery shortening (Bunge India Pvt. Ltd, Mumbai, India), skimmed milk powder (Nandini brand, Karnataka Milk Federation, Mysore, India), vanilla essence (Bush Boake Allen Ltd, Chennai, India), sodium bicarbonate, ammonium bicarbonate (SD Fine Chemicals, India) and dextrose (Fisher Scientific, India) were procured from local market in Mysore, Karnataka, India. The vitamin and mineral mixture were procured from Sisco research laboratories, Bombay and defatted toasted soya flour (Glycine max) were procured from the Sakthi Soya Company, Pollachi, Tamil Nadu, India.
Preparation of MGP and biscuit
The MGP was prepared by mixing 20% each of barley, sorghum, chickpea, pea and soya flour (Kumar et al. 2015b). The biscuits were prepared by replacing wheat flour at an optimised level of 40% with MGP as per the methods of Kumar et al. (2015a). The ingredients used for the preparation of biscuits were, wheat flour 60 g, MGP 40 g, pulverised sugar 35 g, bakery shortening 20 g, sodium chloride 0.5 g, sodium bicarbonate 0.5 g, ammonium bicarbonate 1 g, skimmed milk powder 2 g, dextrose 2 g and vanilla essence 1 ml.
The method of preparation was as follows: Pulverized sugar, bakery shortening, skimmed milk powder, dextrose and vanilla essence were creamed in Hobart mixer (Model N50, Hobart GmbH, Offenburg, Germany) with a flat blade, for 2 min at 61 rpm first, then 3 min at 125 rpm. The cream was mixed with water containing dissolved sodium chloride, sodium bicarbonate, ammonium bicarbonate was separately added and mixed for 5 min at 125 rpm until homogenous cream obtained. Finally, wheat flour was added and mixed at 61 rpm for 2 min. The dough was sheeted to 5 mm thickness using a metal frame and cut into round shape of 55 mm diameter using the circular cutter. The baking was done at 200 °C for 10 min. The biscuits were cooled and stored in an airtight container.
Nutritional characteristics of biscuits
The moisture (method 44-16), protein (method 46-10), fat (method 30-10), ash (method 08-01) were analysed based on AACC (2000) procedures, and results are reported on a dry basis. The soluble and insoluble dietary fibers were estimated according to the methods of Asp et al. (1983), and the total carbohydrate content was determined by the method of difference (Banu et al. 2013). The thiamine and riboflavin vitamins were determined by fluorimeter method as described by AOAC (1999). The mineral content was determined by using an atomic absorption spectrophotometer as per the method of Semwal et al. (1995).
The amino acid composition was measured according to the official method of analysis AACC (2000) using amino acids analyser (Sykam-S7130/Germany) based on high-performance liquid chromatography technique. The fatty acid profile was determined as per AOCS (1990) using gas–liquid chromatography (Model chemito-GC-1000, Mumbai) on a BPX 70 capillary column (60 m × 0.25 mm id) with flame ionisation detector and hydrogen as a carrier gas (1.6 ml/min) with injector split ratio 1:30.
In-vitro protein digestibility of biscuits
The freeze-dried biscuit samples were incubated with pepsin (Sigma Aldrich, P7125) and pancreatin (Sigma Aldrich, P7545) according to the method of Akeson and Stahmann (1964) with slight modifications as made by Susanna and Prabhasankar (2012). Exactly 2 g of sample was incubated with 1.5 mg of pepsin in 15 ml of 0.1 N HCl at 37 °C. After 3 h, the sample was neutralized with 0.5 N NaOH. Then the sample was incubated with pancreatin (4 mg) in 0.2 M phosphate buffer (pH 8), and 0.05 M sodium azide was added. After overnight incubation, 10% trichloroacetic acid was added and centrifuged at 3000 rpm for 20 min. The supernatant was subjected to protein estimation by the Kjeldhal method as per the AACC (2000) procedure.
In-vivo digestibility of biscuits
The protein efficiency ratio (PER) of the multigrain biscuit was carried out according to the method of Serrem et al. (2011). After getting approval from institutional animal ethics committee (IAEC), 20 male albino rats of a single strain, 20–23 days of age were selected and subjected to control casein diet for 7 days for acclimatisation and then randomly divided into two groups comprising of ten rats each. The two groups of rats were maintained on the control and experimental diets respectively. Both diets were kept isoprotein and isocaloric composition and presented in Table 1. All the rats were kept in individual cages and recorded weekly food consumption and body weight gain. PER for each group was calculated according to the following equation: PER = Weight gain (g)/Protein consumed (g).
SDS-PAGE analysis
The SDS PAGE analysis of different grains used, MGP, wheat flour, control and multigrain biscuits were carried out according to the method of Hurkman and Tanaka (2007). A stacking gel of 5% and resolving gel of 10% and gel thickness of 1 mm was used and stained with Coomassie brilliant blue.
Statistical analysis
All the experiments were carried out in triplicates, and the results were expressed as a mean ± standard deviation. All data were statistically analysed by one-way analysis of variance (ANOVA) as per the method of Steel and Torrie (1960).
Results and discussion
Proximate composition
The proximate composition of control and multigrain biscuits are presented in Table 2. Control biscuit had a moisture content of 2.94%, and multigrain biscuits had 3.73%. The moisture content of food indicates dry matter in the food (Adebowale et al. 2012), and both biscuits had a moisture content within the range for biscuits (< 5%) as reported by Pereira et al. (2013).
The increase in moisture content of multigrain biscuit could be attributed to increased protein content, which has more affinity to water and also the presence of high fiber ingredients which absorbs more water during dough development and retains higher moisture content in ultimate products (Sujirtha and Mahendran 2015). A significant increase in ash content of the biscuits was observed due to the presence of whole grain flours. The control biscuit had 1.01% whereas multigrain biscuit had 1.51% ash and this ash content of the product could be used as an indicator of mineral constitute (Sanni et al. 2008). A similar report of increased ash content by the addition of multigrain containing whole grains like chickpea, barley, soya bean and fenugreek seeds on north Indian parotta were reported by Indrani et al. (2011).
The protein content of the biscuits increased more than twofold in multigrain biscuit as compared to control. The increase in protein content of biscuit could be due to the presence of pulse flours like chickpea, pea and the use of defatted toasted soya flour which are known for their high protein content (Chinma and Gernah 2007). The fat content of biscuits was obtained mainly through externally added fat during dough preparation. There was no significant difference in the fat content of control and multigrain biscuits. Serrem et al. (2011) reported that there was no change in the fat content of cereal biscuits and soy flour fortified biscuits as a result of uniform fat content in the formulation. The soluble and insoluble dietary fiber content of biscuits increased from 0.46 to 2.42% and 1.71 to 6.67% respectively. The increase in dietary fiber content of multigrain biscuit was mainly due to the use of whole grains in the preparation of MGP. Due to an increase in protein content in multigrain biscuit, the carbohydrate content of biscuit decreased. Control biscuit had 74.09%, and multigrain biscuit had 63.67% of carbohydrate. There was not much difference in energy content of control (457.15 kcal) and multigrain biscuits (451.36 kcal). The same energy content of biscuits may be due to similar fat content. Indrani et al. (2010) reported that incorporation of a multigrain mix containing soya bean, oats, fenugreek seeds, flax seeds and sesame seeds in bread making at the optimized level of 15%, there was a 1.5, 5.0, 2.5 times increase in protein, fat and dietary fiber when compared to control bread.
Essential amino acid profile of multigrain biscuit
The amino acid composition of control and multigrain biscuit are presented in Table 3. The amino acid profile of multigrain biscuit showed that all the essential amino acids were present in sufficient quantity. The Isoleucine (34.79 g/100 g) and valine (16.58 g/100 g) were predominant amino acids in multigrain biscuits followed by lysine (7.32 g/100 g), threonine (7.20 g/100 g) and leucine (7.16 g/100 g). However, in control biscuit, leucine and isoleucine were not detected, and other amino acids were present in minor quantity. Okoye et al. (2016) studied the amino acid profile of biscuits and reported that lysine and the sulphur containing amino acids; methionine and tryptophan were known to be limiting in cereals and legumes, significantly improved with the addition of soy flour and Bambara groundnut flour.
Vitamin and mineral profile
The vitamin and mineral profile of control and multigrain biscuits were presented in Table 2. A significant increase in thiamine and riboflavin content of multigrain biscuit was observed, and this could be due to the presence of whole grains in the MGP. Earlier, Batifouliera et al. (2005) reported that most of the B group vitamins are present in the bran and germ layer of grains and extensive extraction is liable to a reduction of B group vitamins. The thiamin content in control biscuit was 0.07 mg/100 g and increased to 0.21 mg/100 g in multigrain biscuits, whereas riboflavin in control biscuit was 0.09 mg/100 g and increased to 0.28 mg/100 g. Similar results were also reported by Banu et al. (2012) on steam processed multigrain composite mixes. The important minerals like calcium, iron and zinc were also found to increase with the addition of MGP as compared to control biscuit. The multigrain biscuit had 45.28 mg/100 g of calcium, 3.47 mg/100 g of iron and 1.90 mg/100 g of zinc as compared to control biscuit of 12.89, 1.13 and 1.32 mg/100 g respectively. Sodium and potassium content of biscuit was increased from 544.8 to 684.1 mg/100 g and 281.23 to 485.11 mg/100 g in control and multigrain biscuit respectively. Earlier, Vitali et al. (2009) reported that biscuit containing inulin and soya flour showed the significant increase in calcium (44.4%), iron (44.8%) content and Serrem et al. (2011) reported the twofold increase in minerals particularly, calcium, magnesium, potassium content with the incorporation of soy flour to sorghum biscuits.
Fatty acid profile of multigrain biscuit
There was no significant difference in the fatty acid profile of control and multigrain biscuits. Both the biscuits contained both saturated and unsaturated fatty acids. The saturated fatty acids like lauric, myristics, palmitic and stearic acids and unsaturated fatty acids like oleic and linoleic acids were detected and are presented in Table 3. The control and multigrain biscuits contained palmitic acid at higher concentration. These results are in agreement with Vicario et al. (2003), who studied the fatty acid composition of Spanish cookies and reported that the saturated fatty acids like palmitic and stearic acids were more predominant fatty acids. Similar findings were also reported by Metwal et al. (2011) in their study on debittered and defatted fenugreek–flax seed cookies and reported that significant amounts of stearic, lauric and myristic acids were present in cookies. However, incorporation of MGP increased the polyunsaturated fatty acid content and decreased the saturated fatty acids in multigrain biscuit as compared to control biscuit. The balance between saturated and unsaturated fatty acid was observed in multigrain biscuit. The proportion of unsaturated fatty acids were higher in multigrain biscuit as compared to control biscuit and this can be attributed to the presence of legume flours like chickpea, pea, which were reported to have a higher proportion of polyunsaturated fatty acids (Adsule et al. 1986).
In-vitro protein digestibility
In-vitro protein digestibility is one of the important criteria in the evaluation of protein quality as well as an indicator of protein bio-accessibility (Chinma et al. 2012). The in vitro protein digestibility of multigrain and control biscuits indicated that the proteins present in multigrain biscuit had a high digestibility of 71.73% as compared to control biscuit (38.13%). These findings are in agreement with the results of Serrem et al. (2011), who showed that defatted soy flour fortification to sorghum biscuits significantly increased the protein digestibility as compared to control sorghum biscuits. The increase in the in vitro protein digestibility of multigrain biscuits with the incorporation of MGP could be due to the increase in protein content as reported by Chinma et al. (2012) on unripe plantain and defatted sesame seed flour blended cookies. Earlier, Kiin-Kabari and Giami (2015) also observed increased in vitro protein digestibility of biscuits with the incorporation of Bambara groundnut protein concentrate to plantain flour. Ghumman et al. (2016) observed that the increase in digestibility is also affected by the presence of starch, which increases the digestibility of albumin and globulin proteins by the opening of compact structure of proteins on binding to the surface of starch granules and forming new bonds, hence facilitating easier access of the proteolytic enzymes.
In-vivo protein digestibility
Protein efficiency ratio (PER) is one of the measurable protein quality indices which determinates the effectiveness of proteins through the measurement of growth of rats (Sunday and Julius 2014).
The food intake, gain in body weight by rats and protein efficiency ratio are presented in Table 4. A significant difference in the food intake was observed without affecting protein consumed and gain in body weight. In the first and second week, the rats fed with control casein diet consumed more food compared to the test group. At the beginning of the 3rd week, there was an increased consumption of food by rats fed on the test diet. This may be due to acclimatisation period required for test group animals for test diet containing multigrain biscuit. The rats fed on test group gained comparably more weight by consuming less food. The control rats fed with 10% casein diet showed PER of 2.87, whereas the test diet containing multigrain biscuit showed PER of 3.02, indicating the superior protein quality of the multigrain biscuits than casein. The relatively higher PER in test diet may be due to the balancing of essential amino acid and mineral contents by the addition of MGP which favours test diet to give better weight gain than the control diet. Baskaran et al. (2001) studied the PER of different combinations of supplementary food and compared with skimmed milk powder. They reported that the PER of the combination of wheat flour, Bengal gram dhal and soya flour; and wheat flour and soya flour had the same value (2.8), while the combination of ragi and soya flour had a PER of 2.9 as compared to 10% skimmed milk powder (3.0).
SDS-PAGE analysis
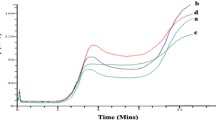
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) of 5 different grains like barley, jowar, pea, chickpea, soya flour and MGP, wheat flour, and both control and multigrain biscuits were performed to obtain information on subunit molecular weight distribution pattern of proteins (Fig. 1). In barley, protein bands around 6.5kD and between 29 and 40 kD were more intense, while in jowar in addition to 6.5 kD bands, more protein bands were seen between 14 and 20 kD regions. Both of these grains had less intense bands between 30 and 90 kD (lane 2 and 3). Compared to other grains, pea and chickpea had similar subunit composition with minor variation in their band intensities. However, protein extracts of these grains showed intense bands between 6.5 and 90 kD (lanes 3–4). The soya flour also showed thick bands between 20 and 90 kD in addition to a band at 6.5kD. In case of MGP presented in lane 7, the thick bands near 14 kD and below are mainly from jowar and chickpea and pea. Medium molecular weight bands from pea and soya flour were also observed between 20 and 66 kD. The high molecular weight protein bands are only from soya flour, which only had thick high molecular weight protein bands. This indicates that prepared MGP is having balanced protein as evidenced by its protein bands distribution pattern from low to high molecular weight.
Wheat flour and control biscuits made with only wheat flour had a similar electrophoretic pattern. These two samples had bands ranging from 6.5 to 116 kD. In addition to a low molecular weight band around 6.5 kD and few prominent bands were seen around 30 kD, which were mainly due to α, β, γ-gliadins and the bands between 85 and 116 kD could be due to the presence high molecular weight glutenin subunits (lanes 8–9). Earlier, Katyal et al. (2017) also reported the presence of major polypeptides ranging from 30 to 68 kD in their study on Indian wheat varieties. The polypeptides of 38–68 kD were classified as ω gliadins, whereas the 30–34 kD were classified as λ-(gamma), β-(beta) and α-gliadins. In lane 9 and 10, representing protein patterns of control and multigrain biscuits, aggregation of proteins bands were observed at high molecular weight region, and this could be due to denaturation of proteins at high temperature used during baking of biscuits. Earlier, Rao et al. (2002) studied denaturation of protein patterns when processing of wheat flour into flakes and observed that decreased number as well as the intensity of the bands in wheat flake compared to wheat flour and attributed this to decreased solubility of wheat flake proteins.
Conclusion
The incorporation of MGP significantly increased the nutritional quality, in vitro and in vivo protein digestibility of multigrain biscuits. The protein content of the biscuits increased more than twofold in multigrain biscuit, and total dietary fiber content increased from 2.17 to 9.09%. The minerals viz. calcium, iron, zinc and vitamins viz. thiamine, riboflavin was also found to increase with the addition of MGP. The in vitro protein digestibility of biscuits increased from 38.13% for control biscuit to 71.73% for optimised multigrain biscuits. The multigrain biscuits had higher values for PER (3.02), and the protein quality of the multigrain biscuit was comparable to casein. The significant increase in the levels of isoleucine (ND-34.79%), methionine (0.04 to 7.65%), tryptophan (0.22 to 5.95%) valine (0.38 to 16.58%), lysine (0.36 to 7.32%), and threonine (0.51 to 7.2%) was also observed. The SDS PAGE analysis showed the presence of all the protein bands from different flour used in MGP. The concept of incorporation of multigrain into traditional bakery products (biscuits) which renders improved protein quality, minerals, dietary fiber etc. opens new dimensions in the development of nutritious energy dense biscuits and other bakery products with variety and boosts the industry to new heights.
References
AACC (2000) Approved methods of the Association of Cereal Chemists International, 10th edn. American Association of Cereal Chemists, St. Paul
Adebowale AA, Adegoke MT, Sanni SA, Adegunwa MO, Fetuga GO (2012) Functional properties and biscuit making potentials of sorghum-wheat flour composite. Am J Food Technol 7:372–379
Adsule RN, Kadam SS, Salunkhe DK, Luh BS (1986) Chemistry and technology of green gram (Vigna radiata [L.] Wilczek). Crit Rev Food Sci Nutr 25:73–105
Akeson WR, Stahmann MA (1964) A pepsin pancreatin digest index of protein quality evaluation. J Nutr 83(3):257–261
AOAC (1999) Official methods of analysis. Association of Official Analytical Chemists, Washington
AOCS (1990) Official methods and recommended practices of the American Oil Chemists Society, 4th edn. AOCS, Champaign
Asp NG, Johabsson CG, Hallmer H, Siljestrom M (1983) Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agric Food Chem 31:476–482
Bansal S, Sudha ML (2011) Nutritional, microstructural, rheological and quality characteristics of biscuits using processed wheat germ. Int J Food Sci Nutr 62:474–479
Banu H, Itagi N, Singh V (2012) Preparation, nutritional composition, functional properties and antioxidant activities of multigrain composite mixes. J Food Sci Technol 49:74–81
Banu H, Itagi N, Singh V, Indiramma AR, Prakash M (2013) Shelf stable multigrain halwa mixes: preparation of halwa, their textural and sensory studies. J Food Sci Technol 50:879–889
Baskaran V, Malleshi NG, Jayaprakashan SG, Lokesh BR (2001) Biological evaluation for protein quality of supplementary foods based on popped cereals and legumes suitable for feeding rural mothers and children in India. Plant Foods Hum Nutr 56:37–49
Batifouliera F, Vernya MA, Chanliaudb E, Remesy C, Demigne C (2005) Effect of different bread making methods on thiamine, riboflavin and pyridoxine content of wheat bread. J Cereal Sci 42:101–108
Chinma CE, Gernah DI (2007) Physicochemical and sensory properties of cookies produced from cassava/soybean/mango composite flours. J Food Technol 5:256–260
Chinma CE, Igbabul BD, Omotayo OO (2012) Quality characteristics of cookies prepared from unripe plantain and defatted sesame flour blends. Am J Food Technol 7:398–408
Gallagher E, Kenny S, Arendt EK (2005) Impact of dairy protein powders on biscuits quality. Eur Food Res Technol 221:237–243
Ghumman A, Kaur A, Singh N (2016) Functionality and digestibility of albumins and globulins from lentil and horse gram and their effect on starch rheology. Food Hydrocoll 61:843–850
Hurkman WJ, Tanaka CK (2007) Extraction of wheat endosperm proteins for proteome analysis. J Chromatogr 849:344–350
Indrani D, Soumya C, Rajiv J, Rao GV (2010) Multigrain bread- its dough rheology, microstructure, quality and nutritional characteristics. J Tex Stud 41(3):302–319
Indrani D, Shwetha P, Soumya C, Rajiv J, Rao GV (2011) Effect of multigrain on the rheological, microstructural and quality characteristics of North Indian parotta—an Indian flatbread. LWT Food Sci Technol 44:719–724
Katyal M, Singh N, Virdi AS, Kaur A, Chopra N, Ahlawat AK, Singh AM (2017) Extraordinarily soft, medium-hard and hard Indian wheat varieties: composition, protein profile, dough and baking properties. Food Res Int 100:306–317
Khanam A, Chikkegowda RK, Swamylingappa B (2013) Functional and nutritional evaluation of supplementary food formulations. J Food Sci Technol 50:309–316
Kiin-Kabari DB, Giami SY (2015) Physico-chemical properties and in vitro protein digestibility of non-wheat cookies prepared from plantain flour and Bambara groundnut protein concentrate. J Food Res 4:78–86
Kotasianins IS, Giannou V, Tzia C (2002) Production and packaging of bakery products using MAP technology. Trends Food Sci Technol 13:319–324
Kumar KA, Sharma G, Khan MA, Govindraj T, Semwal AD (2015a) Development of multigrain premixes—its effect on rheological, textural and microstructural characteristics of dough and quality of biscuits. J Food Sci Technol 52:7759–7770
Kumar KA, Sharma GK, Khan MA, Semwal AD (2015b) Optimization of multigrain premix for high protein and dietary fiber biscuits using response surface methodology (RSM). Food Nutr Sci 6:747–756
Kumar KA, Sharma GK, Khan MA, Semwal AD (2016) A study on functional, pasting and microstructural characteristics of multigrain mixes for biscuits. J Food Meas Charact 10:274–282
Mandge HM, Sharma S, Dar BN (2011) Instant multigrain porridge: effect of cooking treatment on physicochemical and functional properties. J Food Sci Technol 51:97–103
Metwal N, Jyotna R, Jeyarani T, Rao GV (2011) Influence of debittered, defatted fenugreek seed powder and flax seed powder on the rheological characteristics of dough and quality of cookies. Int J Food Sci Nutr 62:336–344
Mridula D, Gupta RK, Manikantan MR (2007) Effect of incorporation of sorghum flour to wheat flour on quality of biscuits fortified with defatted soy flour. Am J Food Technol 2:428–434
Nayak P (2014) Ensuring nutritional enrichment of bakery products. F&B Special. http://www.fnbnews.com/FB-Specials/Ensuring-nutritional-enrichment-of-bakery-products. Accessed 2 May 2017
Okoye J, Philippa O, Anthony U (2016) Amino acid composition and protein quality of wheat flour biscuits fortified with soybean and Bambara groundnut flours. GJESR Res Paper 3:8–19
Pereira D, Correia PM, Guine RP (2013) Analysis of the physical, chemical and sensorial properties of marie type cookies. Acta Chim Slovaca 6:269–280
Rajiv J, Lobo S, Jyothilakshmi A, Rao GV (2012) Influence of green gram flour (Phaseolus Aureus) on the rheology, microstructure and quality of cookies. J Tex Stud 43:350–360
Rao UP, Vatsala CN, Rao HP (2002) Changes in protein characteristics during the processing of wheat into flakes. Eur Food Res Technol 215:322–326
Sanni SA, Adebowale AA, Olayiwola IO, Maziya-Dixon B (2008) Chemical composition and pasting properties of iron-fortified maize flour. J Food Agric Environ 6:172–175
Semwal AD, Murthy MCN, Arya SS (1995) Metal contents in some of the processed foods and their effect on the storage stability of pre-cooked dehydrated flaked Bengal gram dhal. J Food Sci Technol 32:386–390
Serrem CA, de-Kock HL, Taylor JRN (2011) Nutritional quality, sensory quality and consumer acceptability of sorghum and bread wheat biscuits fortified with defatted soy flour. Int J Food Sci Technol 46:74–83
Srivastava Y, Semwal AD, Sharma GK, Bawa AS (2010) Effect of virgin coconut meal (VCM) on the textural, thermal and physicochemical properties of biscuits. Food Nutr Sci 2:38–44
Steel RGD, Torrie JH (1960) Principles and procedures of statistics. McGraw-Hill, New York, pp 99–131
Sujirtha N, Mahendran T (2015) Use of defatted coconut flour as a source of protein and dietary fibre in wheat biscuits. Int J Innov Res Sci Eng Technol 4:7344–7352
Sunday SA, Julius MA (2014) Proximate biological value (BV) and protein efficiency ratio (PER) of processed bush mango (I. gabonensis) kernel (PBMK). IOSR J Pharm Biol Sci 9:18–20
Susanna S, Prabhasankar P (2012) Quality, microstructure, biochemical and immunochemical characteristics of hypoallergenic pasta. Food Sci Technol Int 18:403–411
Vicario IM, Griguol V, Leon-Camacho M (2003) Multivariate characterisation of fatty acid profile of Spanish cookies and bakery products. J Agric Food Chem 51:134–139
Vitali D, Dragojevic IV, Sebecic B (2009) Effects of incorporation of integral raw materials and dietary fibre on the selected nutritional and functional properties of biscuits. Food Chem 114:1462–1469
Yadav DN, Thakur N, Sunooj KV (2012a) Effect of partially de-oiled peanut meal flour (DPMF) on the nutritional, textural, organoleptic and physicochemical properties of biscuits. Food Nutr Sci 3:471–476
Yadav RB, Yadav BS, Dhull N (2012b) Effect of incorporation of plantain and chickpea flours on the quality characteristics of biscuits. J Food Sci Technol 49:207–213
Zaker AMD, Genitha TR, Hashmi SI (2012) Effect of defatted soy flour incorporation on physical, sensorial and nutritional properties of biscuits. J Food Process Technol 3:1–4
Acknowledgements
Authors kindly acknowledge the help of Mr C. Mahesh, in handling atomic absorption spectrophotometer instrument and Mrs A. Padmashree in handling gas chromatography instrument.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ashwath Kumar, K., Sharma, G.K. & Anilakumar, K.R. Influence of multigrain premix on nutritional, in-vitro and in-vivo protein digestibility of multigrain biscuit. J Food Sci Technol 56, 746–753 (2019). https://doi.org/10.1007/s13197-018-3533-z
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3533-z