Abstract
In human diet, the products of starch digestion are a major energy source. Starch is stored as water insoluble granules composed of amylose and amylopectin. The susceptibility of starch granule to digestive enzymes is affected by starch granule size, shape, and composition. In this study, starch characteristics and in vitro enzymatic hydrolysis in three rice (Oryza sativa L.) mutants (RSML 184, RSML 278 and RSML 352) with similar amylose concentration (24.3–25.8%) was compared to parent ADT 43 (21.4%). The three mutants had reduced thousand grain weight and starch concentration but higher protein and dietary fiber concentrations. The mutant RSML 352 had small starch granules and reduced short glucan chains [Degree of polymerization (DP) 6–12] compared to the other two mutants (RSML 184 and RSML 278). The mutant RSML 352 had the highest hydrolytic index (HI) and least concentration of resistant starch (RS) compared to the other two mutants and parent rice ADT 43. The two rice mutants (RSML 184 and RSML 278) had reduced HI and increased RS concentration than the parent ADT 43. The results showed that starch granule size and amylopectin structure influence starch enzymatic digestibility and RS concentration.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice (Oryza sativa L.) is the staple food for more than half of the global population (Boonyaves et al. 2017). The world demand for rice is expected to increase from 676 million tonnes in 2010 to 763 and 852 million tonnes by 2020 and 2035, respectively (Khush 2013). To ensure worldwide food security, rice improvement programs have mainly focused to increase the grain yield and production (Khush 2013). However, rice consumption pattern has changed over time from whole grain brown rice rich in dietary fibre to highly processed polished white rice with reduced dietary fibre, minerals and micronutrients (Sudha et al. 2013).
In Asian countries where rice is the staple cereal, it has been associated with increased incidence of type 2 diabetes (T2D) (Hu et al. 2012) as the glycemic index (GI) of white rice is higher than de-husked brown rice (Shobana et al. 2011). In rice grain, starch is the predominant (~ 90%) storage constituent and main source of dietary energy in humans. Based on enzymatic hydrolysis starch can be divided into three fractions: readily digestible- (RDS), slowly digestible- (SDS), and un-digestible or resistant starch (RS) (Englyst and Hudson 1996). RS is the starch fraction or hydrolysis product which escapes digestion in small intestine, but it is partially or completely fermented by the large intestine microflora (Faraj et al. 2004). Due to incomplete hydrolysis RS concentration can reduce the calorific value of food and it can help persons with diabetes to maintain their postprandial blood glucose concentration. RS has been shown to benefit human health by reducing plasma cholesterol and increasing short chain fatty acids in the large intestine (Bird et al. 2004). Physical and chemical modification methods have been used to develop and/or increase RS concentration in food (Maningat and Seib 2013). Alternatively, genetic approaches have also been used to develop grains with increased RS concentration and reduced starch digestibility (Ahuja et al. 2013).
Starch is a complex glucose polymer stored as discrete water insoluble granules composed of about one-quarter amylose and three quarters amylopectin. Amylose is a predominantly linear glucan polymer composed of α-1,4 glucosidic linkages with sparse branches connected by α-1,6 glucosidic linkages. Amylopectin is a highly branched glucan polymer in which α-1,4 linked glucan chains are interspersed with α-1,6 glucosidic linkages that introduce branches. Starch granule size, shape, composition, structure of amylose and amylopectin determine the functional properties and end use of starch (Buléon et al. 1998). Amylopectin is a highly organized molecule in which the glucan chains are organized into clusters, giving the molecule its architectural (Hizukuri 1986) and genotypic specificity (Jaiswal and Chibbar 2017). Briefly, A-chains (DP 6–12) are the outermost chains, linked to B1 (DP 13–24), B2 (DP 25–36) and B3 + (DP ≥ 37) chains that are attached by α-1,6 glucosidic linkage, to the central C-chain, which carries the sole reducing end of the molecule and within a granule is oriented towards the centre or hilum of the granule (Manners 1989).
Increased amylose concentration has been associated with reduced starch enzymatic hydrolysis in barley (Asare et al. 2011) and rice (Hu et al. 2004), hence positively correlated with RS in grains including corn, wheat and barley (Bird et al. 2004; Regina et al. 2006). Amylopectin fine structure also influences starch enzymatic hydrolysis. It is generally believed that increased proportion of longer chains makes starch more resistant to digestion. A possible reason could be that longer chains form stable helices by hydrogen bonds, distributed over the entire crystalline region and reduce digestibility (Lehmann and Robin 2007). However, gamma irradiation generated rice mutants with increased RS had amylopectin with increased proportion of short chains (DP ≤ 12) and decreased proportion of intermediate chains (DP ≤ 36) and reduced number of long (DP ≥ 37) chains (Shu et al. 2007). Rice amylose extender (ae) and waxy amylose extender (wxae) starches were markedly reduced in small glucan chains (6 ≤ DP ≤ 14) and enriched in medium glucan chains (15 ≤ DP) compared to those from the wild type. The primary structure of rice wxae amylopectin with enriched intermediate chains changed the granular and crystal structure of starch, and resulted in vitro and in vivo resistance to enzymatic digestion (Kubo et al. 2010). Another confounding factor in starch enzymatic hydrolysis is the starch granule size. Several previous studies reported a negative correlation of starch granule size to starch digestibility (Singh et al. 2010; Asare et al. 2011). The starch granules of smaller size are highly amenable for enzymatic hydrolysis due to larger specific surface area for enzymatic binding and reaction (Tester et al. 2006). Besides starch granule size, its shape also influences starch digestibility. The shape of the granule varies from spherical to polyhedral thus affecting the specific surface area of the granule significantly (Singh et al. 2010).
The interaction of grain constituents and starch characteristics on enzymatic hydrolysis of starch was studied in a parent rice cultivar (ADT 43) and gamma (γ) irradiation derived three mutants (RSML 184, RSML 278 and RSML 352) with slightly increased (~ 3%) amylose concentration. However, starch digestibility and hydrolytic index was reduced in two mutants (RSML 184 and RSML 278) but it was increased in the mutant (RSML 352).
Materials and methods
Plant material
Rice (Oryza sativa L.) variety ADT 43 is a popular short duration rice, released in 1998 in Tamil Nadu (India) where it is widely cultivated (~ 250,000 hectares annually). The ADT 43 rice was developed from a cross between IR 50 and Improved White Ponni (IWP) and has acceptable milling and cooking qualities. The mutants RSML 184, RSML 278 and RSML 352 were developed through gamma (γ) irradiation of a rice variety ADT 43 (Agasimani et al. 2013). The selected mutants along with ADT 43 were grown at Paddy Breeding Station (PBS, 11.76° N, 76.44° E), Tamil Nadu Agricultural University (TNAU), Coimbatore, Tamil Nadu India in 2013 crop year. The four genotypes (ADT 43, RSML 184, RSML 278 and RSML 352) were grown in a three-replicate trial using Randomized Complete Block Design (RCBD). For best crop establishment, standard agronomic practices were followed as per the crop production guide 2012, TNAU, Coimbatore (http://agritech.tnau.ac.in/pdf/2013/CPG%202012.pdf). The grain was harvested at maturity and stored in the laboratory for 6 months till the analyses were completed. The biochemical analyses were done with three separate technical replicates from pooled grains from a biological replicate.
Sample preparation
Paddy samples were dehulled (Satake THU35A dehusker, Satake Engineering Co Ltd, Tokyo, Japan) and whole grains were dried to 13% moisture content. A sample (1000 grains) was weighed using a precision electronic balance (Mettler Toledo, USA). Three replicates from each sample were independently weighed to determine the thousand grain weight. The dehulled grain was milled (MC250 Satake one-pass mill, Satake Engineering Co. Ltd, Tokyo, Japan), and aspirated to produce white rice. The white rice grains were ground using a cyclone mill (UDY Corp., Fort Collins, CO, USA) with a 0.5 mm sieve. The ground rice meal was used for biochemical characterization. Moisture content of the meal samples were estimated by hot-air oven method and used to calculate dry weight basis of individual grain constituents.
Ash, starch, protein, dietary fiber, fat and phenolics determination
Ash content of the samples were analyzed using AACC International (AACCI Method 08–01.01) approved method. Total starch content was determined using a stepwise enzymatic degradation of starch (AACCI Approved Method 76–13.01) using a commercial kit (Megazyme International Ireland Ltd., Bray, Ireland). Protein concentration was determined by combustion method using a FP-528 Protein/Nitrogen Analyzer (LECO Corporation, St Joseph, MI) (AACCI Approved Method 46–30.01). Fat concentration was determined by AOCS (AOCS Am 5–04) approved method, using hexane as the extraction solvent. The AOAC approved method (AOAC 991.43) was used to determine total dietary fibre (TDF) in rice grain meal (500 mg). Free phenolics from the meal samples were extracted as reported previously (Adom et al. 2003) with slight modifications. The rice meal samples (1 g) suspended in 80% (v/v) cold ethanol were dispersed for 10 min using a vortex mixer. The suspension was centrifuged at 2500 g for 10 min. This process was repeated, the supernatant was collected and pooled together. The pooled supernatant was concentrated to < 5 mL using a rotary evaporator at 45 °C. In all samples the concentrate was reconstituted to 5 mL with distilled water. The reconstituted samples were stored at − 20 °C until further analysis.
The acid and alkaline soluble phenolics were extracted as previously described (Krygier et al. 1982) with slight modification for hydrolysis. Meal sample (1 g), from which free phenolics had been removed, was mixed with 6 M HCL. The container was purged with nitrogen to minimize the oxidation loss of phenolic compounds. The reaction mixture was kept for shaking in a rotary shaker at 2000 rpm for 4 h. After incubation, pH of the solution was adjusted to two with 6 M NaOH. A 50 mL of 1:1 ratio of diethyl ether and ethyl acetate was added to the mixture and container was inverted 11–12 times and then centrifuged at 1000 g for 10 min. This was repeated, and supernatant was collected and pooled. The pooled supernatant was evaporated at 45 °C to < 5 mL and reconstituted to 5 mL with distilled water. The reconstituted samples were stored at -20 °C until further analysis. For alkaline extracted phenolics, meal sample (1 g) (free and acid phenolics removed) was weighed and mixed with 75 mL of 6 M NaOH. Subsequent steps were similar to the acid extracted phenolics as described above. Phenolic content (free, acid and alkali soluble) of extracted samples was determined (Verma et al. 2008) using the Folin-Ciocalteux reagent by reading the Absorbance at 765 nm with a spectrophotometer (Beckman DU 730 UV/Vis, Beckman Coulter, Fullerton, CA, USA). The phenolic content is expressed as gallic acid equivalent (GAE) per g. The total phenolic acid content was calculated by adding the content of free, acid and alkali soluble phenols.
Starch extraction and physical characterization
Starch extraction was carried out as described (Lumdubwong and Seib 2000) with minor modification. The ground rice meal (1 g) was steeped overnight with 0.01 M NaOH (5 mL) and 100 µL of 1% (w/v) protease at 37 °C, and neutralized using 1 M HCl. The solution was centrifuged at 3000 g and the supernatant was discarded. The pellet was suspended in water (1 mL), layered over 80% (w/v) Cesium Chloride solution (1 mL) and centrifuged at 12,000 g for 20 min. The pellet was suspended in water and filtered through 100 µm pore size nylon filter. Supernatant was discarded, and dark tailing layer was removed with spatula. The starch pellet was washed three times with 1 mL of water and centrifuged at 12,000 g for 10 min, followed by acetone (1 mL) and centrifuged at 12,000 g for 10 min and air dried overnight.
Starch granule size distribution of the extracted starch was determined by laser diffraction (Mastersizer 2000, Malvern Instruments, Malvern, England). The extracted starch (30 mg) was weighed and dispersed in 1 mL of sodium dodecyl sulfate 1% (w/v). Starch slurry (200 µL) was used for size analysis at a pump speed of 1700 rpm (Asare et al. 2011). The starch granule structure was analyzed using scanning electron microscopy. Dried starch samples were adhered to pin stub mount using carbon conductive tabs, and then coated with 20 mm gold using Gold Sputter Coater (Edwards S150B). The prepared specimens were viewed for their morphologies by a scanning electron microscope (Hitachi-SU8000, Japan) at an accelerating voltage of 5 kV.
Amylose concentration was determined in purified starch (5 mg) by enzymatic debranching of purified starch and separation of amylose and amylopectin with size exclusion chromatography (Demeke et al. 1999) using a high-performance liquid chromatography (HPLC) equipped with a differential refractometer (Waters Corporation, Milford, MA, USA). Amylopectin Chain length distribution was determined by fluorophore-assisted capillary electrophoresis (FACE) using a Proteome Lab PA800 (Beckman Coulter, Fullerton, CA, USA) equipped with a 488-nm laser module. A modified debranching protocol (Asare et al. 2011) was used to obtain unit amylopectin chains, which were labelled with 8-aminopyrene-1, 3, 6- trisulfonate in the presence of sodium cyano-borohydride/tetrahydrofuran. The N-CHO capillary with polyvinyl alcohol coating and a pre-burned window (50 μm inner diameter, 50.2 cm total length) was used to separate amylopectin chains of diverse degree of polymerization (DP) in a debranched starch sample. Maltose was used as an internal standard. Samples were injected at 0.5 psi for 3 s and separated at constant voltage of 30 kV for 30 min. Data was recorded and analyzed using 32-karat software (Beckman Coulter, USA). The DP was assessed relative to migration time of maltose.
In-vitro enzymatic hydrolysis of meal and extracted starch
In vitro enzymatic hydrolysis in triplicate for both meal (100 mg) and extracted starch (100 mg) was used to study the enzymatic digestion kinetics as described (Ahuja et al. 2014) essentially based on the method by Englyst et al. (1992). A non-linear model, as described (Goñi et al. 1997) was applied to determine the hydrolytic index of rice meal and extracted starch.
Statistical analysis
All the experiments were done on three technical replicates. The mean comparisons were performed using the Tukey’s range test at P < 0.01 confidence level and Pearson’s correlation were analyzed using MINITAB 16.0 statistical software (Minitab Inc., State College, PA, USA).
Results and discussion
The contribution of carbohydrates to dietary glycemic load and subsequent postprandial blood glucose depends on starch digestibility. Starch concentration, composition and interaction with other grain constituent’s influence starch enzymatic hydrolysis. To improve rice nutritional quality for human health benefits, it is imperative to analyze grain constituents, starch characteristics and their interaction influencing starch enzymatic digestibility.
Grain analysis
The TGW was reduced in the mutant genotypes (11.6–12.9 g) compared to wild type (15.7 g). RSML 352, RSML 184, and RSML 278 had 26.1, 20.3 and 17% lower TGW compared to the wild type, respectively. Mutant genotypes contained lower (P < 0.01) starch concentrations (67.5–79.7%) compared to the wild type (82.0%) (Table 1). The ADT43 had a protein concentration of 8.5% while, the mutants showed higher protein concentrations with RSML 278 had the highest protein concentration (11.0%) followed by RSML 352 (10.1%) and RSML 184 (9.7%). The fat concentration in rice mutants ranged (P < 0.01) from 1.2 to 2.6% which is higher compared to the wild type (0.7%) (Table 1). The total dietary fiber (TDF) concentration of the wild type (ADT 43) was 2.2% but significantly (P < 0.01) increased in the mutants (RSML 278 and RSML 352) and varied from 3.0 to 6.1% (Table 1). RSML 278 had the highest TDF of 6.1% while RSML 184 (3.0%) showed the lowest concentration of TDF.
Phenolic compounds are major secondary metabolites (Klepacka and Fornal 2006) present in both free and bound form in cereal grain (Krygier et al. 1982) cell wall. Rice mutants showed significantly (P < 0.01) higher amount of total phenolic content compared to the wild type. Total phenolic concentration varied from 2.1 to 3.4 mg of GAE g−1 in the mutants while, it was 1.1 mg of GAE g−1 in the wild type (Table 1). RSML 278 and RSML 352 had highest ash concentration of 1.54 and 1.51%, respectively compared to the wild type (0.68%). The increased ash content in the mutants (RSML 278 and RSML 352) suggests increased mineral concentrations. Minerals in starch influence the viscosity, paste clarity and stability (Bao and Bergman 2004).
The TGW was positively correlated (r = 0.59) to starch concentration, and negatively correlated to all other grain constituents (Supporting Table S1). Similarly, starch was also negatively correlated to other grain constituents. Amylose concentration showed positive correlation to fat (r = 0.74, P > 0.01) and total phenolic concentration (r = 0.82, P > 0.01). Protein, total dietary fiber, fat and phenolic acids were positively correlated (Supporting Table S1). In most of the grains starch and protein are negatively correlated as the two compete for photosynthates during seed development. Similar negative correlation between starch and protein concentration has been reported in several grains (Asare et al. 2011; Regina et al. 2006).
Starch characteristics
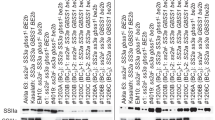
The rice starch granules were angular to polygonal in shape (Supporting Fig. S1), with average diameter around 7 µm for the parent ADT 43, and two mutants RSML 184 and RSML 278. However, the rice mutant RSML 352 starch granules had lower (5 µm) peak diameter (Fig. 1a) and were smaller in size compared to the other two mutants and parent ADT 43 (Table 2). The mutants, RSML 184 (26%) and RSML (28.5%) 278 had significantly (P < 0.01) low proportion of smaller size (< 5 µm) granules, whereas in RSML 352, these small granules contributed more than 50% (Fig. 1a). Amylose concentrations of the three mutants (24.3–25.8%) was slightly higher (~ 3–4%; P < 0.01) than that of the wild type ADT 43 (21.4%) (Table 1).
Characterization of starch from parent ADT 43 and three rice mutants (RSML 184, RSML278, and RSML352) for, a granule size distribution in pure starch, and b amylopectin chain length distribution [Subtraction graph showing DP (degree of polymerization) variation of mutants from the parent genotype ADT 43]
The amylopectin chain length distribution was classified as A chains (DP 6–12), B1 chains (DP 13–24), B2 chains (DP 25–36) and B3 + chains (DP ≥ 37) (Hanashiro et al. 1996). The B1 glucan chains were predominant (> 50%) in all the four rice genotypes, followed by the A chains (24–29%) and the two other types varied from 7 to 8% (Table 2). However, a subtraction curve of the mutants from the parent revealed that RSML 352 had significantly (P < 0.01) higher proportion (29%) of A chains compared to the other two mutants, RSML 184 (24.4%) and RSML 278 (25.8%). However, proportion of B1 and B2 chains were significantly (P < 0.01) higher in RSML 184 (58.1% and 8.6%) and RSML 278 (57.1% and 8.2%) compared to that in RSML 352 (53.9% and 7.2%) (Table 2). The B3 + chains were also reduced in RSML 352 compared to RSML 184 and RSML 278, but the difference was not significant. The enhanced proportion of short chain (DP 6–12) in RSML 352 is probably due to overexpression of starch synthase (SSI), whereas down regulated in RSML 184 and RSML 278 (Crofts et al. 2017). The amylopectin chain variation observed in all three mutant ranged from DP 12–50, we speculated that SS genes may plays a partially/fully overlapping role in the synthesis of various DP of amylopectin and it may have complementary effect from other genes involved in starch synthesis. Therefore, future genetic analysis on the rice mutants will be useful to understand the role of starch synthases in amylopectin synthesis.
In-vitro enzymatic starch hydrolysis
In vitro enzymatic hydrolysis of starch in meal and purified form showed significant difference between the mutants and wild type (Table 3). Kinetic analyses of starch enzymatic hydrolysis in rice grain meal showed that RSML 352 (43.3%) contained significantly (P < 0.01) higher proportion of RDS compared to the wild type (29.4%) and the other two mutants RSML 184 (23.4%) and RSML 278 (24.5%). The SDS, ranged from 52.0 to 57.0%, but did not differ significantly among mutants and parent. RS remains un-hydrolyzed ever after 4 h, showed higher concentrations in RSML 184 (25%) and RSML 278 (20%) compared to the parent (14.5%) whereas, RSML 352 (4.4%) had the least RS concentration (Table 3). The in vitro hydrolysis index (HI) was maximum for RSML 352 (66.4%), followed by the parent (50.0%), RSML 278 (46.0%), and RSML 184 (35.9%), respectively.
The extracted starch also followed the same pattern for RDS, SDS, RS and HI as in grain meal but with different numerical values. The RDS proportions (31.0–52.6%) were higher whereas, SDS (38.9–52.9%) and RS (3.8–20.5%) proportions were lower in extracted starch samples compared to that of in grain meal (23.4–43.3%, 52.0–57.0%, and 4.4–25.2% of RDS, SDS and RS, respectively). The HI of the extracted starch (50.3 and 46.0%) did not differ significantly from the grain meal samples (49.1 and 45.4%) in the parent and RSML 278 whereas, the other two mutants RSML 184 and RSML 352 showed higher HI in the extracted starch (46.8 and 71.7%) than in the grain meal (35.9 and 66.4%), respectively (Table 3).
Starch in vitro enzymatic hydrolysis was affected by starch composition and structure and its interaction with grain constituents. The parent rice ADT 43 and the three mutants showed differences in grain composition, starch characteristics and starch in vitro enzymatic hydrolysis. The rice ADT 43 and RSML 278 grain meal and extracted starch had similar HI ~ 50% and 45%, respectively (Table 3). The other two rice mutants (RSML 184 and RSML 352) the grain meal had lower HI compared to the extracted starch, suggesting that grain constituents affected starch digestibility. The meal contained all the grain constituents including protein, dietary fiber, fat and phenols that have been reported to inhibit activities of major starch metabolizing enzymes, α-amylase and amyloglucosidase, in cereal grains including rice thus reduce starch digestibility (Slavin 2003). Proteins present in the meal could limit the rate of starch hydrolysis by blocking the adsorption sites and therefore influencing the enzyme binding (Singh et al. 2010; Tester et al. 2006). Proteins can also form a protective network with starch granules reducing the rate of starch digestion (Lehmann and Robin 2007). In addition to this, proteins network (disulfide-linked polymers) in cereal grains may also possibly reduce the starch digestion and glycemic response (Singh et al. 2010). The rice mutant RSML 278 had increased SDS concentration, and it also had the highest grain protein concentration among the four genotypes in the study. The rice RSML 278 also had the highest concentration of TDF, which in aqueous solutions forms gel and increase the viscosity of solutions (Castro et al. 2012). The increased protein and TDF concentration can reduce starch digestibility thus increasing concentration of SDS in the meal compared to extracted starch (Table 3). Fat and total phenolic concentrations were also high in the RSML 278. All the four grain constituents (Protein, TDF, fat and total phenolics) present in higher concentrations in RSML 278 showed negative correlation to meal SDS (Table 4). The lipids can interact with amylose and amylopectin chains making them less accessible to α-amylase (Asp et al. 1996; Crowe et al. 2000). Fat can also stick on the surface of the starch granule minimizing its contact with α-amylase that can reduce digestibility compared to free amylose (Tester et al. 2004). In addition to this, phenolic compounds in grains can form carbohydrate esters that may also inhibit the reaction between α-amylase and starch (Singh et al. 2010).
All the three mutants had similar increase in amylose concentration but differed in starch enzymatic hydrolysis and RS concentration. Similar results showing that rice varieties (IR 42, IR 36 and IR 62) with similar amylose concentration (26.7–27%) varied in their in vitro starch digestibility rates and glycemic and insulin responses (Panlasigui et al. 1991). Rice IR 42 had the highest starch digestion and the glycemic and insulin response, followed by IR 36 and then IR 62. In the present report, the three mutants showed similar amylose concentration but the starch granule size was reduced with the most significant decrease observed in the RSML 352. The RSML 352 showed the highest HI both in grain meal and extracted starch with majority of the starch granules less than 5 µm in diameter and starch granules more than 15 µm could not be detected (Table 2). HI in extracted starch showed significant (r = 0.98, P < 0.01) positive relationship with small (< 5 µm) starch granule size and negative correlation (r = 0.98, P < 0.01) with medium (5–15 µm) granule size. An inverse square relationship between starch digestibility coefficient and starch granule size was reported for potato and maize which have a unimodal size distribution (Dhital et al. 2010). However, in species such as wheat, barley, rye and triticale with bi-modal starch granules, the small B-type starch granules were more rapidly hydrolyzed compared to large A-type starch granules (Ahuja et al. 2014; Asare et al. 2011).
The two mutants RSML 184 and RSML 278 starch was enriched in short glucan chains (DP 6–12) but reduced in intermediate and long chain glucans (Fig. 1b) showed reduced starch HI and an increase in RS compared to the parent ADT 43. On the other hand, RSML 352, showed a reduction in short glucan chains, and an increase in the intermediate and long glucan chains caused an increase in HI and reduction in RS. These results concur with an earlier study of rice mutants (Shu et al. 2007) and barley (Asare et al. 2011). However, in corn, an increase in either highly branched short chains or long chains resulted in an increase in SDS (Zhang et al. 2008), thus inferring that starch digestibility is specific to botanical source. Increased RS concentration in the rice mutant showed significant positive correlation with all the medium and long chains [DP 13–24 (r = 0.99, P < 0.01), DP 25–36 (r = 0.95, P < 0.01), and DP ≥ 37 (r = 0.89, P < 0.01)] amylopectin. Consequently, HI showed significant negative correlation with medium and long chains amylopectin (Table 5). DP 6–12 showed significantly negative (r = − 0.96, P < 0.01) and positive (r = 0.75, P < 0.01) relationship with RS concentration and HI of starch, respectively. The changes in starch composition and structure could be due to alterations in starch biosynthetic gene structure and/or expression (Ahuja et al. 2013). Recently, in another study mutations in starch synthases (SSI, SSIIa and SSIIIa) of rice have been associated with enhanced ratio of short glucan chains in amylopectin and changes in resistant starch concentration (Raja et al. 2017). Future genetic studies on the rice mutants will be helpful in understanding the role of starch synthases in amylopectin structure.
Conclusions
Starch digestibility in three rice mutants with similar amylose concentration revealed that starch granule size and amylopectin structure were important determinants of starch digestibility. The mutant RSML 352 with the highest HI had increased proportion of small size starch granules and reduced amount of small and increased large glucan chains. The two mutants with increased small glucan chains and reduced amount of long glucan chains had low HI and high RS concentration compared to the parent ADT 43. The results show the need for detailed analyses of starch composition and structure to identify rice genotypes with desired starch hydrolytic properties. The two rice mutants RSML 184 and RSML 278 can be used in a rice improvement program to develop rice varieties with reduced starch digestibility for improved human health and well-being.
References
Adom KK, Sorrells ME, Liu RH (2003) Phytochemical profiles and antioxidant activity of wheat varieties. J Agric Food Chem 51:7825–7834. https://doi.org/10.1021/jf030404l
Agasimani S, Selvakumar G, Joel AJ, Ganesh Ram S (2013) A simple and rapid single kernel screening method to estimate amylose content in rice grains. Phytochem Anal 24:569–573. https://doi.org/10.1002/pca.2433
Ahuja G, Jaiswal S, Chibbar RN (2013) Starch biosynthesis in relation to resistant starch. In: Shi YC, Maningat CC (eds) Resistant starch sources, applications and health benefits. Wiley, Chichester, pp 1–15
Ahuja G, Jaiswal S, Hucl P, Chibbar RN (2014) Differences in starch granule composition and structure influence in vitro enzymatic hydrolysis of grain meal and extracted starch in two classes of Canadian wheat (Triticum aestivum L.). Cereal Chem 91:233–239. https://doi.org/10.1094/CCHEM-07-13-0139-R
Asare EK, Jaiswal S, Maley J, Båga M, Sammynaiken R, Rossnagel BG, Chibbar RN (2011) Barley grain constituents, starch composition, and structure affect starch in vitro enzymatic hydrolysis. J Agric Food Chem 59:4743–4754. https://doi.org/10.1021/jf200054e
Asp NG, Van Amelsvoort JMM, Hautvast JGAJ (1996) Nutritional implications of resistant starch. Nutr Res Rev 9:1–31. https://doi.org/10.1079/NRR19960004
Bao J, Bergman CJ (2004) The Functionality of Rice Starch. In: Eliassion CA (ed) Starch in food: structure, function and applications. Woodhead Publishing Limited/CRC Press LLC, New York, pp 258–294
Bird AR, Flory C, Davies DA, Usher S, Topping DL (2004) A novel barley cultivar (Himalaya 292) with a specific gene mutation in starch synthase IIa raises large bowel starch and short-chain fatty acids in rats. J Nutr 134:831–835. https://doi.org/10.1093/jn/134.4.831
Boonyaves K, Wu TY, Gruissem W, Bhullar NK (2017) Enhanced grain iron levels in rice expressing an iron-regulated metal transporter, nicotianamine synthase, and ferritin gene cassette. Front Plant Sci 8:130. https://doi.org/10.3389/fpls.2017.00130
Buléon A, Colonna P, Planchot V, Ball S (1998) Starch granules: structure and biosynthesis. Int J Biol Macromol 23:85–112. https://doi.org/10.1016/S0141-8130(98)00040-3
Castro A, Bergenståhl B, Tornberg E (2012) Parsnip (Pastinaca sativa L.): dietary fibre composition and physicochemical characterization of its homogenized suspensions. Food Res Int 48:598–608. https://doi.org/10.1016/j.foodres.2012.05.023
Crofts N, Sugimoto K, Oitome NF, Nakamura Y, Fujita N (2017) Differences in specificity and compensatory functions among three major starch synthases determine the structure of amylopectin in rice endosperm. Plant Mol Biol 94:399–417. https://doi.org/10.1007/s11103-017-0614-8
Crowe TC, Seligman SA, Copeland L (2000) Inhibition of enzymic digestion of amylose by free fatty acids in vitro contributes to resistant starch formation. J Nutr 130:2006–2008. https://doi.org/10.1093/jn/130.8.2006
Demeke T, Hucl P, Abdel-Aal ES, Båga M, Chibbar RN (1999) Biochemical characterization of the wheat waxy A protein and its effect on starch properties. Cereal Chem 76:694–698. https://doi.org/10.1094/CCHEM.1999.76.5.694
Dhital S, Shreshta AK, Gidley MJ (2010) Relationship between granule size and in vitro digestibility of maize and potato starches. Carbohydr Polym 82:480–488. https://doi.org/10.1016/j.carbpol.2010.05.018
Englyst HN, Hudson GJ (1996) The classification and measurement of dietary carbohydrates. Food Chem 57:15–21. https://doi.org/10.1016/0308-8146(96)00056-8
Englyst HN, Kingman SM, Cummings JH (1992) Classification and measurement of nutritionally important starch fractions. Eur J Clin Nutr 46:S33–S50
Faraj A, Vasanthan T, Hoover R (2004) The effect of extrusion cooking on resistant starch formation in waxy and regular barley flours. Food Res Int 37:517–525. https://doi.org/10.1016/j.foodres.2003.09.015
Goñi I, García-Alonso A, Saura-Calixto F (1997) A starch hydrolysis procedure to estimate glycemic index. Nutr Res 17:427–437. https://doi.org/10.1016/S0271-5317(97),00010-9
Hanashiro I, Abe JI, Hizukuri S (1996) A periodic distribution of the chain length of amylopectin as revealed by high-performance anion-exchange chromatography. Carbohydr Res 283:151–159. https://doi.org/10.1016/0008-6215(95)00408-4
Hizukuri S (1986) Polymodal distribution of the chain lengths of amylopectins, and its significance. Carbohydr Res 147:342–347. https://doi.org/10.1016/S0008-6215(00)90643-8
Hu P, Zhao H, Duan Z, Linlin Z, Wu D (2004) Starch digestibility and the estimated glycemic score of different types of rice differing in amylose contents. J Cereal Sci 40:231–237. https://doi.org/10.1016/j.jcs.2004.06.001
Hu EA, Pan A, Malik V, Sun Q (2012) White rice consumption and risk of type 2 diabetes: meta-analysis and systematic review. BMJ 344:1454. https://doi.org/10.1136/bmj.e1454
Jaiswal S, Chibbar RN (2017) Amylopectin small chain glucans form structure fingerprint that determines botanical origin of starch. Carbohydr Polym 158:112–123. https://doi.org/10.1016/j.carbpol.2016.11.059
Khush GS (2013) Strategies for increasing the yield potential of cereals: case of rice as an example. Plant Breed 132:433–436. https://doi.org/10.1111/pbr.1991
Klepacka J, Fornal Ł (2006) Ferulic acid and its position among the phenolic compounds of wheat. Crit Rev Food Sci Nutr 46:639–647. https://doi.org/10.1080/10408390500511821
Krygier K, Sosulski F, Hogge L (1982) Free, esterified, and insoluble-bound phenolic acids. 1. Extraction and purification procedure. J Agric Food Chem 30:330–334. https://doi.org/10.1021/jf00110a028
Kubo A, Colleoni C, Dinges JR, Lin Q, Lappe RR, Rivenbark JG, Meyer AJ, Ball SG, James MG, Hennen-Bierwagen TA, Myers AM (2010) Functions of heteromeric and homomeric isoamylase-type starch debranching enzymes in developing maize endosperm. Plant Physiol 153:956–969. https://doi.org/10.1104/pp.110.155259
Lehmann U, Robin F (2007) Slowly digestible starch–its structure and health implications: a review. Trends Food Sci Technol 18:346–355. https://doi.org/10.1016/j.tifs.2007.02.009
Lumdubwong N, Seib PA (2000) Rice starch isolation by alkaline protease digestion of wet-milled rice flour. J Cereal Sci 31:63–74. https://doi.org/10.1006/jcrs.1999.0279
Maningat CC, Seib PA (2013) RS4-type resistant starch: chemistry, functionality and health benefits. In: Shi YC, Maningat CC (eds) Resistant starch: sources, applications and health benefits. Willey Blackwell, West Sussex, pp 43–78
Manners DJ (1989) Recent developments in our understanding of amylopectin structure. Carbohydr Polym 11:87–112. https://doi.org/10.1016/0144-8617(89)90018-0
Panlasigui LN, Thompson LU, Juliano BO, Perez CM, Yiu SH, Greenberg GR (1991) Rice varieties with similar amylose content differ in starch digestibility and glycemic response in humans. Am J Clin Nutr 54:871–877. https://doi.org/10.1093/ajcn/54.5.871
Raja RB, Agasimani S, Jaiswal S, Thiruvengadam V, Sabariappan R, Chibbar RN, Ram SG (2017) EcoTILLING by sequencing reveals polymorphisms in genes encoding starch synthases that are associated with low glycemic response in rice. BMC Plant Biol 17:13. https://doi.org/10.1186/s12870-016-0968-0
Regina A, Bird A, Topping D, Bowden S, Freeman J, Barsby T, Kosar-Hashemi B, Li Z, Rahman S, Morell M (2006) High-amylose wheat generated by RNA interference improves indices of large-bowel health in rats. Proc Natl Acad Sci USA 103:3546–3551. https://doi.org/10.1073/pnas.0510737103
Shobana S, Malleshi NG, Sudha V, Spiegelman D, Hong B, Hu FB, Willett WC, Krishnaswamy K, Mohan V (2011) Nutritional and sensory profile of two Indian rice varieties with different degrees of polishing. Int J Food Sci Nutr 62:800–810. https://doi.org/10.3109/09637486.2011.585962
Shu X, Jia L, Gao J, Song Y, Zhao H, Nakamura Y, Wu D (2007) The influences of chain length of amylopectin on resistant starch in rice (Oryza sativa L.). Starch-Stärke 59:504–509. https://doi.org/10.1002/star.200700640
Singh J, Dartois A, Kaur L (2010) Starch digestibility in food matrix: a review. Trends Food Sci Technol 21:168–180. https://doi.org/10.1016/j.tifs.2009.12.001
Slavin J (2003) Why whole grains are protective: biological mechanisms. Proc Nutr Soc 62:129–134. https://doi.org/10.1079/PNS2002221
Sudha V, Spiegelman D, Hong B, Malik V, Jones C, Wedick NM, Hu FB, Willett W, Bai MR, Ponnalagu MM, Arumugam K (2013) Consumer acceptance and preference study (CAPS) on brown and undermilled Indian rice varieties in Chennai, India. J Am Coll Nutr 32:50–57. https://doi.org/10.1080/07315724.2013.767672
Tester RF, Karkalas J, Qi X (2004) Starch—composition, fine structure and architecture. J Cereal Sci 39:151–165. https://doi.org/10.1016/j.jcs.2003.12.001
Tester RF, Qi X, Karkalas J (2006) Hydrolysis of native starches with amylases. Anim Feed Sci Technol 130:39–54. https://doi.org/10.1016/j.anifeedsci.2006.01.016
Verma B, Hucl P, Chibbar RN (2008) Phenolic content and antioxidant properties of bran in 51 wheat cultivars. Cereal Chem 85:544–549. https://doi.org/10.1094/CCHEM-85-4-0544
Zhang G, Ao Z, Hamaker BR (2008) Nutritional property of endosperm starches from maize mutants: a parabolic relationship between slowly digestible starch and amylopectin fine structure. J Agric Food Chem 56:4686–4694. https://doi.org/10.1021/jf072822m
Acknowledgements
Monsanto’s Beachell-Borlaug International Scholars Program is acknowledged for an award to BRR for his Ph.D. studies. Canada Research Chairs program (RNC) is acknowledged for financial support.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ramadoss, B.R., Gangola, M.P., Agasimani, S. et al. Starch granule size and amylopectin chain length influence starch in vitro enzymatic digestibility in selected rice mutants with similar amylose concentration. J Food Sci Technol 56, 391–400 (2019). https://doi.org/10.1007/s13197-018-3500-8
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-018-3500-8