Abstract
Nisin is a lantibiotic exhibiting antimicrobial activity against a wide range of Gram-positive bacteria, or some Gram-negative bacteria when used in combination with other preservative agents. The objective of the present work was to study the effect of nisin treatment on biogenic amines occurrence and shelf life of refrigerated (4 °C) vacuum packaged rainbow trout samples. For this purpose samples were divided in two batches: the experimental batch (CB-N), consisting of samples immersed in sterilized broth formulated with soy milk 1.4% (v/v) and whey powder 11.2% (w/v) dissolved in deionized water with addition of nisin (500 mg L−1); the control batch (CB), consisting of samples immersed in the former broth without addition of nisin. A positive effect of nisin resulted on colour stability; in fact, the global colour index ΔE remained constant during the storage of treated rainbow trout samples, while it increased in the control. However, the behaviour of microbiota, texture, odour and biogenic amines were comparable between fillet samples treated with nisin broth and with control medium (without nisin). No inhibitory effects of nisin on biogenic amines accumulation was observed; conversely, the decline of histamine content (about 30%), observed only in fishes of the control batch, may be correlated to the presence of histamine-degradating bacteria (Pseudomonas species). Further studies are necessary to investigate nisin action mechanism on the colour, an important physical characteristic involved in the product quality and consumer acceptability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last years, aquaculture has increased considerably throughout the world and in Italy rainbow trout (Oncorhynchus mykiss) is the principal product (FAO 2014). Enzymatic and chemical reaction are usually responsible for the initial loss of freshness, while microbial activity is responsible for subsequent spoilage (Mohan et al. 2008).
In addition to microbial spoilage, the occurrence of unpleasant volatile compounds and physical changes of texture and colour influence the quality deterioration of rainbow trout fillets. In particular, the most prominent visual quality characteristic of salmonid fish including the trout is the red/pink flesh colour.
Several strategies have been used to extend the shelf life of fresh fish products and, during the recent years, the combined treatment through creation of multiple hurdles to control microbial growth in food products is a very prominent strategy. In particular, vacuum-packaging (VP) associated to refrigeration, has become increasingly popular as preservation techniques, which has brought major changes in storage, distribution, and marketing of raw and processed products to meet consumer demands (Özogul et al. 2004). In addition to combination of chilling with modified atmosphere packaging and treatment with permitted additives, the use of edible coatings has been studied to increase the shelf life of rainbow trout (Raeisi et al. 2014).
Nisin is a lantibiotic that was discovered before penicillin, exhibiting antimicrobial activity against a wide range of Gram-positive bacteria (vegetative cells and spores); in addition it may be useful against some Gram-negative bacteria when used in combination with other preservative agents (Balciunas et al. 2013). This bacteriocin has been accepted for commercial application, being a Generally Regarded as Safe (GRAS) compound, approved as a natural food preservative by around 50 countries, as well as by the Food and Agriculture Organization/World Health Organization and the European Union (FDA 1988; EU 2004). Nisin is an efficient tool in the hurdle technology for food preservation and it was included as biopreservative ingredient in the European food additive list with the number E234 (EFSA 2006).
In the last years many researchers have applied nisin to inhibit the pathogens related to food manufacturing, however only few researches have been carried out on the effect of nisin (combined with other preservative technologies) on the shelf life of species with high commercial value such as freshwater rainbow trout (Oncorhynchus mykiss) (Nykänen et al. 2000; Vázquez et al. 2006). Kakatkar et al. (2017) showed that combination of nisin and irradiation can extend the shelf life of minimally processed seer fish steaks in chilled storage.
In food systems, the antibacterial activity of nisin decreases due to many factors, such as enzymatic degradation, interaction with food components or lability at higher pH values; therefore various delivery systems were developed, based on the coating of nisin using food grade carbohydrates, alginate or lipids (Gruskiene et al. 2017). Use of nisin in its free form is associated with loss of activity, so some encapsulation methods have been introduced to overcome in the best way these limitations. In particular, Imran et al. (2015) observed that liposomes formulation made from soy lecithin may provide an efficient nanodelivery system for nisin. In a previous study, Montalvo Rodríguez (2014) investigated the optimal formulation of culture broth for nisin lactic acid bacteria producers, demonstrating an effect of soy milk and whey protein on nisin activity, which was more stable than nisin in acqueous solution.
The objective of the present work was to implement a mild technology treatment to extend the shelf life of a highly perishable raw material, such as rainbow trout fillets, that also had a low impact both on the cost of the final product and on the environment (eco-sustainable). For this purpose, the effect of the nisin broth treatment on biogenic amines and the shelf life of rainbow trout fillets was investigated, monitoring the main quality properties during storage time of vacuum packaged and refrigerated (4 °C) samples.
Materials and methods
Approximately 450–500 g rainbow trouts (n = 33) were collected from a commercial farm (geografically located in Giulianova, Italy): fishes were caught, slaughtered (with electrical stimulation immediately after death), gutted, washed and manually filleted and transported to laboratory (in no more than 10 min) in expanded polyester boxes with ice.
All chemicals were of analytical reagent grade and supplied by Carlo Erba (Milan, Italy). Standards were obtained from Sigma (Bellefonte, USA).
All methods applied in the study (for sample treatment and analytical determination) and apparatus are described below.
Fish treatment
Trout fillets (n = 60) were divided into two differently treated experimental groups: a) the control batch (CB), consisting of 30 fillets immersed in sterilized broth formulated, in according to Montalvo Rodríguez (2014), with soy milk 1.4% (v/v) (Soyadrink Valsoia, Bologna, Italy) and food grade whey powder 11.2% (w/v) (Farmalabor s.r.l., Canosa di Puglia, Italy) dissolved in deionized water.; b) the experimental batch (CB-N), consisting of 30 fillets immersed in the former broth with addition of nisin 500 mg L−1 (Nisin from Lactococcus lactis, 2.5% balance sodium chloride and denatured milk solids, EC No 215-807-5, Sigma).
Both treatments were carried out on fillets for 30 min at 12 °C; after immersion the fillets were drained, gently dried on paper towel and then under vacuum packed in vacuum bags (Nylon coupled with PA/PE, thickness of 80 µ) by belt vacuum chamber machines Orved VM18 (ORVED S.p.A., Musile di Piave, Italy).
Samples of the two experimental batches CB and CB-N were analysed prior to treatment (characterization of raw fishes quality) and during refrigerated storage (4 °C). Samples were taken after treatment (t = 0 days) and after 1, 5, 12, and 21 days, and were immediately analysed for microbiological, sensory, physical, physico-chemical and chemical analyses.
Analytical procedure
pH, water activity and moisture content
Water activity was measured at 25 °C using a dew point hygrometer AquaLab CX 2 (Aqualab Scientific Pty Ltd., Castle Hill, NSW) on nine replicates samples of each treatment. Calibration with different saturated salt solutions at different Equilibrium Relative Humidity (ERH %) was performed before the analyses.
The pH values were measured by a pH-meter 3510 model (JENWAY, Bibby Scientific Limited, Staffordshire, ST15 OSA, UK).
The moisture content was determined as % on samples (2 g) dried in oven (105 °C overnight) followed AOAC procedure (AOAC 2002).
Microbiological analysis
Ten grams of each trout fillet were suspended in sterile 0.1% (w/v) peptone-water solution and homogenized with a Stomacher Lab-Blender 400 (PBI International Milan, Italy) for 2 min. The following microbial groups were determined: total aerobic mesophilic and psychrotrophic bacteria on Plate Count Agar (PCA) (Oxoid Ltd, Basingstoke, Hampshire, England) at 30 and 6 °C for 48 and 72 h respectively, enterococci on Slanetz & Bartley agar (S&B, Oxoid) at 37 °C for 48 h; total enterobacteria on Violet Red Bile glucose Agar (VRGBA, Oxoid) at 37 °C for 24 h; total coliforms on Violet Red Bile Agar (VRBA, Oxoid) at 37 °C for 24 h; micrococci and staphylococci on Mannitol Salt Agar (MSA, Oxoid) at 30 °C for 72 h; yeasts and moulds on Yeast extract-Peptone-Dextrose agar (YPD, Oxoid), added of 150 ppm chloramphenicol, at 30 °C for 72 h.
Texture
Rainbow trout fillets texture was measured with an Instron dynamometer mod. 5542-H5036 (Instron International Limited, High Wycombe, UK) equipped with a Warner–Bratzler cell. The shear strength (N) required to shear through the sample was determined using a Warner–Bratzler blade (blade speed: 50 mm min−1) with samples (at 4 °C) cut into rectangular pieces (15 × 20 × 10 mm), taken from trout fillets perpendicularly to the muscle fibre length. At least ten specimens were taken (two for each differently processed fillets) and analysed by force/deformation graphs, according to Bourne (Bourne 2002).
Colour
Colour investigation was performed following the guidelines of American Meat Science Association (AMSA 2012). Ten of each differently treated vacuum packaged fillet (stored at 4 °C) were analysed using a Spectrophotometer Chroma Meter CM-508d (Minolta Co., Ltd., Osaka, Japan), with illuminant D65 and a 10° observer angle and set with the specular component excluded (SCE mode); the colourimeter was calibrated using the white porcelaine calibration plate; colour was expressed as L* (lightness, intensity of white colour), a* (+a, red; −a, green) and b* (+b, yellow; −b, blue) values, determined in different locations of fillet samples; moreover, these values were used for the calculation of the hue angle value [=arctan (b*/a*)] and chroma or saturation index [=(a*^2 + b*^2) ^1/2]; colour differences were also determined using the following formula [ΔE* = (ΔL*^2 + Δa*^2 + Δb*^2)^1/2] (Sanchez-Zapata et al. 2011).
Biogenic amines
The following eight biogenic amines were detected, identified and quantified: ethylamine (ETY), β-phenylethylamine (β-PHE), putrescine (PUT), cadaverine (CAD), histamine (HIS), tyramine (TYR), spermidine (SPD) and spermine (SPM).
The procedure of amines extraction and derivatization was carried out as described by Martuscelli et al. (2009): an aliquot of 4 g muscle trout was homogenized (in Stomacher Lab blender 400, International PBI, Milan, Italy) with 10 mL of 5% trichloracetic acid (TCA) containing 10 mg L−1 of internal standard (1,7-diaminoheptane) (Fluka, Milano, Italia) and centrifuged at 10.000 rpm for 20 min at 4 °C (refrigerated centrifuge ALC4237R, ALC International srl); the supernatant was recovered and the extraction was performed with 5% TCA acid. The two acid extracts were mixed and made up to 50 mL with 5% TCA acid; the final acid extract was filtered through Whatman 54 paper (Carlo Erba).
For derivatization of the samples, an aliquot of each acid extract (0.5 mL) was mixed with 150 μL of a saturated NaHCO3 solution and the pH was adjusted to 11.5 with about 150 μL NaOH 1.0 M. Dansyl chloride (Fluka) solution (2 mL of 10 mg mL−1 dansyl chloride/acetone) was added to the alkaline amine extract. Derivatized extracts were transferred to an incubator and kept for 60 min at 40 °C under agitation (195 stokes) (Dubnoff Bath-BSD/D, International PBI, Milano, Italy). The residual dansyl chloride was removed by adding 200 mL of 300 g L−1 ammonia solution (Carlo Erba). After 30 min at 20 ± 1 °C and protected from light, each sample was brought up to 5 mL with acetonitrile (Carlo Erba) and filtered through a 0.22 µm PTFE filter (Alltech, Sedriano, Italy). HPLC analysis was performed on 10 µl of each samples.
The separation of the analytes was carried out using a Waters Spherisorb C18 S3ODS-2 column (3 µm particle size, 150 mm × 4.6 mm I.D.), equipped with a Waters Spherisorb S5ODS-2 guard column. Identification of the biogenic amines was based on their retention times.
The calibration curves, i.e. the peak area versus concentration, were linear in the range of concentration between 0.5 and 50 mg L−1. The lines of regression calculated have been used to compute the amount of the analytes in samples by interpolation, using external standard method.
Limit of detection, precision and accuracy of the method were assessed. The accuracy, evaluated by recovery experiments and performed on the same spiked samples used for assessing precision, was found to be between 78 and 85% for all analytes with the exception of tyramine (76%), spermidine (72%) and spermine (68%). To check if the method was applied efficiently, quality control was carried out on the day of each round analysis by spiking fish samples with biogenic amines and running the analyses, obtaining recoveries always in agreement with the data previously recorded.
Biogenic amines analysis was carried out by a chromatographic system consisting in an HPLC Waters Alliance equipped with a Waters 2695 separation module connected to a Waters 2996 photodiode array detector. The system was governed by Waters Empower personal computer software.
Sensory evaluation
Sensory analysis was carried out by a group of five panellists, trained for evaluation of freshness and quality assessment of refrigerated rainbow trout fillets. The panel had no background information about the samples.
Sensory evaluation was carried out on trout fillets during storage (0, 1, 5, 12 and 21 days); the appearance of unopened packages and the stiffness and odour characteristics in just opened packages were evaluated by panellists; samples were coded, the order of samples was randomized and a quality scale ranging from 0 to 3 was used (0—excellent, no defects; 3—rejected).
Many authors have indicated a good correlation between sensory characteristics and biochemical parameters as total volatile basic nitrogen (TVB-N) and trimethylamine (TMA) (Ruiz-Capillas and Moral 2001), so the purpose of the present research was to evaluated only odour characteristic as suitable indirect index for changes in TVB-N and TMA during storage time.
Statistical analysis
All analyses were conducted on each fillet rainbow trout sample; six fillets samples were analysed for each investigated parameter, before treatment and during the storage time.
Analysis of variance (ANOVA) and multiple mean comparisons with least significant differences (LSD) test were used to test the significance of difference caused by two factor: nisin treatment and time storage.
Data were processed by using Microsoft Excel 2011 for Mac and Statistica 8.0 for Windows (StatSoftTM, Tulsa, UK).
Results and discussion
Characterization of raw rainbow trout fillets
In order to characterize the raw material, rainbow trout fillets were evaluated for chemical, physical–chemical, physical and microbiological parameters after arrival to the laboratory.
Table 1 shows data of weight, moisture content, water activity, pH and some instrumentally measured organoleptic properties (colour, texture). Collected data demonstrated the uniformity of rainbow trout fillets employed for this study. The pH values of 6.6 ± 0.07 and moisture content were comparable to literature data (Chytiri et al. 2004).
In Table 1, it can be observed that the values of L*, a* and b* before treatment were comparable with those observed in other studies on the same matrix (Choubert and Baccaunaud 2010); these values were used as reference in the formula for calculating the ΔE* (during time storage of both of the differently treated samples). Furthermore, the high values of L* (48.79 ± 2.69) are attributable to the slaughter technology used (with electrical stimulation immediately after death) (Robb et al. 2000).
As evidenced in Table 1, the low levels of microorganisms at the beginning of storage indicate the good quality of the raw material. Psichrotrophic bacteria counts were similar to mesophilic bacteria, both related to the presence of Pseudomonadaceae. Although some microbial groups were around 5 Log CFU g−1, this values could be considered within the microbiological limits for fresh fish: guidelines, standards and specifications as part of purchase agreements accept a total aerobic psychrotrophic count of 6 Log CFU g−1 for human consumption of chilled fish, whilst the spoilage generally becomes organoleptically detectable when a total aerobic psychrotrophic count of 7–8 Log CFU g−1 occurs (Broekaert et al. 2011).
In fishery products, many bacterial species are potential producers of toxic compounds as biogenic amines (BAs), when strains with amino acid decarboxylase enzymes occur (Bulushi et al. 2009). Data of literature reported strains of Escherichia, Salmonella, Clostridium and Bacillus with high histidine decarboxylase activity (Ordonez et al. 1999). The formation of histamine (HIS), tyrosine (TYR), and cadaverine (CAD) seems to be related to mesophilic microbiota, Enterobacteriaceae and coliforms (Veciana-Nogués et al. 1997).
The quantification of biogenic amines production in the fish muscle can be used as index of microbial spoilage: histamine has been traditionally used as a quality indicator for histidine-rich fish (dark-muscle fish); on the other hand, putrescine and cadaverine are the most objective indicators of quality of histidine-poor fish (white-muscle fish), shellfish and fermented seafood products, in fact putrescine <10÷14 mg kg−1 was suggested as a limit for acceptable quality of rainbow trout (Prester, 2011). In the present study the putrescine content in raw fish was 6.4 ± 0.9 mg kg−1, confirming a state of excellent freshness of the raw fillets employed in this research.
Change of quality indexes during the storage of treated rainbow trout fillets
Fresh fish, including rainbow trout, is more susceptible to deterioration than meat. The chemical and enzymatic reactions are generally responsible for the initial loss of freshness, while microbial activity is the cause of the consequent alteration (Mohan et al. 2008).
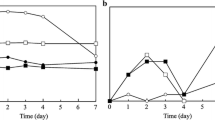
Figure 1 shows the behaviour of moisture content (%), aw and pH values in the samples during storage. It is possible to evidence that moisture did not change significantly after immersion (Fig. 1 a) with respect to raw fillets (see Table 1). However, there was a decline in both samples in the latter storage stages, which could be associated with the decrease in pH approximating the isoelectric point of the fish proteins (especially in the control sample). The biochemical phenomena, mainly of proteolysis, which occur in the course of fish deterioration cause changes of pH values, generally resulting in an increase due to the release of basic amino acids (Mohan et al. 2008). Observing Fig. 1b, it appears evident that the pH values in the sample treated with nisin (CB-N) remained constant, unlike the samples treated with control medium (CB) in which the progressive decline up to 5.96 can be attributed to the activity of lactic acid bacteria (7 log CFU g−1).
As showed in Fig. 1c, the aw trends were similar in both control and nisin sample.During storage time, mesophilic and Enterobacteriaceae counts gradually increased and the limit values associated to the microbial spoilage (Arashisar et al. 2004) were reached after 5 days in both the investigated batches (6 log CFU g−1 and 3 log CFU g−1 respectively); furthermore a dominance of psychrotrophic bacteria was observed, with 7.8 ± 0.4 log CFU g−1 in CB and 7.4 ± 0.2 log CFU g−1 in CB-N. Up to the fifth day of storage, the Pseudomonadaceae counts resulted considerable, a little higher in control samples (6.4 ± 0.3 log CFU g−1) compared to those treated with nisin (5.9 ± 0.4 log CFU g−1), without significant difference (p < 0.05). These bacteria are considered as Specific Spoilage Organisms (SSO) in fish stored in refrigeration. From these data, it can be concluded that the trout fillets in this study had a shelf life of 5 days. In the vacuum refrigerated fishes, the species involved in the spoilage association mainly consist of Gram positive bacteria (Gram et al. 2002); in fact, the lack of oxygen should stop the development of extremely competitive microorganisms such as Pseudomonas spp., favouring other spoiling bacteria not needing oxygen (including Shewanella putrefaciens and Photobacterium phosphoreum). In this work no significant effect of nisin treatment was observed on Gram negative bacteria studied in refrigerated vacuum packaged fillets; on the contrary, a little effect was observed in lactic acid bacteria count, that remained constant during storage time with respect to untreated controls, in which LAB increased up to 7 log CFU g−1. Studies performed by Gao et al. (2014), using an aqueous solution of nisin (5% w/v) on fish fillets, evidenced a reduction of approximately 1 log CFU g−1 of total mesophilic bacteria, during storage at 4 °C. On the other hand, Abdollahzadeh et al. (2014) observed a weak inhibition against L. monocytogenes in minced fish samples treated with nisin at concentration of 500 IU g−1.
One of the hypothesized mechanisms for the antibacterial action of nisin considers the possibility of formation of pores in the bacterial membrane through which the monomers of the nisin are joined and polymerize, altering the cells osmotic pressure (Sobrino-López and Martín-Belloso 2008). It is well known that Gram positive bacteria are more susceptible to nisin effect than Gram negative ones (Balciunas et al. 2013). In general nisin can induce cell autolysis and inhibit outgrowth of bacterial spores (Gut et al. 2008); in addition it can inhibit peptidoglycan synthesis (Brötz et al. 1998). Furthermore, nisin activity against Gram positive bacteria (such as LAB) is due to an interaction with phospholipid membrane without specific receptor. Lins et al. (1999) analysed the interactions between nisin and phospholipid membrane by molecular modelling; results suggested that N-terminal part of nisin is implied in the insertion of nisin in lipids, while the C-terminal part could be involved in the interaction with membrane surface. As can be observed from Fig. 2, the accumulation of amines has been considerable in all the vacuum packaged trout samples. Most of the analysed biogenic amines showed an increase during storage; remarkable was the accumulation of histamine, tyramine, cadaverine, putrescine and ethylamine in both the investigated batches (Fig. 2a, b). These data seem to disagree with studies of Prester (2011) that reported putrescine limit for the quality assesment during rainbow trout shelf life.
High histamine levels in species of the Sgombroidae family have been explained with their naturally high free histidine contents (Bulushi et al. 2009), however, researches carried out on other fishes, such as freshwater ones, have also demonstrated the increase of histamine and other biogenic amines during storage time. Kuley et al. (2011) investigated the biogenic amines occurrence in trout fillets and observed that brine solution had an inhibitory effect on histamine, putrescine and cadaverine, whilst lactic acid bacteria did not seem to play an important role on biogenic amines production by pathogenic bacteria.
Results of the present study showed no inhibitory effects of nisin on biogenic amines accumulation. These data did not seem to disagree with studies of Kurt and Zorb (2010) in which nisin (500 or 1000 IU g−1) decreased counts of lactic acid bacteria and increased the putrescine, cadaverine, and spermidine levels in Turkish dry fermented sausage.
However, detail of the present study is the behaviour of histamine. In Fig. 2 it is possible to notice that, although histamine increased in both samples up to the twelfth day of sampling, subsequently a decline (about 30%) was detected only in fishes of the control batch (CB, Fig. 2a), whilst in CB-N ones (Fig. 2b) histamine continued to accumulate (increasing by 80% from the twelfth to the twenty-first day of observation). Histamine has been correlated to histidine decarboxylase activity occurring in several pathogenic bacteria (e.g. Escherichia spp., Salmonella spp., clostridia and Bacillus spp.) (Ordonez et al. 1999). It should be considered that, within the same species, the presence, the activity and the specificity of decarboxylases enzymes are strain-specific (Bover-Cid and Holzapfel 1999). However, the histamine decomposition was also observed in other studies on fish stored at low temperature (Guizani et al. 2005); these results may be correlated to the presence of histamine-degradating bacteria, such as Pseudomonas species (Sato et al. 1995), which were found in greater numbers in the control sample CB.
Some authors have proposed the Biogenic Amines Index (BAI) in order to assess fish deterioration during storage time. Karmas (1981) established the quality of fish products taking into account the values of five biogenic amines according to the formula: [HIS + PUT +CAD]/[1 + SPM + SPD]. This index is expressed as a score from 1 to 10 and has a good correlation with the organoleptic test: ≤10 acceptable product; ≥10 decomposed product. Veciana-Nogués et al. (1997) also proposed a formula for BAI calculation as the sum of HIS + TYR + CAD + PUT, which showed good correlation with both time of storage and organoleptic assessment (limit of acceptance for tuna: <50 mg kg−1).
The BAI values were calculated on the fillet samples during storage: at initial storage time, the BAI using both the formula of Karmas (1981) and of Veciana-Nogués et al. (1997) resulted of 0.41 and 11.5 mg kg−1 values respectively, below the limits considered to be of top quality. Results showed that BAI values were upper than the limit of quality assurance after 5 days of storage (Fig. 3), when odour acceptability was beginning to decrease (Table 2). Histamine has also been studied in correlation to sensory changes, even if Lopez-Sabater et al. (1996) did not demonstrate a direct relationship between HIS and deterioration of sensory quality in tuna fish. Texture is an important quality characteristic in refrigerated fish. Both sensory and instrumental analyses have monitored a decrease in the consistency during storage of both differently treated samples, but without any significant effect of nisin on this quality parameter. In fact, a similar score has been assigned by the judges about the stiffness, being moderately acceptable (score 1) from the fifth day onward (Table 2); instrumentally, at the end of storage time (21 days) the maximum cutter force resulted at 7.8 ± 1.4 and 8.3 ± 1.9 N, in CB and CB-N samples respectively. This result is explainable by the proteolytic phenomena occurring by endogenous enzymes activity as well as by microbiological spoilage (e.g. Pseudomonas spp.) (Godiksen et al. 2009; Sato et al. 1995). Several authors have studied the role of endogenous proteolytic enzymes in the degradation process that occurs with a softening of muscle in fish products. In particular lysosomal cathepsins, especially cathepsin B, cathepsin L and cathepsin D (particularly active in the fish) are considered as responsible for the breakup of the myofibrillar structures (Delbarre-Ladrat et al. 2006).
The values of L*, a* and b*, instrumentally monitored during chilled preservation of vacuum packaged samples CB and CB-N, are reported in Table 3; the data showed no significant effect of the nisin treatment and time storage on a* and b* variables, whilst L* value significantly increased in the control sample (CB) during the storage, unlike the sample treated with nisin (CB-N). No significant differences were observed on hue (h) and chroma (C*) values (data not shown).
One variable that has received little attention but may be useful is ΔE* (Table 3) which measures the total colour change by accounting for combined changes in L*, a* and b*;
It is assumed that when ΔE* is higher than 3–5 units, a color variation can be perceived by the human eye (Ghidouche et al. 2013). Table 3 shows that ΔE* values remained constant in the samples treated with nisin, while increased in the control (CB), up to the end of shelf life; on the other hand, the deterioration for the other investigated parameters (sensory, texture, BAI, microbiota) was comparable between CB and CB-N samples (data previously discussed). To our knowledge this is the first report that suggested an action of nisin on colour stability.
The colour of salmonids is primarily due to deposits of carotenoids in lipid tissues. The carotenoids in the diet are digested and absorbed by the plasma, bound to serum lipoproteins, then they are transported and distributed in various tissues and organs, giving to salmon trout the typical colouration. Choubert & Baccaunaud (2010) have reported concentrations of 0.2–0.3 mg carotenoids g−1 of lipid in fillets of salmon trout. It is easily understood that the processes that promote the oxidation of lipids can cause the change of colour.
Processing or storage affect the colour of a product through changes in the nature of the pigments and/or their physical state (Hutchings 1999).
Studies carried out on Atlantic salmon showed that the keto-carotenoids are non-specifically linked to the hydrophobic pocket in the actomyosin protein-complex by weak interactions, and keto-groups help to stabilize these complexes (Henmi et al. 1989). During storage the carotenoids may also interact with other compounds, thus, on the basis of these considerations, the colour stabilization observed in this study (ΔE* constant) in the samples treated with nisin (CB-N) could be due to the formation of hydrophobic bonds between carotenoids and the apolar fraction of nisin (Lins et al. 1999).
A further hypothesis that can be formulated about nisin effect on colour stabilization, considers the pigments location in the cellular structure and their ease of diffusion in the cell. Therefore it is possible to speculate that nisin, through the formation of pores (Sobrino-López and Martín-Belloso 2008), could facilitate the higher diffusion and mobility of pigmented compounds in the fish tissue cells, thus preventing the depreciation of the product due to changes in appearance.
Conclusion
The quality properties of rainbow trout fillets were monitored during storage time. Results showed no inhibitory effects of nisin on biogenic amines accumulation; however, this preliminary study sets the basis for a possible use of nisin as food preservative, not only for its antagonistic activity against bacteria but also for a possible effect on other quality characteristics, such as colour in rainbow trout. This result might have a strong impact on the quality of a product such as rainbow trout, for which the colour is one of the most important parameters from the commercial point of view (for both consumers and producers). The role of nisin in colour stability of rainbow trout could be ascribed to the possible formation of hydrophobic bonds between carotenoids and the apolar fraction of nisin; another hypothesis concerns the possibility of nisin of facilitating a level mechanical/structural distribution of carotenoids in the cell lumen. However further studies are needed to confirm the formulated hypotheses; in addition, further investigations are necessary to determine the optimal technological conditions of nisin activity in liquid media for the treatment of fish products and the extension of their shelf-life.
References
Abdollahzadeh E, Rezaei M, Hosseini H (2014) Antibacterial activity of plant essential oils and extracts: the role of thyme essential oil nisin and their combination to control Listeria monocytogenes inoculated in minced fish meat. Food Control 35:177–183
AMSA (2012) Meat color measurement guidelines. American Meat Science Association, Champaign, IL, USA. http://www.meatscience.org
AOAC (2002) Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Gaithersburg, MD
Arashisar Ş, Olcay H, Mükerrem K, Telat Y (2004) Effects of modified atmosphere and vacuum packaging on microbiological and chemical properties of rainbow trout (Oncorynchus mykiss) fillets. Int J Food Microbiol 97:209–214
Balciunas EM, Martinez FAC, Todorov SD, De Melo Franco BDG, Converti A, De Souza Oliveira RP (2013) Novel biotechnological applications of bacteriocins: a review. Food Control 32:134–142
Bourne MC (2002) Food texture and viscosity. Concept and measurement. Academic Press, New York, pp 134–138
Bover-Cid S, Holzapfel WH (1999) Improved screening procedure for biogenic amine production by lactic acid bacteria. Int J Food Microbiol 59:391–396
Broekaert K, Heyndrickx M, Herman L, Devlieghere F, Vlaemynck G (2011) Seafood quality analysis: molecular identification of dominant microbiota after ice storage on several general growth media. Food Microbiol 28:1162–1169
Brötz H, Josten M, Wiedemann I, Schneider U, Götz F, Bierbaum G, Sahl HG (1998) Role of lipid-bound peptidoglycan precursors in the formation of pores by nisin epidermin and other lantibiotics. Mol Microbiol 30:317–327
Bulushi IA, Poole S, Deeth HC, Dykes GA (2009) Biogenic amines in fish: roles in intoxication spoilage and nitrosamine formation—a review. Crit Rev Food Sci Nutr 49(4):369–377
Choubert G, Baccaunaud M (2010) Effect of moist or dry heat cooking procedures on carotenoid retention and color of fillets of rainbown trout (Oncorhynchus mykiss) fed astaxanthin or canthaxantin. Food Chem 119:265–269
Chytiri S, Chouliara I, Savvaidis IN, Kontominas MG (2004) Microbiological chemical and sensory assessment of iced whole and filleted aquacultured rainbow trout. Food Microbiol 21:157–165
Delbarre-Ladrat C, Chéret R, Taylor R, Verrez-Bagnis V (2006) Trends of postmortem aging in fish: Understanding of proteolysis and disorganization of the myofibrillar structure. Crit Rev Food Sci Nutr 46:409–421
EFSA (2006) Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the commission related to the use of nisin (E 234) as a food additive. Eur Food Saf Auth J 314:1–16. http://www.efsa.eu.int/science/afc/afc_opinions/catindex_en.html
EU (2004) Regulation (EC) No. 1935/2004 European Parliament and the Council of 27 October 2004 on materials and articles intended to come into contact with food repealing
FAO (2014) The state of world fisheries and aquaculture. FAO, Rome
FDA (1988) Federal Register, Nisin preparation: affirmation of GRAS status as a direct human food ingredient. 21 CFR Part 184. Fed Reg 53:11247–11251
Gao M, Feng L, Jiang T, Zhu J, Fu L, Yuan D, Li J (2014) The use of rosemary extract in combination with nisin to extend the shelf life of pompano (Trachinotus ovatus) fillet during chilled storage. Food Control 37:1–8
Ghidouche S, Rey B, Michel M, Galaffu NA (2013) Rapid tool for the stability assessment of natural food colours. Food Chem 139:978–985
Godiksen H, Morzel M, Hyldig G, Jessen F (2009) Contribution of cathepsins B L and D to muscle protein profiles correlated with texture in rainbow trout (Oncorhynchus mykiss). Food Chem 113:889–896
Gram L, Ravn L, Rasch M, Bruhn JB, Christensen AB, Givskov M (2002) Food—interaction between food spoilage bacteria. Int J Food Microbiol 78:79–97
Gruskiene R, Krivorotova T, Sereikaite J (2017) Nisin-loaded pectin and nisin-loaded pectin-inulin particles: comparison of their proteolytic stability with free nisin. LWT Food Sci Technol 82:283–286
Guizani N, Al-Busaidy M, Al-Belushi I, Mothershaw A, Rahman M (2005) The effect of storage temperature on histamine production and freshness of yellowfin tuna (Thunnus albacares). Food Res Int 38:215–222
Gut IM, Prouty AM, Ballard JD, van der Donk WA, Blanke SR (2008) Inhibition of Bacillus anthracis spore outgrowth by nisin. Antimicrob Agents Chemother 52:4281–4288
Henmi H, Hata M, Hata M (1989) Astaxantin and/or canthaxanthin-actomyosin complex in salmon muscle. Nippon Suisan Gakkaishi 55:1583–1589
Hutchings JB (1999) Food color and appearance, 2nd edn. Aspen publishers Incorporated, Frederick, MD, USA, pp 347–351, 512–535
Imran M, Revol Junelles AM, Paris C, Guedon E, Linder M, Desobry S (2015) Liposomal nanodelivery systems using soy and marine lecithin to encapsulate food biopreservative nisin. LWT Food Sci Technol 62:341–349
Kakatkar AS, Gautam RK, Shashidhar R (2017) Short communication: combination of glazing, nisin treatment and radiation processing for shelf-life extension of seer fish (Scomberomorous guttatus) steaks. Radiat Phys Chem 130:303–305
Karmas E (1981) Biogenic amines as indicator of seafood in freshness. Food Sci Technol 17:20–23
Kuley E, Özogul F, Özogul Y, Akyol I (2011) The function of lactic acid bacteria and brine solutions on biogenic amine formation by foodborne pathogens in trout fillets. Food Chem 129:1211–1216
Kurt S, Zorb O (2010) Biogenic amine formation in Turkish dry fermented sausage (sucuk) as affected by nisin and nitrite. J Sci Food Agric 90(15):2669–2674
Lins L, Ducarne P, Breukink E, Brasseur R (1999) Computational study of nisin interaction with model membrane. Biochim Biophys Acta 1420:111–120
Lopez-Sabater E, Rodriguez-Jerez J, Hernandez-Herero M, Roig-Sagues A, Mora-Ventura M (1996) Sensory quality and histamine formation during controlled decomposition of tuna (Thunnus thynnus). J Food Prot 59:167–174
Martuscelli M, Pittia P, Camassima LM, Manetta AC, Lupieri L, Neri L (2009) Effect of intensity of smoking treatment on the free amino acids and biogenic amines occurrence in dry-cured ham. Food Chem 116:955–962
Mohan CO, Ravishankar CN, Srinivasagopal K (2008) Effect of O2 scavenger on the shelf-life of catfish (Pangasius sutchi) steaks during chilled storage. J Sci Food Agric 88:442–448
Montalvo Rodríguez C (2014) “Impregnación al vacío de filetes de tilapia (oreochromosis sp.) con bacterias ácido lácticas y su efecto sobre las características de calidad en almacenamiento refrigerado”. Dottorado in Ingegniería. Universidad del Valle (Colombia)
Nykänen A, Weckman K, Lapveteläinen A (2000) Synergistic inhibition of Listeria monocytogenes on cold-smoked rainbow trout by nisin and sodium lactate. Int J Food Microbiol 61(1):63–72
Ordonez JA, Hierro EM, Bruna JM, de la Hoz L (1999) Changes in the components of dry-fermented sausages during ripening. Crit Rev Food Sci Nutr 39:329–367
Özogul F, Polat A, Özogul Y (2004) The effects of modified atmosphere packaging and vacuum packaging on chemical sensory and microbiological changes of sardines (Sardina pilchardus). Food Chem 85(1):49–57
Prester L (2011) Biogenic amines in fish, fish products and shellfish: a review. Food Addit Contam Part A 28(11):1547–1560
Raeisi M, Tajik H, Aliakbalu J, Valipour S (2014) Effect of carboxymethyl cellulose edible coating containing Zataria multiflora essential oil and grape seed extract on chemical attributes of rainbow trout meat. Vet Res Forum 5:89–93
Robb DHF, Kestin SC, Warris PD (2000) Muscle activity at slaughter: I. Changes in flesh colour an gaping in rainbow trout. Aquaculture 182:261–269
Ruiz-Capillas C, Moral A (2001) Correlation between biochemical and sensory quality indices in hake stored in ice. Food Res Int 34:441–447
Sanchez-Zapata E, Fuentes-Zaragoza E, Navarro-Rodriguez de Vera C, Sayas E, Sendra E, Fernández-López J, Pérez-Álvarez JA (2011) Effects of tuna pâté thickness and background on CIEL*a*b* color parameters and reflectance spectra. Food Control 22(8):1226–1232
Sato T, Okuzumi M, Masuda T, Fujii T (1995) Distribution and genus/species composition of histamine-decomposition bacteria during storage of common mackerel. Fish Sci 61:83–85
Sobrino-López A, Martín-Belloso O (2008) Use of nisin and other bacteriocins for preservation of dairy products—review. Int Dairy J 18(4):329–343
Vázquez JA, González MP, Murado MA (2006) Preliminary tests on nisin and pediocin production using waste protein sources: factorial and kinetic studies. Bioresour Technol 97(4):605–613
Veciana-Nogués MT, Mariné-Font A, Vidal-Carou MC (1997) Relationships with microbial counts ATP-related compounds volatile amines and organoleptic change. J Agric Food Chem 45:2036–2041
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chaves López, C., Serio, A., Montalvo, C. et al. Effect of nisin on biogenic amines and shelf life of vacuum packaged rainbow trout (Oncorhynchus mykiss) fillets. J Food Sci Technol 54, 3268–3277 (2017). https://doi.org/10.1007/s13197-017-2773-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2773-7