Abstract
The study quantified the major phenolics in different fractions of Syzygium cumini seeds and evaluated their cardioprotective efficacy. Gallic acid, ellagic acid, cinnamic acid, quercetin, syringic acid and ferulic acid were the major polyphenols present in different fractions of Syzygium cumini seeds. The cardioprotective effect of Syzygium cumini seed fractions in modulating angiotensin converting enzyme (ACE), HMG-CoA reductase, LDL oxidation and tertiary butyl hydrogen peroxide (TBHP) induced oxidative stress in H9c2 cardiac cell lines were investigated. Syzygium cumini effectively attenuated the cellular oxidative stress in H9c2 cardiomyoblasts. These fractions possessed inhibitory potential against ACE, HMG-CoA reductase and LDL oxidation. Molecular docking studies of the predominant polyphenols with ACE and HMG-CoA proteins revealed the binding interactions of these compounds, thus confirming their modulation of activity. The present study demonstrated the cardioprotective efficacy of Syzygium cumini seed fractions which can be attributed to the presence of phenolic acids and flavonoids.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cardiovascular disease (CVD) is the leading global cause of death, accounting for 17.3 million deaths per year, a number that is expected to grow to more than 23.6 million by 2030 (Mozaffarian et al. 2015). Therefore, preventive strategies focusing factors associated with CVD are the major tasks of health care professionals and researchers.
Hypertension is quantitatively the most independent risk factor for the development of cardiac diseases for all age/race/sex groups. The use of synthetic hypotensive drugs like captopril, enalapril and lisinopril are reported to have adverse effects such as developing a dry cough, taste disturbances, skin rashes, as well as alterations in serum lipid metabolism (Wijesekara and Kim 2010). Therefore, search for natural products with minimum side effects as an alternative approach to synthetic drugs is on rise worldwide.
ROS is a connective term used for a group of oxidants, which are either free radicals or molecular species capable of generating free radicals (Varsha Tegeli 2014). Oxidative stress reflects an imbalance between the systemic manifestation of reactive oxygen species and a biological system’s ability to readily detoxify the reactive intermediates or to repair the resulting damage. Oxidative stress, or the generation of ROS, are known to be a contributing factor to the progression of many cardiovascular diseases (Rahman et al. 2012). Antioxidants are employed to protect biomolecules from the damaging effects of such ROS. Low intake of antioxidants has been suggested to be an increased risk factor for the development of CVD (Pandey and Rizvi 2009). Polyphenols present in fruits and vegetables provide protection against oxidative stress and thus they can act as powerful antioxidants.
The relationship between dietary factors and cardiovascular disease (CVD) was exclusively dependent on lipid consumption and metabolism leading to increased serum cholesterol; in particular, low-density lipoprotein (LDL) levels resulting in atherosclerotic problem (Schroeder et al. 2015). High fruit and vegetable consumption have been reported to reduce cardiovascular and cerebrovascular diseases due to the antioxidant potential of flavonoids and phenolic acids present in them (Habauzit and Morand 2012; Yang et al. 2011). Therefore, use of plant fractions as natural cardioprotective agents may offers enormous hope for the prevention of chronic human diseases and could enhance the long-term health of a person with atherosclerotic dysfunctions.
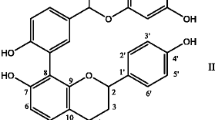
Syzygium cumini (commonly known as black plum or Jamun) is a well-known Indian medicinal plant tested for many therapeutic properties and had been extensively used for the treatment of diabetes (Ayyanar et al. 2013). The plant is reported to possess acetyl oleanolic acid, triterpenoids, ellagic acid, isoquercetin, quercetin, kaempferol and myricetin (Ayyanar and Subash-Babu 2012). Antimicrobial, anti-diabetic, anti-inflammatory, hepatoprotective, and diuretic properties of leaves, stem, bark and fruit pulp of S. cumini have been evaluated extensively (Saroj et al. 2015). Compounds such as jambosine, gallic acid, ellagic acid, corilagin, 3,6 hexahydroxydiphenoylglucose, 3-galloylglucose, quercetin, kaempferol, myricetin, and β-sitosterol were identified from Syzygium cumini seeds (Baliga et al. 2011).
However, to date, research on the beneficial effect of different fractions of Syzygium cumini seed against cardiovascular diseases is limited. A very few studies supporting antihyperlipidaemic effect of Syzygium cumini seeds in streptozotocin induced diabetic rat and against glucose induced oxidative stress were reported (Patel Soncharan et al. 2010). The present study has been designed to systematically quantify the major phenolics in the fractions of Syzygium cumini seeds and the activity of fractions against tertiary butyl hydrogen peroxide induced oxidative stress and key enzymes involved in hypertension, cholesterol biosynthesis. This is the one of the first study that compares the activity of ethyl acetate, methanol, 70% methanol and water fractions of Syzygium cumini seeds.
Materials and methods
Chemicals
Lipoprotein low density (LDL) from human plasma, thiobarbituric acid (TBA), trichloro acetic acid (TCA), angiotensin converting enzyme (ACE) from rabbit lung, Furylacryloyl-Phenylalanyl-Glycyl-Glycine (FAPGG) as a substrate peptide of ACE, HMG-CoA reductase assay kit, Dulbecco’s Modified Eagle’s Media (DMEM), antibiotic–antimycotic mix, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) and ter-butyl hydrogen peroxide (TBHP) were purchased from Sigma–Aldrich Chemicals (St. Louis, MO, USA). Foetal bovine serum (FBS) was purchased from Gibco-BRL (Auckland, NZ). Glutathione assay kit, catalase assay kit (Cat No. 706002) and super oxide dismutase (SOD) activity kit were procured from Cayman Chemicals, USA. Glutathione peroxidase assay kit (K762-100) was obtained from Biovision (USA). Methanol and acetic acid of HPLC grade were supplied by Merck, Germany. H9c2 rat cardiac myoblast cells were purchased from the American Type Culture Collection (Rockville, MD, USA). BCA protein assay kit was procured from Pierce Biotechnology, Rockford, USA. All other chemicals used were of the standard analytical grade.
Plant material
The fully mature Syzygium cumini fruits were collected from Trivandrum, Kerala, India. The samples were authenticated by Dr. E. S. Santhosh Kumar, Technical Officer, Jawaharlal Nehru Tropical Botanic Garden and Research Institute (JNTBGRI), Palode, Trivandrum, Kerala and voucher specimen (collection No: SC-APNP-CSIR-100) was deposited in the herbarium of JNTBGRI, Palode, Trivandrum, Kerala, India. Samples were collected during the month of April and fruits were stored at −80 °C until processed.
Preparation of plant fractions
Syzygium cumini seeds were separated from fruits and washed well using distilled water. The seeds thus obtained were dried in the oven at 40 °C, seed coats were removed, and seeds were coarsely powdered using a motor and pestle. The powdered seeds were extracted sequentially with hexane (for defatting), ethyl acetate, methanol, 70% methanol and water at room temperature (27 ± 1 °C). The extraction process was repeated till each solvent became colourless. These fractions were filtered through Whatman No. 1 filter paper. The fractions were evaporated in rota vapor (BUCHI R215, Switzerland) and stored at −80 °C for further analysis.
HPLC–DAD analysis of phenolic compounds
The identification and quantification of phenolic compounds present in ethyl acetate (EA), methanol (ME), 70% methanol (70% ME) and water fractions (WE) of Syzygium cumini were performed with a Shimadzu HPLC system containing two LC-8A preparative liquid chromatography pump units, a C18 reverse phase column (Phenomenex, 5 μm, 250 × 4.6 mm2 dia.), and a diode array detector (DAD; SPD-M10A VP) with a wavelength range of 200-450 nm. The active fractions and 13 reference standards viz. gallic acid, chlorogenic acid, caffeic acid, syringic acid, coumaric acid, ferulic acid, ellagic acid, cinnamic acid, catechol, myricetin, quercetin, kaempferol and apigenin were prepared in HPLC grade methanol at a concentration of 1 mg/mL and filtered through a 0.45 μm filter. Each sample (20 µL) were injected, and the HPLC analysis was done according to the method of Chen et al. (2001) with slight modifications. The mobile phase used was Water: Acetic acid (98:2, v/v) as Solvent A and Methanol: Acetic acid (98:2, v/v) as solvent B with a time program of 0– 15 min 15% B, 16–20 min 50% B, 21–35 min 70%B, 36–50 min 100% B. The flow rate was 1 mL/min and the column temperature was set at 30 °C. Identification and quantification of the phenolic compounds were done by comparing the retention time and characteristic absorption spectra from the DAD with those of the authentic standards. In order to minimize variation in quantification, samples were taken in triplicates. Data acquisition and analysis were carried out using SHIMADZU- CLASS-VP version 6.14 SP1 software.
In-vitro cell based assay
Maintenance of cell lines
H9c2 rat cardiac myoblast cells were cultured in DMEM supplemented with 10% FBS, 1% antibiotic–antimycotic mix at 37 °C under 5% CO2 atmosphere. After attaining 70–80% confluency, cells were washed with phosphate buffered saline (PBS) and trypsinised using 1% trypsin and were subcultured and seeded in 24 well plates.
Experimental design
In the experiments involving H9c2, cells without any treatment served as control cells. Cells treated with only TBHP served as TBHP control cells. All the pretreated cells with Syzygium cumini were also treated with TBHP.
Cytotoxicity assay
The cytotoxicity of H9c2 was assessed by MTT assay (Mosmann 1983). The H9c2 cells were seeded (1 × 104 cells/well) in a 96-well plate. The cells were treated with various concentrations of fractions (1, 10, 100, 250, 500 µg) (sample). After 24 h incubation, cells were washed and 100 μl of MTT (5 mg/mL), dissolved in DMEM, was added to each well and incubated at 37 °C in a CO2 incubator. Cells without any treatment served as control. After 4 h incubation, DMSO was added to each well, and the plate was kept on a shaker at 12 rpm for 45 min. The change in colour was monitored using a micro-plate reader (BIOTEK-USA) at 570 nm. Results were expressed as percentage of cytotoxicity:
where Ac-absorbance of control, As-absorbance of the sample.
Detection of reactive oxygen species (ROS) production by flow cytometry
The intracellular level of ROS was determined using the fluorescent probe 2,7-dichlorodihydrofluorescein diacetate (LeBel et al. 1992) (DCFH-DA). H9c2 cells were pre-treated with different concentrations of fractions (1, 10 and 100 μg). Cells without fractions serve as control cells and cells treated with only TBHP serves as TBHP control cells. After 24 h, the culture medium was removed, and 100 μM TBHP in PBS was added and incubated for 30 min. The cells were then washed twice with cold PBS, trypsinized, resuspended in ice-cold PBS and subjected to flow cytometry. Samples were analyzed using BD FACS Aria II (BD Biosciences) at FITC range (excitation 490 nm, emission 525 nm band pass filter). The mean fluorescence intensity of different groups was analyzed by BD FACS Diva software and corrected for auto-fluorescence from unlabelled cells.
Antioxidant assays
H9c2 cells cultured in six-well plates at a density of 1 × 106 cells/well were pre-treated with 100 µg concentration of fractions for 24 h, followed by TBHP (100 μM) for 30 min at 37 °C. After incubation, cells were washed with PBS and lysed using respective enzyme specific buffer. The lysed cells were used to determine the antioxidant activity. Cells without fractions were used as control and the cells with only TBHP serves as TBHP control.
Catalase
Catalase enzyme helps in promoting the conversion of hydrogen peroxide to water and molecular oxygen. After incubation, cells were washed with PBS and lysed using cold catalase assay buffer. The supernatant was used to determine the intracellular catalase activity using catalase activity colorimetric assay kit according to the manufacturer instructions. The results were expressed as mU/mL.
Superoxide dismutase assay
Superoxide is one of the most effective intracellular enzymatic antioxidants, and it catalyzes the conversion of superoxide anions to dioxygen and hydrogen peroxide. After incubation, cells were washed with PBS and lysed using cold HEPES buffer (20 mM, pH 7.2). The supernatant obtained after cell lysis was used for assaying SOD activity and was done using Cayman kit according to the manufacturer instructions. The results were expressed as units/mL.
Glutathione peroxidase assay
Glutathione peroxidase acts in association with tripeptide glutathione (GSH), which is present in high concentrations in cells and catalyzes the conversion of hydrogen peroxide or organic peroxide to water or alcohol while simultaneously oxidizing GSH. It also competes with catalase for hydrogen peroxide as a substrate and is the major source of protection against low levels of oxidative stress. After incubation, cells were washed with PBS and homogenized using cold glutathione peroxidase assay buffer. Glutathione peroxidase activity was evaluated as per manufacturer’s instructions (Biovision). The results were expressed as mU/mL.
Glutathione reductase assay
Glutathione reductase can recycle back to glutathione. After incubation, cells were washed with PBS and lysed using cold glutathione buffer. The lysed cells were used to determine the reduced glutathione. The assay was performed using the kit according to the manufacturer instructions. The results were expressed in microgram.
In-vitro chemical assays
Inhibition of human LDL oxidation
Oxidation of LDL leads to the production of malondialdehyde (MDA) which was measured by reaction with TBA according to the method of Chidambara Murthy et al. 2002 with slight modification. LDL (50 μg/mL) was incubated with different concentrations of the fractions, and the oxidation of LDL was initiated by the addition of 50 μl copper sulphate (2 mM) at 37 °C for 2 h. The final volume of the reaction mixture was made up to 1.5 mL with phosphate buffer (pH 7.4). Reaction mixture (500 μl) was mixed with 250 of μl TBA (1% in 50 mM of NaOH) and TCA (0.28%). Samples were again incubated at 95 °C for 45 min. After cooling and centrifugation at 2000 rpm (10 min), the fluorescence of the supernatant was taken at 515 nm excitation and 553 nm emission. The result was expressed as percent of inhibition of LDL oxidation.
where Ac-absorbance of control, As-absorbance of sample.
Determination of HMG-CoA reductase inhibitory activity
The assay is based on the spectrophotometric measurement of the decrease in absorbance at 340 nm, which represents the oxidation of NADPH by the catalytic subunit of HMGR in the presence of the substrate HMG-CoA. The HMG-CoA reductase assay kit from Sigma-Aldrich (St. Louis, MO, USA) with the catalytic domain of the human enzyme (recombinant GST fusion protein expressed in E. coli) was used, under conditions recommended by the manufacturer, to identify the most effective fraction of plant fractions. The concentration of the purified human enzyme stock solution (Sigma) was 0.52 – 0.85 mg protein/mL. Reference statin drug pravastatin from Sigma was used as positive control. To characterize HMG-CoAreductase inhibition under defined assay conditions, reactions containing 4μL of NADPH (to obtain a final concentration of 400 μM) and 12 μL of HMG-CoA substrate in a final volume of 0.2 mL of 100 mM potassium phosphate buffer, pH 7.4 were added. The reaction was initiated by the addition of 2 μL of the catalytic domain of human recombinant HMG-CoA reductase and incubated at 37∘C in the presence or absence (control) of 1 μL aliquots of fractions dissolved in DMSO. The rates of NADPH consumed were monitored every 20 s for up to 15 min by scanning spectrophotometrically.
IC50 was calculated as:
where Ac-absorbance of control, As-absorbance of the sample.
Determination of ACE inhibition using HPLC method
To 10 μg of inhibitor solution/fractions, 30 μl of buffer (50 mMTris–HCl pH 7.5 containing 0.3 MNaCl) and 100 μl of substrate solution (0.25 mM FAPGG in the same buffer) were added. The reaction was started by addition of 20 μl of ACE enzyme (0.3 U/mL), and the mixture was incubated in a water bath at 37 °C for 45 min. The reaction was then stopped by addition of 100 μl of methanol, and the reaction mixture was then analyzed by HPLC. ACE inhibition was determined by measuring the level of FAP with and without inhibitor under same conditions. The analysis was performed with a Shimadzu HPLC system containing two LC-8A preparative liquid chromatography pump units, a C18 reverse phase column (Phenomenex, 5 μm, 250 × 4.6 mm2 dia.), and a diode array detector (DAD; SPD-M10A VP). FAP and FAPGG were separated using the mobile phases water containing 0.1% TFA as solvent A and acetonitrile containing 0.1% TFA as solvent B with a time program of 0% B to 100% B for 25 min. The flow rate was 1 mL/min and the column temperature was set at 35 °C. Data acquisition and analysis were carried out using SHIMADZU- CLASS-VP version 6.14 SP1 software. ACE inhibition was evaluated based on the comparison between the concentration of FAP in the presence or absence of an inhibitor. The FAP peak areas obtained in the two cases were measured, and the percentage of ACE inhibition was calculated using the equation:
where Ac-absorbance of control, As-absorbance of the sample.
Molecular docking studies
The inhibitory potential of Syzygium cumini fractions against HMG-CoA and ACE were performed. Following these assays, molecular docking studies were carried out to understand the binding interaction of major phenolics in Syzygium cumini seeds with angiotensin converting enzyme and HMG-CoA reductase enzyme. Docking studies were performed using Autodock 4.2 (Mahindroo et al. 2006; Morris et al. 2009; The PyMOL Molecular Graphics System 2015). The 3D model of angiotensin converting enzyme (PDB id: 4CA6) and HMG-CoA reductase enzyme (PDB id: 1DQ8) were retrieved from the Brookhaven Protein Data Bank (PDB) (http://www.rcsb.org/pdb/). Gallic acid (ChemSpider ID: 361), ellagic acid (ChemSpider ID: 4445149), cinnamic acid (ChemSpider ID: 392447), quercetin (ChemSpider ID: 4444051), ferulic acid (ChemSpider ID: 393368), syringic acid (ChemSpider ID: 10289), pravastatin (ChemSpider ID: 54687) and captopril (ChemSpider ID: 40130) structures were downloaded from Chemspider (http://www.chemspider.com/) and converted to PDB file using Chem3D Pro 10.
Statistical analysis
All measurements were replicated three times, and one-way analysis of variance (ANOVA) was used. The Duncan’s multiple range tests were used to test the significant differences (p ≤ 0.05). All statistical analyzes were performed with SPSS 11.0 (Statistical Program for Social Sciences, SPSS Corporation, Chicago, IL) for Windows.
Results
Quantification of phenolics
The phenolic profile of all fractions were quantified using HPLC–DAD (Table 1). All fractions were individually spiked with each standard and recorded an increased peak height with similar retention times, indicating the presence of those compounds. The results showed that EA fraction possessed the highest quantity of quercetin, cinnamic acid, ferulic acid and syringic acid. The ME fractions had high concentrations of gallic acid (531.1 mg/g DW), ellagic acid (199.1 7852 mg/DW) and myricetin (100.5 mg/g DW). The highest concentration of ellagic acid (222.2 mg/g DW) and chlorogenic acid (42.6 mg/g DW) were in 70% ME fractions (Table 1). WE showed the presence of very minute quantity of gallic acid, myricetin, kaempferol, chlorogenic acid, syringic acid and apigenin.
Assessment of cytotoxicity
The cytotoxicity of each fractions in H9c2 cell lines were determined by MTT assay. The concentration of fractions up to 100 µg was found to be less than 30% toxic for a period of 24 h (Suppl. Table 1). Concentration below 30% toxicity was used for further experiments.
Determination of ROS generation using DCFH-DA
To demonstrate the extent of cellular oxidative stress the intracellular ROS production was estimated in the present study (Fig. 1). Cells treated with TBHP showed a significant increase in ROS generation over time as compared to untreated control. H9c2 cells pre-treated with Syzygium cumini seed fractions showed significant (p < 0.05) reduction in ROS generation in a dose-dependent manner in presence of TBHP. The highest reduction of ROS generation was demonstrated by ME fractions (100 µg) pre-treated cells. In addition, 70% ME and EA fractions also significantly prevented ROS generation in TBHP treated cells.
Fluorescent analysis of ROS by flow cytometry. FACS analysis of intracellular ROS production in H9c2 cell lines by plotting cell count against FITC. The groups contained a control cells, b cells treated with Tertiary butyl hydrogen peroxide (TBHP), c ethyl acetate fractions (100 µg), d methanol fractions (100 µg), e 70% methanol fractions (100 µg), f water fractions (100 µg)
Antioxidant enzyme levels
The most evident enzymatic antioxidants catalase, superoxide dismutase, glutathione peroxidase and glutathione reductase levels in H9c2 cells were determined by pretreating the fractions and compared with cells treated with only TBHP, served as TBHP control cells (Fig. 2). The level of antioxidant enzymes is higher in control cells. Treatment of fractions increased the antioxidative potential of cells when compared with TBHP control. The pretreatment of 70% ME, ME and EA fractions demonstrated significant increase (p < 0.05) in catalase (Fig. 2a) and peroxidase (Fig. 2c) activity as compared to TBHP group. The SOD activity (Fig. 2b) also showed a similar trend in fractions pre-treated cells. The level of reduced glutathione (Fig. 2d) was decreased significantly in TBHP treated cells, but pre-treatment with different fractions helped the cells in reverting the glutathione levels to near normal (p > 0.05).
Antioxidant activity in H9c2 cell lines pre-treated with different fractions of Syzygium cumini (100 µg). Values are mean ± SD (standard deviation) of triplicate samples. Significance test compared with TBHP treated cells was determined by using one way ANOVA followed by Duncan’s multiple range test and the significance accepted at p ≤ 0.05. a Catalase activity, b Superoxide dismutase activity, c Glutathione peroxidase activity, d Reduced glutathione, *p ≤ 0.05 versus TBHP,# p ≤ 0.05 versus control (EA ethyl acetate fractions, ME methanol fractions, 70% ME 70% methanol fractions, WE water fractions)
LDL oxidation inhibition
Compared with the standard, ascorbic acid (IC50 – 28.7 µg/mL), our results demonstrated that 70% ME fractions (0.156 µg/mL) and ME fractions (15.46 µg/mL) of Syzygium cumini significantly (p < 0.05) inhibited LDL oxidation (Fig. 3). Polyphenols such as ellagic acid and gallic acid found to be present in the fractions of Syzygium cumini in the present study were independently reported to inhibit lipid peroxidation, while ellagic acid has been shown to inhibit LDL oxidation specifically (Safari et al. 2003; Baba et al. 2007). In the present study, the gallic acid concentration was markedly high in ME and 70% ME fractions which might have attributed to their relatively high LDL oxidation inhibitory potential.
LDL oxidation inhibitory potential of Syzygium cumini. Values are mean ± SD (standard deviation) of triplicate samples. Significance test compared with the standard was determined by using one way ANOVA followed by Duncan’s multiple range test and the significance accepted at p ≤ 0.05. *p ≤ 0.05 versus ascorbic acid. (EA ethyl acetate fractions, ME methanol fractions, 70% ME 70% methanol fractions, WE water fractions)
HMG-CoA reductase inhibition
HMG-CoA reductase inhibition of methanol fractions (0.2units/mg) is highly significant when compared with positive control, pravastatin (Fig. 4a).
Enzyme inhibitory potential of Syzygium cumini seeds. a HMG-CoA reductase potential of Syzygium cumini and b ACE inhibitory potential of Syzygium cumini (100 µg). Values are mean ± SD (standard deviation) of triplicate samples. Significance test compared with positive control pravastatin for HMG-CoA reductase and captopril for ACE inhibition was determined by using one way ANOVA followed by Duncan’s multiple range test and and the significance accepted at p ≤ 0.05. *p ≤ 0.05 versus positive control. (EA ethyl acetate fractions, ME methanol fractions, 70% ME 70% methanol fractions, WE water fractions)
ACE inhibition
In the present study, the role of Syzygium cumini seed fractions in inhibiting the activity of rabbit lung ACE was investigated (Fig. 4b). The EA, ME and 70% ME fractions of the seeds of Syzygium cumini showed significantly higher ACE inhibition (71.04, 93.11, 84% respectively) as compared to that of positive control, captopril (62.65%).
Molecular docking
In order to confirm the HMG-Co A and ACE inhibitory potential of Syzygium cumini seed fractions, docking studies with ACE and HMG-Co A were conducted to identify the binding interactions of the major identified compounds that we identified in Syzygium cumini fractions. The minimum binding energy from the docking studies indicated that the ACE and HMG-CoA reductase (target enzymes) were successfully docked with the compounds, gallic acid, ellagic acid, quercetin, cinnamic acid, syringic acid and ferulic acid (Suppl. Table 2).
The best conformation of the ligand binding to the receptor and also the residues on the receptor to which the ligand can bind in Autodock are represented in Fig. 5. All the predominant compounds showed relatively higher binding affinity to HMG CoA reductase protein when compared to the positive control, pravastatin (minimum binding energy of −5.46 kcal/mol). The lowest binding energy was shown by gallic acid (−7.92 kcal/mol) and quercetin (-7.94 kcal/mol) that may attribute to the HMG-CoA reductase inhibitory property of methanol fractions of Syzygium cumini (Fig. 5-i). Against ACE, the compounds, namely quercetin (−6.92 kcal/mol) and ellagic acid (−7.46 kcal/mol) demonstrated higher binding affinity which proves the ACE inhibitory potential of met fractions (Fig. 5-ii).
Molecular docking images of predominant phenolics with HMG- Co A and ACE. a Representing the residues on HMG-CoA reductase enzyme, b representing the residues on ACE enzyme binding to (i) gallic acid (ii) ellagic acid (iii) cinnamic acid (iv) quercetin (v) syringic acid (vi) ferulic acid (vii-a)pravastatin (vii-b) captopril using Autodock 4.2
Discussion
Cardiovascular diseases are currently one of the leading cause of morbidity and mortality globally (Lloyd-Jones et al. 2010). Oxidation of LDL, excess of cholesterol deposits and increased blood pressure play vital role in the development of cardiovascular diseases (Zafar 2015). Natural products are emerging as a promising target to address the increasing risk of this disease. Any natural product that can act against these three major factors can provide immense benefit to mankind that can overcome the side effects of synthetic drugs. Syzygium cumini is widely used in traditional medicine to treat various diseases. However, apart from a few isolated studies on the cardioprotective effects of Syzygium cumini (Atale et al. 2013), the scientific evidence is limited. Thus in the present study we explored the cardio protective efficiency of Syzygium cumini seeds.
The results from the present study demonstrate that 70% ME, ME and EA fractions of Syzygium cumini can retrieve the natural antioxidant enzymes which was drastically reduced under TBHP exposure which signifies the antioxidant property of the fractions of Syzygium cumini. The retrieval of antioxidant enzymes in the present study were similar to the reported study on cardioprotective role of Syzygium cumini against glucose-induced oxidative stress in H9c2 cardiac myocytes (Atale et al. 2013).
Atherosclerosis is the hardening and narrowing of arteries which leads to heart attacks, stroke and other cardio vascular diseases. Evidence suggests that LDL oxidation and ROS play a crucial role in the pathogenesis of atherosclerotic complications including coronary heart diseases (Kuo et al. 2011; Pashkow 2011). Oxidised LDL accumulates near blood vessels and vascular plaques are formed which ultimately leads to cardio vascular diseases. Our result suggests that 70% methanol and methanol fractions of Syzygium cumini have a significant inhibitory action on LDL oxidation. The inhibitory potential shown by the fractions may be due to the presence of pharmacologically valuable phenolics like quercetin, myricetin, gallic acid, ellagic acid and cinnamic acid in Syzygium cumini seeds (Priya et al. 2017). The presence of antioxidants like phenolics and flavonoids in plants are known to suppress LDL oxidation and delay the development of heart diseases (Itabe et al. 2011). Based on the earlier reports and results from the present study it can be suggested that Syzygium cumini fractions possess significant potential against LDL oxidation and all its deleterious effects in the vessel wall.
Hypercholesterolemia is another risk factor for the development of atherosclerosis and attempts have been made for blocking the biosynthesis of cholesterol by inhibiting the activity of the key enzyme of cholesterol biosynthetic pathway, the 3-hydroxy-3-methyl glutaryl Coenzyme A (HMG-CoA) reductase. HMG-CoA reductase is the rate controlling enzyme of the mevalonate pathway, the metabolic pathway that produces cholesterol and other isoprenoids. In the present study, 70% methanol and methanol fractions inhibited HMG-CoA reductase activity demonstrating their ability to reduce the cholesterol level. The presence of phenolics (such as, ferulic acid, gallic acid, ellagic acid, quercetin and myricetin) in Syzygium cumini fractions may be attributing to high HMG-CoA reductase inhibitory potential, since these phenolics have been reported for the inhibition of HMG-CoA reductase (Santhosh et al. 2008; Paiva et al. 2013).
ACE is a dipeptidylcarboxypeptidase that plays a crucial role in the regulation of blood pressure. ACE promotes the conversion of angiotensin-I to the potent vasoconstrictor angiotensin-II as well as inactivates the vasodilator bradykinin, which has a depressor action in the renin-angiotensin system. The results demonstrated that methanol and 70% methanol significantly inhibited ACE. The inhibitory potential of the fractions might be due to the presence of apigenin, chlorogenic acid, catechin and quercetin, as these compounds are reported to have potent ACE inhibitory activity (Kumar et al. 2010). The presence of these compounds in Syzygium cumini seeds may attribute to the inhibition of rabbit lung ACE by competing with the substrate for their active sites.
The inhibitory studies of the phenolics, detected in the Syzygium cumini seed fractions, against HMG-CoA reductase and ACE were confirmed through molecular docking studies. Molecular docking experiments with major phenolic acids and flavonoids reveal the binding interaction of these compounds with HMG-CoA and ACE which indirectly stating that these phenolics and flavonoids may be the attributing factor for HMG-CoA and ACE inhibitory potential of Syzygium cumini seed fractions.
The present study’s findings shed light into the cardio protective benefits of Syzygium cumini seeds, by exploring the potential of seeds against tertiary butyl hydrogen peroxide induced oxidative stress and its ability to inhibit HMG Co-A reductase, LDL oxidation and ACE. The study also quantified the major phenolics present in different fractions of Syzygium cumini. Our molecular docking studies demonstrated a positive correlation between the phenolics and key enzymes in preventing cardio vascular diseases. These findings are of greatest importance as it provides a novel therapeutic strategy to overcome the risk of cardiovascular disease.
Conclusion
The present study is one of the first study that reports the importance of sequential fractions of Syzygium cumini seeds in demonstrating cardioprotective potential. The present study also highlighted the mode of action of these fractions for their cardioprotective effect. The presence of phenolic acids and flavonoids may be the reason for potent cardioprotective action shown by these fractions. This study clearly showed the role of different fractions of Syzygium cumini seeds in modulating the level of various antioxidant enzymes, cholesterol biosynthesis, blood pressure thus demonstrating cardioprotective potential.
References
Atale N, Chakraborty M, Mohanty S, Bhattacharya S, Nigam D, Sharma M, Rani V (2013) Cardioprotective role of Syzygium cumini against glucose-induced oxidative stress in H9c2 cardiac myocytes. Cardiovasc Toxicol 13(3):278–289
Ayyanar M, Subash-Babu P (2012) Syzygium cumini (L.) Skeels: a review of its phytochemical constituents and traditional uses. Asian Pac J Trop Biomed 2(3):240–246. doi:10.1016/S2221-1691(12)60050-1
Ayyanar M, Subash-Babu P, Ignacimuthu S (2013) Syzygium cumini (L.) Skeels a novel therapeutic agent for diabetes: folk medicinal and pharmacological evidences. Complement Ther Med 21(3):232–243. doi:10.1016/j.ctim.2013.03.004
Baba S, Osakabe N, Kato Y, Natsume M, Yasuda A, Kido T, Kondo K (2007) Continuous intake of polyphenolic compounds containing cocoa powder reduces LDL oxidative susceptibility and has beneficial effects on plasma HDL-cholesterol concentrations in humans. Am J Clin Nutr 85(3):709–717
Baliga MS, Bhat HP, Baliga BRV, Wilson R, Palatty PL (2011) Phytochemistry, traditional uses and pharmacology of Eugenia jambolana Lam. (black plum): a review. Food Res Int 44(7):1776–1789. doi:10.1016/j.foodres.2011.02.007F
Chen H, Zuo YG, Deng YW (2001) Separation and determination of flavonoids and other phenolic compounds in cranberry juice by high-performance liquid chromatography. J Chromatogr A 913(1–2):387–395. doi:10.1016/S0021-9673(00)01030-X
Chidambara Murthy KN, Singh RP, Jayaprakasha GK (2002) Antioxidant activities of grape (Vitis vinifera) pomace fractions. J Agric Food Chem 50(21):5909–5914
Habauzit V, Morand C (2012) Evidence for a protective effect of polyphenols-containing foods on cardiovascular health: an update for clinicians. Ther Adv Chronic Dis 3(2):87–106
Itabe H, Obama T, Kato R (2011) The dynamics of oxidized LDL during atherogenesis. J Lipids. doi:10.1155/2011/418313
Kumar R, Kumar A, Sharma R, Baruwa A (2010) Pharmacological review on natural ACE inhibitors. Der Pharmacia Lettre 2(2):273–293
Kuo MY, Ou HC, Lee WJ, Kuo WW, Hwang LL, Song TY, Sheu WH (2011) Ellagic acid inhibits oxidized low-density lipoprotein (OxLDL)-induced metalloproteinase (MMP) expression by modulating the protein kinase C-alpha/extracellular signal-regulated kinase/peroxisome proliferator-activated receptor gamma/nuclear factor-kappaB (PKC-alpha/ERK/PPAR-gamma/NF-kappaB) signaling pathway in endothelial cells. J Agric Food Chem 59(9):5100–5108. doi:10.1021/jf1041867
LeBel CP, Ischiropoulos H, Bondy SC (1992) Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol 5(2):227–231
Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A (2010) Heart disease and stroke statistics—2010 update. Circulation 121(7):e46–e215
Mahindroo N, Wang CC, Liao CC, Huang CF, Lu IL, Lien TW, Hsieh HP (2006) Indol-1-yl acetic acids as peroxisome proliferator-activated receptor agonists: design, synthesis, structural biology, and molecular docking studies. J Med Chem 49(3):1212–1216. doi:10.1021/jm0510373
Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, Olson AJ (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J Comput Chem 30(16):2785–2791. doi:10.1002/jcc.21256
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65(1–2):55–63. doi:10.1016/0022-1759(83)90303-4
Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Subcomm SS (2015) Heart disease and stroke statistics-2015 update a report from the american heart association. Circulation 131(4):E29–E322. doi:10.1161/Cir.0000000000000152
Paiva LBd, Rosana G, Santos WD, Squina FMS (2013) Ferulic acid and derivatives: molecules with potential application in the pharmaceutical field. Braz J Pharm Sci 49(3):395–411
Pandey KB, Rizvi SI (2009) Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev 2(5):270–278
Pashkow FJ (2011) Oxidative stress and inflammation in heart disease: do antioxidants have a role in treatment and/or prevention? Int J Inflam 11:2011
Patel Soncharan STS, Somasundaram I, Niladri Maity (2010) Protective effect of Syzygium cumini seeds against doxorubicin induced cardiotoxicity in rats. Int J Pharm Life Sci 1(6):343–349
Priya SH, Prakasan N, Purushothaman J (2017) Antioxidant activity, phenolic-flavonoid content and high-performance liquid chromatography profiling of three different variants of Syzygium cumini seeds: a comparative study. J Intercult Ethnopharmacol 6(1):107
Rahman T, Hosen I, Towhidul Islam MM, Shekhar HU (2012) Oxidative stress and human health. Adv Biosci Biotechnol 3:997–1019
Safari MR, Rezaie M, Sheikh N (2003) Efects of some flavonoids on the susceptibility of low-density lipoprotein to oxidative modification. Indian J Biochem Biophys 40(5):358–361
Santhosh M, Ramarethinam S, Latha K (2008) Comparative analysis of HMG CoA inhibitors via computational tools. JCIB 1:119–128
Saroj A, Pragadheesh VS, Palanivelu Yadav A, Singh SC, Samad A, Chanotiya CS (2015) Anti-phytopathogenic activity of Syzygium cumini essential oil, hydrocarbon fractions and its novel constituents. Ind Crops Prod 74:327–335. doi:10.1016/j.indcrop.2015.04.065
Schroeder N, Park YH, Kang MS, Kim Y, Ha GK, Kim HR, Caballero B (2015) A randomized trial on the effects of 2010 dietary guidelines for Americans and Korean diet patterns on cardiovascular risk factors in overweight and obese adults. J Acad Nutr Diet 115(7):1083–1092. doi:10.1016/j.jand.2015.03.023
The PyMOL Molecular Graphics System (2015) (Version 1.8 Schrödinger, LLC)
Varsha Tegeli PK (2014) Importance of free radical and antioxidant on human health. Int J Pharma Chem Biolo Sci 4(4):1038–1050
Wijesekara I, Kim SK (2010) Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: prospects in the pharmaceutical industry. Mar Drugs 8(4):1080–1093
Yang Y, Chan SW, Hu M, Walden R, Tomlinson B (2011) Effects of some common food constituents on cardiovascular disease. ISRN cardiol 16:2011
Zafar R (2015) A new insight into pathogenesis of cardiovascular diseases: stress induced lipid mediated, vascular diseases. J Cardiovasc Dis Diagn 3:197. doi:10.4172/2329-9517.1000197
Acknowledgements
The authors are thankful to Department of Science and Technology (INSPIRE), India, and Council for Scientific and Industrial Research, India for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest exists among the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Syama, H.P., Arya, A.D., Dhanya, R. et al. Quantification of phenolics in Syzygium cumini seed and their modulatory role on tertiary butyl-hydrogen peroxide-induced oxidative stress in H9c2 cell lines and key enzymes in cardioprotection. J Food Sci Technol 54, 2115–2125 (2017). https://doi.org/10.1007/s13197-017-2651-3
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-017-2651-3