Abstract
Ghee forms an important component of the diet of human beings due to its rich flavor and high nutritive value. This high priced fat is prone to adulteration with cheaper fats. ATR-FTIR spectroscopy coupled with chemometrics was applied for determining the presence of goat body fat in ghee (@1, 3, 5, 10, 15 and 20% level in the laboratory made/spiked samples). The spectra of pure (ghee and goat body fat) and spiked samples were taken in the wavenumber range of 4000–500 cm−1. Separated clusters of pure ghee and spiked samples were obtained on applying principal component analysis at 5% level of significance in the selected wavenumber range (1786–1680, 1490–919 and 1260–1040 cm−1). SIMCA was applied for classification of samples and pure ghee showed 100% classification efficiency. The value of R2 was found to be >0.99 for calibration and validation sets using partial least square method at all the selected wavenumber range which indicate that the model was well developed. The study revealed that the spiked samples of goat body fat could be detected even at 1% level in ghee.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Milk fat is one of the most important dietary components for supplying nutrients. The composition of milk fat is unique as it contains various bioactive components like butyric acid, cis and trans palmitoleic acid, phytanic acid, conjugated linoleic acid (CLA), alpha-linolenic acid (ALA) which are potentially identified as positive predisposing factors for human health (Belury 2002; McBain et al. 1997; Parodi 1994). Butyric acid and linolenic acid are reported to have anticarcinogenic properties. Linolenic acid is also shown to prevent hypertension and heart related diseases and is involved in the improvement of vision (Parodi 2003). CLA is reported to induce antithrombotic effect (Truitt et al. 1999), enhance immunological functions (Pariza et al. 2001), decrease total and low density lipoprotein (LDL) cholesterol (Lee et al. 1994) and inhibit carcinogenesis (Ip et al. 1999). Ghee (heat clarified milk fat) is reported to be the richest natural source of CLA (Sserunjogi et al. 1998). Most dietary guidelines recommend the consumption of milk and dairy products as an important part of a healthy well-balanced diet (Gidding et al. 2009 and United States Department of Agriculture Department of Health and Human Services 2010). Milk fat is valued higher over other fats and oils. Report from National Sample Survey Office: Level and Pattern of Consumer Expenditure (2011–2012) revealed that higher income brackets spend more on dairy products (Mani and Intodia 2014).
India is the world’s largest milk producing nation. However, there is a gap between the demand and supply as the growth in milk production is 3.3%, while there is 5% growth in the consumption (http://www.dairyuniverseindia.com/Marketdate.html) (last accessed on 19th June, 2015). Similarly, the supply of ghee (most consumed after fluid milk among dairy products), also falls short of its demand (Das 2012; Mani and Intodia 2014). The higher cost of ghee (4–5 times) over the other edible fats and oils and the limited supply of this high premium fat attract the unscrupulous manufacturers to adulterate it with cheaper alternatives, especially in the Indian subcontinent. A wide range of literature is available on the incidences of adulteration of ghee with foreign fats (Gandhi 2009; Headlines Today 2009; Ramkumar 2014). Although, this malpractice of adulterating the ghee is not new. A statistical survey of 13 years, 1960–1972, on ghee revealed that 16% of the samples investigated were adulterated, with average level of adulteration being 12%. The more recent reports suggest a higher percent of adulteration in the ghee samples. Gahlawat et al. (2012) reported that all of the ghee samples collected from Delhi region, India contained vanaspati (hydrogenated vegetable oils) as an adulterant. In another survey, Amrutha Kala (2013) reported that up to 43% branded and 87.5% of unbranded ghee samples did not conform to triglyceride profiles of pure ghee based on triglyceride profile and standardized values. Hence, such adulteration is of major concern as it leads to fraud by overcharging (Upadhyay et al. 2015). This practice can affect the nutritional value of original product, its technological properties and most importantly it violates the consumer rights (Nunes 2014). By adding animal body fats in ghee, one of the sacred foods, the oblivious ghee traders lacking honesty not only fleece the public, but also play with their religious sentiments, especially of the devourer and vegetarian sect of the society. The consumption of animal body fat (from red meat) is reported to have adverse health implications. Willett et al. (1990) and Giovannucci and Willett (1994) reported that its consumption increase the risk of colon cancer. Trans fatty acids are reported to be present in animal body fat and are associated with deleterious effects on serum lipoproteins leading to increase in the risk of cardiovascular and cerebrovascular diseases (Stender and Dyerberg 2004).
The detection of adulteration of ghee with animal body fat is one of the priority areas of research in the field of food technology as only limited methods are available for their detection. The available tests are mainly based on planar chromatography technique (Ramachandra and Dastur 1959; Chakrabarty et al. 1968) that involves hazardous chemicals; determination of fatty acid composition of triglycerides and 2-monoglycerides (Soliman and Younes 1986). But, these tests suffer from repeatability problems or do not give clear cut range of determination of adulteration. The other methods are based on techniques like differential scanning calorimetry (Lambelet and Ganguli 1983; Coni et al. 1994) which are reported to detect the presence of foreign animal body fat in ghee only at the higher levels.
In the light of above facts, there is an increased interest of researchers in finding a solution to the problem of detection of adulteration of animal body fat in ghee. Fourier transform infrared (FTIR) spectroscopy, a fingerprint technique, is rapid, simple, sensitive, advanced and powerful analytical tool that finds its application in the wide areas of detection of adulteration in various food commodities (Sivakesava and Irudayaraj 2001; Ozen and Mauer 2002; Jha and Matsuoka 2004; Rodriguez-Saona and Allendorf 2011). FTIR spectroscopy is also reported to provide information about the composition of dairy product from the complex spectra (Albanell et al. 1998; Sørensen and Jepsen 1998; Rodriguez-Otero et al. 1997; Lerma-García et al. 2010; Nicolaou et al. 2010). The technique has been widely applied, because once the instrument has been calibrated; it can be used for routine analyses. FTIR coupled with chemometric techniques had been extensively used to discriminate different edible oils and fats (Safar et al. 1994; Rohman and Man 2010; Nurrulhidayah et al. 2013). However, no work has been done to distinguish ghee from goat body fat using FTIR spectroscopy. Thus, present study was aimed with the objective of detection of adulteration of goat body fat in pure mixed ghee (heat clarified milk fat) by employing FTIR spectroscopy coupled with chemometric techniques.
Materials and methods
Collection of Sample
Pure cow milk and pure buffalo milk were separately collected from their respective pooled milk from the Institute Farm, Guru Angad Dev Veterinary and Animal Sciences University, Ludhiana, Punjab (India). The goat adipose tissues were separately procured from the local slaughterhouse of Ludhiana, Punjab (India).
Preparation of sample
Pure ghee from cow milk and buffalo milk were prepared by employing direct cream method (De 2012). The ghee thus obtained was subjected to filtration using four folds of the filter paper to get clear ghee devoid of ghee residues. The two ghee vis-à-vis pure cow ghee and pure buffalo ghee were mixed together in the ratio of 1:1 to get the pure mixed ghee (PMG). The samples were stored in glass bottles (of Borosil make) under refrigerated conditions (4–6 °C) until further use. Goat body fat (GBF) was prepared from the adipose tissue procured from the local slaughter house by dry rendering process at 152 ± 2 °C till the complete extraction of fat (Upadhyay et al. 2014). For preparation of adulterated samples, GBF was spiked into PMG at a level of 1, 3, 5, 10, 15 and 20% to make the binary system comprising of PMG and GBF. Infrared spectra were collected for each sample.
Spectra Acquisition
The fats and oils are mainly composed of triglycerides and can be directly applied in their neat form to ATR crystal (Nurrulhidayah et al. 2013). Thus, using an auto-pipette, fat samples obtained were placed in direct contact with Diamond crystal cell attenuated total reflectance (ATR) crystal (Path length: 1.66 µm; 7.2 Build, Bruker, Model-Alpha, Germany). The absorption spectra of the samples were acquired using Fourier transform infrared (FTIR) spectrometer at resolution of 4 cm−1 and scan speed of 0.2 cm s−1. Twenty four scans of each sample maintained at 70 °C (so as to maintain the sample in completely molten stage) were taken in the wavenumber range of 4000–500 cm−1 and connected to software OPUS (7.2 Build, Bruker Optik GmbH) for collection of data. The reference (background) spectra using blank ATR crystal were recorded at an interval of 35 spectra of 24 scans each. The reference spectra were used by the instrument in rationing the spectra of the samples in the absorbance unit. After each scan, the ATR crystal was carefully cleaned by wiping in situ with a soft tissue paper using isopropanol and dried completely before taking the next spectra of the sample.
Chemometric analysis
All the spectra were exported to the Unscrambler software (version 10.2; CAMO AS, Trondheim, Norway) and divided into two groups of calibration (training) and validation (testing) sets, taking every third sample as validation set. This amounted to be around 187 and 93 samples in respectively, calibration and validation sets of the PMG adulterated with GBF. Care was taken that control (PMG) and spiked samples (PMG containing GBF) were well represented in calibration and validation sets to avoid the confusion of calibration samples in validation sets. Absorbing regions of the spectra dictate the number of variables used as input in the latent variables based methods such as Principal Component Analysis (PCA) and partial least square (PLS) regression modeling. As a thumb rule, the spectral range should include regions where information pertaining to variation in the concentration of analyte to be modeled and any other matrix constituents are well represented. However, the spectral range should exclude regions that are dominated by artifacts or noise. The raw spectra were plotted and peaks were looked into them so as to identify and select the suitable region. PCA was performed so as to examine the clustering of samples in different groups and also for the purpose of identifying the outliers, if any.
Classification of samples
Initially a global model was built followed by development of class model for control and spiked samples using calibration set of samples. Soft Independent Modeling of Class Analogy (SIMCA) thereafter was used to predict the class memberships using validation set of samples at 5% level of significance. Test samples were classified to one of the established class models on the basis of their best fit to the respective model. False negative and false positive samples were identified to calculate the classification efficiencies. The samples which belonged to a specific class but did not classify as such by the class model were referred to as false negative, while those samples which were incorrectly identified in the relevant class model but did not belong to that specific class were referred to as false positive.
Validation of results
PLS regression using full cross validation option present in the Unsrambler software was performed on the peaks and depressions of the selected spectral window (1786–1680, 1490–919 and 1260–1040 cm−1) for developing the model for predicting spiked GBF in PMG. The best model was selected based on the standard error of calibration (SEC), coefficient of determination (R2) and standard error of prediction (SEP) (Jaiswal et al. 2012; Jha and Matsuoka 2004; Jha et al. 2015).
Results and discussion
Natural fats contain a wide range of triacylglycerol species with fatty acids of different substitution patterns, chain length, degree of unsaturation and other minor components. Milk fat is one of the unique naturally occurring fats of animal origin containing almost 70% of saturation of which around 11% comprises of short-chain fatty acids, approximately half of which is butyric acid. Around 25% of the fatty acids in milk fat are mono-unsaturated, while 2.3% are poly-unsaturated (Månsson 2008). Goat depot fats are reported to contain around 55% saturated fatty acid, 39% monounsaturated fatty acid and 4% polyunsaturated fatty acid (Casey and Van Niekerk 1985). Although the type of fatty acids present in different fats was more or less same but the concentration of these varies. In past, few researchers attempted to use Ultraviolet spectra for differentiation of pure ghee from the ghee containing animal body fat. Singhal (1973) and Sharma (1989) dissolved cow ghee, buffalo ghee and animal body fats (buffalo, goat, pig and sheep) in n-hexane and scanned the Ultraviolet spectra of these between 200 and 320 nm. They reported a maximum absorption between 220 to 230 nm. However, Kumar et al. (2010) and Sharma (1989) reported that adulterated ghee could not be differentiated from pure ghee using the above method. On the contrary, FTIR can be considered a fingerprint technique as no two fats and oils have superimposing FTIR spectra and specific functional groups show specific absorption maxima (Bendini et al. 2007).
FTIR spectral analysis
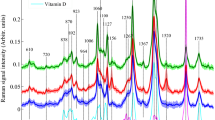
The representative FTIR spectra of control, spiked and GBF samples in the region of 4000–500 cm−1 is shown in Fig 1 in Electronic Supplementary Material (ESM). It revealed that the peak height for PMG and GBF were non-overlapping, while the peaks of the spiked samples fell between the two as per the level of spiking in entire range of the region of the spectra. The peaks of samples that had more percentage of spiking were observed to be near to peak of GBF, while the samples that contained lower percentage of spiking were near to PMG. However, the differences were more distinct in the wavenumber regions of 1786–1680 and 1490–919 cm−1 (Fig. 1). The peaks in the smaller spectral range of 1260–1040 cm−1 also showed distinct differences among the pure and spiked samples of GBF in PMG. This could be due to the differences in the biochemical composition of PMG and GBF as explained below.
The absorptions in the FTIR spectroscopy were attributed to the particular spectrum absorbed by the specific functional group. For example, the functional group associated with –C=O (ester) i.e. carbonyl group from the ester linkage of triacylglycerol is attributed to the wavenumber of 1745 cm−1. The wavenumbers 1460, 1377, 1161, 1061, 1117 and 1097 cm−1 have been reported to be associated with different functional groups/bonds found in the fatty acids i.e. –C–H–(CH2,CH3), =C–H–(cis), –C–O–CH2–, –C–H– and –C–H– bending, respectively (Nurrulhidayah et al. 2010; Vlachos et al. 2006; Guillén and Cabo 1997; Safar et al. 1994). Therefore, in the present study, the peaks observed in the region of 1786–1680 cm−1 might be attributed to the ester group of the triglycerides present in the fat samples, while the peaks observed in the spectral region of 1490-919 cm−1 might be associated with the different functional groups present in the fatty acid. Earlier workers, Guillén and Cabo (1997) suggested bands near 1400 and 1097 cm−1 as the fingerprint regions, while Nurrulhidayah et al. (2013) reported a wider range of 1500–1000 cm−1 as the fingerprint regions for detection of beef fat in butter.
Principal component analysis
Principal Component Analysis (PCA) used the combinations of absorbance at the specified wavenumbers that explained most of the variance present in the data set. PCA of the spectral data carried out in the selected range of wavenumber i.e. 1786-1680 cm−1 so as to observe the clustering of samples for the control, spiked and GBF samples is represented in Fig. 2a. It can be observed from the figure that PMG and GBF showed well separated clusters. The variance as explained by Principal Component 1 and 2 were 91% and 9%, respectively indicating the role of FTIR in the detection of GBF in PMG at 5% level of significance. With the increase in the level of spiking, the clusters of the spiked samples moved away from the cluster of PMG and approached to the cluster of GBF. In fact, even the lowest level of spiking showed separate clusters of the sample than the PMG. This could again be explained by the difference in the fatty acid composition of pure mixed ghee and goat body fat. Similar results were obtained when the PCA was applied to the other range of the selected wavenumbers i.e. 1490-919 cm−1 and 1260-1040 cm−1 (Fig. 2b, c), where the Principal Component 1 and 2 contributed respectively, 92 and 8% and 97 and 3%. Recently, Nicolaou et al. (2010) used FTIR spectroscopy and multivariate analysis for detection of presence of sheep and goat milk in cow milk. However, in the present investigation, FTIR coupled with chemometics was used for detection of goat body fat in pure mixed ghee with the purpose of detection of adulteration of cheaper body fat in the high priced mixed ghee.
Classification of sample
Soft Independent Modeling of Class Analogy (SIMCA) approach at the selected wavelengths was adopted for predicting probable class membership among the PMG, PMG spiked with GBF at different levels and GBF. As a result of SIMCA class projections of the spectra, it was evident that PMG was always classified under its class with the classification efficiency of 100% at all the selected wavenumbers (Table 1). Similarly, GBF was always classified in its own class at all the wavenumber ranges studied giving a classification efficiency of 100%. It was further interesting to note that although the spiked samples were misclassified at times but they never classified in the PMG indicating the scope of the methodology for detecting the presence of GBF in PMG. Minimum classification efficiency for the test samples (those spiked with GBF) was obtained to be 90.90% for 1490–919 and 1260–1040 cm−1 spectral ranges for the samples spiked with up to 5% of the GBF. However, in case of 1786–1680 cm−1 spectral range, the minimum class efficiency was observed to be 92.67% for the samples spiked with up to 3% of GBF in PMG. However, the classification efficiency was 100% at the higher levels. Thus, it can be concluded that even the lowest level of 1% GBF in PMG can be detected using SIMCA approach considering the results that none of the spiked samples were classified in the PMG class.
Calibration and validation
Partial least square (PLS) was used for constructing calibration model in selected ranges of wavenumbers and the effect of different spectral windows on spectral data modeling are presented (Table 2). The results revealed R2 closer to unity (i.e. >0.99) in all the selected spectral ranges, which further signify the better relationship between actual and FTIR predicted values of the analyte of interest (GBF in our case). Also, according to International Conference on Harmonization (ICH 1994), R2 value higher than 0.99 is acceptable for such relationship which was observed in all the selected wavelengths. RMSEC refers to the calibration uncertainty. The difference between RMSEC and RMSEV was less, which indicate that the calibration model was accurately developed. Also, the slope was observed to be around 45°, while the intercept was negligible for the scatter plots of both calibration and validation sets of sample (Fig. 2 in ESM) confirming the predictability of the analyte i.e. GBF in PMG. It can also be said that all the selected wavenumber ranges (1786–1680, 1490–919 and 1260–1040 cm−1) are useful for determining the presence of GBF in PMG as these are associated with the specific groups present in the fat as mentioned previously. Infra-red spectroscopy was also used by some workers (Konevets et al. 1987; Sharma 1989) in the past who reported a level of detection of 10% for the animal body fats in milk fat, which is much higher than the one reported in the present study.
Conclusion
The difference in the absorption values of pure mixed ghee, goat body fat and the pure mixed ghee spiked with goat body fat in the spectral region of 1786–1680, 1490–919 and 1260–1040 cm−1 is clearly evident from the peaks in the said regions. Using SIMCA, almost 97% of the test samples could accurately classify themselves into their respective class in the models developed without preprocessing the data. The efficiency of the coefficient of determination using PLS for quantitatively predicting the data was also found to be high for both calibration and validation sets (>0.99) at all the wavelengths studied (1786–1680, 1490–919 and 1260–1040 cm−1). The difference in the value of RMSEC and RMSEV was less, indicating that the model was well developed. The study concludes that the method is rapid, non-destructive and has great precision and accuracy. Attenuated Total Reflectance- Fourier Transform Infra-red spectroscopy has the potential of determining the adulteration of even 1% of goat body fat in pure mixed ghee.
References
Albanell E, Cáceres P, Caja G, Molina E, Gargouri A (1998) Determination of fat, protein and total solids in ovine milk by near-infrared spectroscopy. J AOAC Int 82(3):753–758
Amrutha Kala AL (2013) Detection of possible adulteration in commercial ghee samples using low-resolution gas chromatography triglyceride profiles. Int J Dairy Technol 66(3):346–351
Belury MA (2002) Dietary conjugated linoleic acid in health: physiological effects and mechanisms of action 1. Annu Rev Nutr 22(1):505–531
Bendini A, Cerretani L, Di Virgilio F, Belloni P, Bonoli-Carbognin M, Lercker G (2007) Preliminary evaluation of the application of the FTIR spectroscopy to control the geographic origin and quality of virgin olive oils. J Food Quality 30(4):424–437
Casey NH, Van Niekerk WA (1985) Fatty acid composition of subcutaneous and kidney fat depots of Boer goats and the response to varying levels of maize meal. S Afr J Anim Sci 15(2):60–62
Chakrabarty MM, Bandyopadhyay C, Bhattacharyya D, Gayen AK (1968) Detection of adulteration of fats by thin layer chromatography of trisaturated glycerides: I. Detection of hydrogenated groundnut oil, tallow and Mohua (Mowrah) oil in butter fat (ghee). J Chromatogr A 36:84–90
Coni E, Di Pasquale M, Coppolelli P, Bocca A (1994) Detection of animal fats in butter by differential scanning calorimetry: a pilot study. J Am Oil Chem Soc 71(8):807–810
Das G (2012) Adulteration of ghee. http://www.lakshitmadhukalp.in/lakshit/cow-products/adulteration-ghee/index.html. Last accessed on 19th June, 2015
De S (2012) Indian dairy products. In: De S (ed) Outlines of dairy technology. Oxford University Press, New Delhi, pp 382–466
Gahlawat S, Kumari S, Boora N, Sharma YVRK (2012) Screening of adulterants in the milk and dairy products of Delhi region—a case study. Int J Pharm Technol 4(4):4889–4897
Gandhi M (2009) http://www.bihartimes.in/maneka/gheeadulteration.html. Accessed on 1 Dec 2015
Gidding SS, Lichtenstein AH, Faith MS, Karpyn A, Mennella JA, Popkin B, Rowe J, Whitsel L (2009) Implementing American Heart Association Pediatric and Adult Nutrition Guidelines A Scientific Statement From the American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Cardiovascular Disease in the Young, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Nursing, Council on Epidemiology and Prevention, and Council for High Blood Pressure Research. Circulation 119(8):1161–1175
Giovannucci E, Willett WC (1994) Dietary factors and risk of colon cancer. Ann Med 26(6):443–452
Guillén MD, Cabo N (1997) Infrared spectroscopy in the study of edible oils and fats. J Sci Food Agr 75(1):1–11
Headlines Today (2009) http://indiatoday.intoday.in/story/There’s+poison+in+the+ghee+you+consume/1/48111.html. Accessed on 1 Dec 2015
International Conference on Harmonisation (ICH) (1994) Validation of analytical procedures: text and methodology. Downloaded from: http://www.ich.org/fileadmin/Public/Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1_Guideline.pdf, 19/04/2011
Ip C, Banni S, Angioni E, Carta G, McGinley J, Thompson HJ, Barbano D, Bauman D (1999) Conjugated linoleic acid-enriched butter fat alters mammary gland morphogenesis and reduces cancer risk in rats. J Nutr 129(12):2135–2142
Jaiswal P, Jha SN, Bharadwaj R (2012) Non-destructive prediction of quality of intact banana using spectroscopy. Sci Hort 135:14–22
Jha SN, Matsuoka T (2004) Detection of adulterants in milk using near infrared spectroscopy. J Food Sci Technol -Mysore 41(3):313–316
Jha SN, Jaiswal P, Borah A, Gautam AK, Srivastava N (2015) Detection and quantification of urea in milk using attenuated total reflectance-Fourier transform infrared spectroscopy. Food Bioprocess Technol 8(4):926–933
Konevets VI, Roganoua ZA, Smolyanski AL (1987) Use of infra-red spectroscopy for analysing fats of different origin. Izvestiya Vyrshikh O Chebnykh Zavadenii Pischevaya Tekhnologiya 1:64–67 [Cited: Dairy Sci Abstract. (1988) 50:1973]
Kumar A, Kumar A, Lal D, Seth R, Sharma V (2010) Validation of ultra-violet and visible spectroscopy methods for detection of milk fat adulteration. J Dairy Foods Home Sci 29(1):8–14
Lambelet P, Ganguli NC (1983) Detection of pig and buffalo body fat in cow and buffalo ghees by differential scanning calorimetry. J Am Oil Chem Soc 60(5):1005–1008
Lee KN, Kritchevsky D, Parizaa MW (1994) Conjugated linoleic acid and atherosclerosis in rabbits. Atherosclerosis 108(1):19–25
Lerma-García MJ, Gori A, Cerretani L, Simó-Alfonso EF, Caboni MF (2010) Classification of Pecorino cheeses produced in Italy according to their ripening time and manufacturing technique using Fourier transform infrared spectroscopy. J Dairy Sci 93(10):4490–4496
Mani R, Intodia V (2014) Dairy and products annual. Global Agricultural Information Network Report Number: IN4089, Approved by: Joshua Emmanuel Lagos. http://gain.fas.usda.gov/Recent%20GAIN%20Publications/Dairy%20and%20Products%20Annual_New%20Delhi_India_10-15-2014.pdf. Accessed on 1 Dec 2015
Månsson HL (2008) Fatty acids in bovine milk fat. Food Nutr Res 52:1–3. doi:10.3402/fnr.v52i0.1821
McBain JA, Eastman A, Nobel CS, Mueller GC (1997) Apoptotic death in adenocarcinoma cell lines induced by butyrate and other histone deacetylase inhibitors. Biochem Pharmacol 53(9):1357–1368
Nicolaou N, Xu Y, Goodacre R (2010) Fourier transform infrared spectroscopy and multivariate analysis for the detection and quantification of different milk species. J Dairy Sci 93(12):5651–5660
Nunes CA (2014) Vibrational spectroscopy and chemometrics to assess authenticity, adulteration and intrinsic quality parameters of edible oils and fats. Food Res Int 60:255–261. doi:10.1016/j.foodres.2013.08.041
Nurrulhidayah AF, Che Man YB, Rohman A, Amin I, Shuhaimi M, Khatib A (2013) Authentication analysis of butter from beef fat using Fourier transform infrared (FTIR) spectroscopy coupled with chemometrics. Int Food Res J 20(3):1383–1388
Ozen BF, Mauer LJ (2002) Detection of hazelnut oil adulteration using FT-IR spectroscopy. J Agr Food Chem 50(14):3898–3901
Pariza MW, Park Y, Cook ME (2001) The biologically active isomers of conjugated linoleic acid. Prog Lipid Res 40(4):283–298
Parodi PW (2003) Anti-cancer agents in milkfat. Aust J Dairy Technol 58(2):114
Ramachandra BV, Dastur NN (1959) Application of paper chromatography to differentiate ghee from other fats. 1. Behaviour of unsaponifiable matter on chromatograms. Indian J Dairy Sci 12(3/4):139–148
Ramkumar P (2014) http://timesofindia.indiatimes.com/city/coimbatore/50-of-ghee-adulterated-say-food-safety-officers/articleshow/45486643.cms. Accessed on 1 Dec 2015
Rodriguez-Otero JL, Hermida M, Centeno J (1997) Analysis of dairy products by near-infrared spectroscopy: a review. J Agr Food Chem 45(8):2815–2819
Rodriguez-Saona LE, Allendorf ME (2011) Use of FTIR for rapid authentication and detection of adulteration of food. Annu Rev Food Sci Technol 2:467–483
Rohman A, Man YC (2010) Fourier transform infrared (FTIR) spectroscopy for analysis of extra virgin olive oil adulterated with palm oil. Food Res Int 43(3):886–892
Safar M, Bertrand D, Robert P, Devaux MF, Genot C (1994) Characterization of edible oils, butters and margarines by Fourier transform infrared spectroscopy with attenuated total reflectance. J Am Oil Chem Soc 71(4):371–377
Sharma SK (1989) Studies on unsaponifiable matter of milk fat (clarified butterfat) and animal body fats with a view to detect adulteration, Ph.D. Thesis. Submitted to National Dairy Research Institute (Deemed University), Karnal, India
Singhal OP (1973) Studies on ghee (clarified butterfat) and animal body fats with a view to detect adulteration. Ph.D. Thesis. Submitted to Punjab University, Chandigarh
Sivakesava S, Irudayaraj J (2001) Detection of inverted beet sugar adulteration of honey by FTIR spectroscopy. J Sci Food Agric 81(8):683–690
Soliman MA, Younes NA (1986) Adulterated butterfat: fatty acid composition of triglycerides and 2-monoglycerides. J Am Oil Chem Soc 63(2):248–250
Sørensen LK, Jepsen R (1998) Assessment of sensory properties of cheese by near-infrared spectroscopy. Int Dairy J 8(10):863–871
Sserunjogi ML, Abrahamsen RK, Narvhus J (1998) A review paper: current knowledge of ghee and related products. Int Dairy J 8(8):677–688
Stender S, Dyerberg J (2004) Influence of trans fatty acids on health. Ann Nutr Metab 48(2):61–66
Truitt A, McNeill G, Vanderhoek JY (1999) Antiplatelet effects of conjugated linoleic acid isomers. BBA-Mol Cell Biol L 1438(2):239–246
United States Department of Agriculture Department of Health and Human Services (2010) Dietary Guidelines for Americans
Upadhyay N, Kumar A, Gandhi K, Goyal A, Lal D (2014) Standardization of solvent fractionation technique for detection of adulteration by enriching animal body fat and vegetable oil in different fractions. Indian J Dairy Sci 67(4):323–327
Upadhyay N, Kumar A, Rathod G, Goyal A, Lal D (2015) Development of a method employing reversed-phase thin-layer chromatography for establishing milk fat purity with respect to adulteration with vegetable oils. Int J Dairy Technol 68(2):207–217
Vlachos N, Skopelitis Y, Psaroudaki M, Konstantinidou V, Chatzilazarou A, Tegou E (2006) Applications of Fourier transform-infrared spectroscopy to edible oils. Anal Chim Acta 573:459–465
Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE (1990) Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med 323(24):1664–1672
Acknowledgements
The first author acknowledges Dr. A. K. Srivastava, Director, ICAR-National Dairy Research Institute, Karnal, Haryana and Dr. R. K. Gupta, Director, ICAR-Central Institute of Post Harvest Engineering and Technology, Ludhiana for granting necessary permission for undergoing the professional attachment training in the research laboratory of Dr. S. N. Jha leading to this work. First author would also like to thank Dr. H. G. Ramya (Research Associate) for timely and useful suggestions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors do not have any conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Upadhyay, N., Jaiswal, P. & Jha, S.N. Detection of goat body fat adulteration in pure ghee using ATR-FTIR spectroscopy coupled with chemometric strategy. J Food Sci Technol 53, 3752–3760 (2016). https://doi.org/10.1007/s13197-016-2353-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2353-2