Abstract
The rheological property of Shanxi aged vinegar (SAV) was determined by rheometer, and its effects on release in eight key aroma components of SAV was investigated by SPME–GC–MS. In order to simulate the change of rheological property of SAV, a modified SAV system was developed from a finished SAV using carboxymethylcellulose, pectin, glucose, fructose, sodium chloride and tannic acid at indicate levels. The consistency coefficients (K) of SAV ranged from 1.09e−5 to 0.0137, which was correlated to glucose, polyphenol, acids and oBx. SAV changed from shear-thickening to Newtonian fluid during long-time ageing. In the modified SAV system, the K values increased significantly, and two modified vinegar became quasi-Newtonian fluids too. Furthermore, release of the eight key aroma compounds decreased significantly and decreased was pronounced, for acetic acid, furfural and tetramethylpyrazine. The results demonstrated rheological property correlated to the concentrate of sugar, salt, polyphenol, acids and macromolecule, which significantly affected the release of major aroma compounds.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shanxi aged vinegar (SAV) is the most famous traditional vinegar in northern China. SAV is a product from a solid-state fermentation process, including five successive stages, i.e. (1) starter preparation, (2) alcohol fermentation, (3) acid fermentation, (4) thermal process and (5) ageing (ranged from 1 to 20 years; Liu et al. 2004). The aroma perception, which is an important quality factor of SAV, is determined by aroma composition and the release of aroma compounds (Poinot et al. 2013). More than 56 aroma compounds, including acids, esters, aldehydes, ketones, alcohols, and volatile phenols have been identified in SAV (Wang et al. 2012a; Xiao et al. 2011). Nineteen compounds have been identified as key aroma compounds of SAV. Among them, propanoic acid, acetic acid, trimethyl oxazole, butanoic acid, acetoin, 3-methylbutanoic acid and furfural were the most powerful odorants (Zhu et al. 2016).
The rheological property of food is related to aroma release, but the relationship between them was complicated (Lubbers et al. 2004; Muñoz-González et al. 2014). It was found that the quantity of aroma compounds in the headspace of strawberry fat-free stirred yogurt was drastically reduced, while the apparent viscosity was significantly increased during storage (Lubbers et al. 2004). However, Decourcelle et al. (2004) found that adding locust bean gum to fat-free stirred yogurt increased the viscosity of yogurt, which resulted in the increase of flavor release. Furthermore, it was reported that perceived volatile fruity flavor was significantly different in model oil/water emulsions with similar rheological property (Bayarri et al. 2007). Although the relationship between rheological property and aroma release was complicated, it is critical to study in SAV. The reason was two-fold: (1) the rheological property could be a simple and reliable quality indicator of vinegar (Falcone et al. 2008); (2) rheological property is closely related to sensory property of fluids food, such as mouth-feel (Bourne 2002) and the intensity of flavor (Godshall 1997). Viscosity is also known to influence other taste perception, including saltiness, sweetness, bitterness, flavor and astringency (Christensen 1980). Therefore, the effect of rheological property on aroma release is needed to be investigated.
Rheological property of vinegar was influenced by several factors, including sugar content, NaCl, polyphenol and polymerization during ageing. The glucose concentration as well as the ratio of glucose to fructose have proven to be the most significant factors affecting the steady shear viscosity and flow activation energy of vinegar (Falcone et al. 2007). Flow behavior index is found to be negatively related to both glucose and fructose concentration and oBx of vinegar (Falcone et al. 2008). In addition, polymerization during long-time ageing process led to alteration of rheological property of vinegar, which is from Newtonian to shear-thinning flow behavior (Falcone and Giudici 2008). A modified SAV system would be developed by adding sugar, salt, polyphenol and polysaccharide into SAV in this work so as to simulate the change of rheological property and analyze the changes of aroma release while keeping aroma composition unchanged.
Static headspace analysis was widely used to analyze aroma release. Solid phase microextraction (SPME) coupled with GC–MS, was carried out to analyze the air/product partition coefficient of the compounds (Mitropoulou et al. 2011). Based on previous works (Wang et al. 2012a; Xiao et al. 2011; Zhu et al. 2016), eight compounds (propanoic acid, acetic acid, trimethyl oxazole, acetoin, 3-methylbutanoic acid, furfural, tetramethylpyrazine and 2-methylphenol), which were identified as key aroma in SAV, would be quantified by SPME–GC–MS in this study. The aim of our research is to study the rheological property of SAV, and effect of rheological property variation on the release of key aroma compounds of SAV.
Materials and methods
Chemical
Chemical standards of the aroma compounds, including propanoic acid, acetic acid, trimethyl oxazole, acetoin, 3-methylbutanoic acid, furfural, tetramethylpyrazine and 2-methylphenol, were supplied by Sigma-Aldrich (Shanghai, China) and J&K (Beijing, China). Tannic acid (reagent grade), CMC (Carboxymethyl Cellulose) and pectin were also purchased from Sigma-Aldrich (Shanghai, China). Glucose, fructose and NaCl were purchased from J&K (Beijing, China). Pure water was obtained from a Milli-Q purification system (Millipore, Bedford, MA, USA).
Sample preparation and experimental design
Four kinds of SAV made from sorghum, tartary buckwheat, wheat and millet were purchased from Shanxi Ziyuan Microorganism R&D Co., Ltd. (Shanxi, China). The ageing time of these four SAV samples was 3 years. Other seven SAV samples, which have continuous aging time (young, 3, 5, 8, 10, 16 and 20 years), were obtained from Donghu Co., Ltd. (Shanxi, China). The raw material of these seven vinegar samples was sorghum.
A modified SAV system was developed by adding nonvolatile components into the tartary buckwheat SAV, which was used as the control, to simulate the change of rheological property in SAV. Six nonvolatile components (CMC, pectin, glucose, fructose, sodium chloride and tannic acid) were added into each tartary buckwheat SAV at two levels (low and high), respectively (Table 1).
Chemical analysis
Sugar content was determined by ion chromatograph; model ICS-5000 (Dionex, USA). The chromatographic conditions were as follows: CarboPac PA10 (4 × 250 mm) and Guard, BorateTrap; 1.0 mL/min flow rate; isocratic separation with 52 mM NaOH; detected on integrated amperometry, quadruple waveform; gold electrode; 1 mL injection volume. The content of acetic acid and lactic acid was analyzed by the method reported in our previous research (Zhu et al. 2016). Total polyphenolic content was measured by the Folin-Ciocalteu method (Wang et al. 2012b). The content of NaCl was determined according to AOAC Official Method 971.27, Sodium Chloride in Canned Vegetables, Method III (Potentiometric Method). The soluble solids content in vinegar was measured using a Leica temperature compensating oBx scale (0–30) refractometer (Leica Inc., Buffalo, NY). The pH value of the vinegar was measured by a pH meter (InoLab 720, WTW99 GmbH, Weilheim, Germany). Density was measured according to the method of AOAC (1990).
Rheological measurement
Rheological behavior was studied by a controlled-strain rotational viscosimeter (ARES model, TA Instruments, New Castle, USA) equipped with a force rebalance transducer (model 1KFRTN1, 1–1000 g cm, 200 rad/s, 2–2000 gmf) and a couette tool, a concentric cylinder geometry (diameter of cup and bob, 34 and 32 mm, respectively). The steady temperature was controlled with an accuracy of ±0.1 °C by means of a controlled fluid bath unit and an external thermostatic bath. Three replicates of flow experiment were performed for each sample. Steady shear stress was measured over a range of shear rates of 0.1–500 s−1. The flow experiment was conducted at 20 °C. In order to prevent acetic acid evaporation, the couette tool was sealed by a cover tool during tests.
SPME extraction and gas-chromatographic analysis
Eight volatile compounds, including propanoic acid, acetic acid, trimethyl oxazole, acetoin, 3-methylbutanoic acid, furfural, tetramethylpyrazine and 2-methylphenol were chosen as aroma indicator as reported earlier (Wang et al. 2012a; Zhu et al. 2016). The analysis of aroma compounds was performed by SPME–GC–MS. Five milliliters of SAV was transferred into a 10 mL vial and the vial was tightly capped with a PTFE/silicon septum (Bellefonte, PA, USA). The incubation time was 15 min, during which the samples were magnetically agitated at 37 °C. The solutions were heated at 37 °C corresponding to the average temperature in the oral cavity. After equilibration, the headspace of the samples was sampled using a DVB/CAR/PDMS (divinylbenzene-carboxen-polydimethylsiloxane 50/30 μm) coated fiber. The extraction time was 20 min exactly. Then, it was placed into the GC injection port for 5 min in splitless mode. Additionally, the SPME fiber was conditioned for 30 min at 230 °C in the GC injector before the first daily analysis.
Gas chromatographic analysis was carried out with a VARIAN GC system coupled with 210 mass spectrometer. The separation was performed using a HP-innowax fused capillary column (30 m × 0.25 mm, and 0.25 μm film thickness). Oven temperature started from 40 °C and held for 3 min, increased to 120 °C at a rate of 5 °C/min, increased to 200 °C at a rate of 10 °C/min, and then held for 5 min. The injection temperature was 230 °C. Helium was used as the carrier gas at a constant rate of 1.0 mL/min. Each analysis was undertaken three times and average values were calculated. The identification method of the target aroma compounds in SAV was done according to Wang et al. (2012a) and Zhu et al. (2016). The relative content of aroma compounds in the headspace was represented by the peak area of GC–MS.
Statistical analysis
Statistical analysis was performed using SPSS 18.0 software. A Pearson correlation test was conducted to determine the correlation between variables. Duncan’s significance test at a significance level of p < 0.05 was carried out to determine significant differences among mean values.
Results and discussion
Rheological property of Shanxi aged vinegar
Flow experiments provided a wide range of rheological responses among the investigated vinegar samples including the shear-thickening and Newtonian behavior. The rheogram of shear stress and shear rates of investigated SAV is given in Online Resource 1. Rheological data of SAV fitted to power law model, and the determination coefficient, (R2) was above 0.99 in present study. The values of two rheological parameters, consistency coefficient (K) and flow behavior index (n) obtained from the power law curve, were presented in Table 2. Higher K value indicates higher viscosity. There was a wide range of K values among different raw material and ageing time (from 1.09e−4 to 0.0137). K value increased along with longer ageing time. The n values of all SAV were higher than 1, indicating their SAV were shear-behavior. However, three vinegar samples with longer ageing time exhibited Newtonian behavior.
The result indicated that the K values of SAV varied in a wide range due to different raw material and ageing time. K values of SAV were much lower than those of balsamic vinegar and soursop juice, which ranged from 0.001 to 12 (Falcone et al. 2008; Quek et al. 2013). According to the flow behavior index, the SAV samples were classified into two groups. Vinegar samples with shorter ageing time (below 10 years) were shear-thickening fluids, while vinegar samples with longer ageing time (above 10 years) exhibited Newtonian behavior. This indicated long-time ageing process of vinegar might result in the variation of microstructure. The previous study proposed that the formation of polydisperse melanoidins was responsible for change of rheological property (from Newtonian to shear-thinning fluid) of traditional balsamic vinegar (Falcone et al. 2012). Therefore, the contribution of certain macromolecule formed in SAV during the ageing process to its rheological property (both K and n value) can not be ruled out.
From the perspective of rheological property, SAV was different from gels, emulsions and balsamic vinegar. The gel is solid-in-liquid colloid in which the solid phase forms a network structure and produces solid-like property (Tabilo-Munizaga and Barbosa-Cánovas 2005). Compared to gel, the viscoelasticity of emulsion was much lower (K values are around 0.01), while it was still stronger than that of SAV (Arancibia et al. 2011). The oil, which was not found in SAV, may have been responsible for the viscosity of emulsion (Tabilo-Munizaga and Barbosa-Cánovas 2005).
Chemical analysis of Shanxi aged vinegar
The SAV was a complicated liquid system, the main components of SAV were sugar, sodium chloride, organic acid and polyphenol. Chemical compositions of eleven SAV were reported in Table 3. Glucose content of SAV ranged from 7.40 to 54.40 g/L, and increased significantly (p < 0.05) during the ageing process. However, fructose cannot be detected in vinegar if ageing time was too long. The total sugar content of all SAV was much lower than that of balsamic vinegar (ranged from 410 to 470 g/L) (Masino et al. 2008). Total polyphenol content ranged from 0.88 to 25.94 g/L, and the highest level was found in SAV with 20 ageing years. The phenolic content of SAV was comparable to that of wine and other Chinese vinegar, and ranged from 0.38 to 4.51 g/L (Budak and Guzel-Seydim 2010; Fan et al. 2011). In particular, the content of glucose, total polyphenol, NaCl, acetic acid, lactic acid and values of pH, density and oBx of SAV all increased with ageing time. This may be due to the loss of a large amount of water, such as evaporation in summer and ice-removing in winter during the ageing process. The condensation effect of SAV ageing was proven in a previous study, which indicated most conventional ingredients (organic acids, free amino acids, carbohydrates) were increased during ageing process (Chen et al. 2013). Besides the increasing trends, the content of acetic acid was fluctuating during the early stage of ageing. In fact, the content of acetic acid depended on condensation effect of SAV ageing and evaporation of acetic acid. The former factor led to the increasing of acetic acid, while the latter one resulted in the decrease. So the contents of acetic acid may be affected by the packaging season as well.
The composition of vinegar was related to the rheological property (Falcone et al. 2007). The apparent viscosity of non-Newtonian vinegar was found to be positively related to the fructose concentration (Falcone et al. 2008). Person coefficients of correlation relationships between composition and K values of SAV were listed in Table 3. Positive correlations were found between K values and the concentration of glucose, polyphenol, acetic acid, lactic acid and oBx. The oBx value, glucose and fructose content also contributed to the vinegar viscosity (Falcone et al. 2008). It is reasonable that K values of SAV were lower than those of balsamic vinegar (from 0.898 to 12), because the oBx values of balsamic vinegar (around 70 oBx) (Falcone et al. 2008) were much higher. It could be concluded that variation of main components in SAV could lead to the change of rheological property.
Rheological property of Shanxi aged vinegar with modification
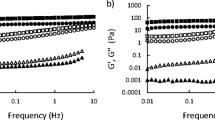
The rheological property of buckwheat SAV added with CMC, pectin, glucose, fructose, sodium chloride and tannic acid are presented in Fig. 1. The rheological data fitted to power law model showed, R2. As shown in Fig. 1a, the K values of all the modified SAV increased significantly. The highest K value (0.0428) was obtained by adding pectin, followed by adding CMC (0.0359). CMC and pectin were widely used in emulsion and gel system to increase the viscosity (Decourcelle et al. 2004; Roberts et al. 1996). Interestingly, among the SAV added with glucose, fructose and sodium chloride, the K values depended on the addition level rather than the components added. Figure 1b shows the n values of all modified SAV decreased, compared with control. Minimum n values (0.93 and 0.94) were obtained in the sample with CMC and pectin at high level. This indicated these two modified vinegar samples were quasi-Newtonian fluid, which was similar to SAV with longer ageing time (over 10 years). The variation of n values of glucose, fructose and NaCl groups were similar. Specifically, n values decreased slightly at the low addition level and further decreased close to 1 (still >1) at high addition level. The addition of tannic acid led to the increase of K value and decrease of n value. Unlike the other five components, tannic acid at low level (0.5 %) has a greater influence on K and n values than at high level (1 %), indicating the effect of tannic acid on the rheological property of SAV could be more complex.
This modification system provided evidence for our two hypotheses. First, a small amount of macromolecules, namely CMC and pectin, could increase K values and change the flow behavior of SAV towards Newtonian fluids. Secondly, the increasing of sugar, sodium chloride and polyphenol could increase the K values of SAV. Furthermore, this modified SAV system was suitable for analysis of aroma release because the volatile composition of modified SAV did not change by the addition of non-volatile components. Therefore, the modified SAV system was developed in this study successfully, which simulated the change of rheological property.
Relationship between rheological property and aroma release of Shanxi aged vinegar
The variation of the release of each aroma compound in as percent rate of content of counterpart compounds in control was shown in Table 4. Compared to control, the total content of these eight aroma compounds was decreased. The SAV with high CMC addition level showed the lowest aroma release content, followed by SAV with high level of pectin addition. Moreover, the key aroma release decreased along with the increase of viscosity. These results were in agreement with studies on yogurt, model solutions and custard. The release of aroma compounds in the headspace of strawberry fat-free stirred yogurt was significantly weaker during storage, as the apparent viscosity of the products significantly increased (Lubbers et al. 2004). Meanwhile, the highly volatile compounds showed a significant decrease in maximum abundance (aroma release determined by APCI-MS) as the viscosity increased in CMC, guar and sucrose solutions (Roberts et al. 1996). In addition, custard with lower apparent viscosity showed a higher kinetic release of aroma compounds under in-mouth simulated conditions (Lubbers and Butler 2010). According to mass transfer theory, an increase in matrix viscosity would lead to the decrease of aroma release in amount (Crank 1979). This relationship between viscosity and aroma release was also predicted by the Stokes–Einstein and Wilke–Chang equations (Wilke and Chang 1955).
In the vinegar modified by CMC and pectin (high level), most aroma compounds were retained. Therefore, the release of aroma in SAV decreased when it converted to Newtonian fluid. Even if a small amount (around 1 %) of polysaccharide were formed during ageing, the rheological property of SAV would be changed and the release of aroma compounds was significantly reduced accordingly. As for sodium chloride addition, the release of some aroma compounds, such as trimethyl oxazole, tetramethylpyrazine, propanoic acid and 3-methyl-butanoic acid, increased. This might be due to the salting-out effect of sodium chloride, which could increase the release of aroma compounds significantly (Paula Barros et al. 2012). In the tannic acid group, the release of acetic acid, furfural, tetramethylpyrazine and 2-methylphenol was reduced more than those of other four aroma compounds. The previous study reported that π–π stakes could be involved in direct interactions between polyphenols, such as tannic acid and catechin, and aroma compounds with aromatic cycles (Muñoz-González et al. 2014). Moreover, the release of three aroma compounds, namely acetic acid, furfural and tetramethylpyrazine, was reduced significantly (p < 0.05) in most modified vinegar samples, except for the vinegar modified by 20 % sodium chloride. According to our previous studies, these three aroma compounds accounted for the majority of volatile compounds of SAV and were the most powerful odorants (Zhu et al. 2016). Thus, alteration of rheological property, which referred to increasing K values and decreasing n values, could slow the release of major aroma compounds and improve the quality of SAV.
Conclusion
The rheological property and its effect on aroma release of SAV were studied. SAV showed shear-thickening and Newtonian fluids behavior. Vinegar aged for shorter period (less than 10 years) were shear-thickening fluids, while those aged for longer period (above 10 years) exhibited Newtonian behavior. The K values of SAV varied largely (1.09e−4 to 0.0137) according to raw material used and length of ageing process. Chemical analysis demonstrated a positive correlation between the content of glucose, polyphenol, acetic acid, lactic acid and oBx and K values of SAV. A modified SAV system, which simulated the change of rheological property, was successfully developed by adding different levels of CMC, pectin, glucose, fructose, NaCl and polyphenol. In the modified system, CMC and pectin modified SAV became quasi-Newtonian fluid, and the K values of all modified SAV increased significantly. This modification system proved that a small amount of macromolecules could change rheological property (both K and n value) of SAV significantly, and increasing amount of sugar, NaCl and polyphenol could increase the K values of SAV. The SPME–GC–MS analysis indicated the total key aroma compounds released for modified SAV was lower than that of control. Formation of small amount (around 1 %) of polysaccharide in SAV led to significant reduction the release of aroma compounds. Moreover, the release of aroma compounds, namely acetic acid, furfural and tetramethylpyrazine, decreased significantly in modified vinegar which also showed an increase in the viscosity.
References
AOAC (1990) Official methods of analysis. AOAC International, Washington
Arancibia C, Jublot L, Costell E, Bayarri S (2011) Flavor release and sensory characteristics of o/w emulsions. Influence of composition, microstructure and rheological behavior. Food Res Int 44:1632–1641. doi:10.1016/j.foodres.2011.04.049
Bayarri S, Smith T, Hollowood T, Hort J (2007) The role of rheological behaviour in flavour perception in model oil/water emulsions. Eur Food Res Technol 226:161–168
Bourne M (2002) Food texture and viscosity: concept and measurement. Academic, Cambridge
Budak HN, Guzel-Seydim ZB (2010) Antioxidant activity and phenolic content of wine vinegars produced by two different techniques. J Sci Food Agric 90:2021–2026
Chen T, Gui Q, Shi JJ, Zhang XY, Chen FS (2013) Analysis of variation of main components during aging process of Shanxi aged vinegar. Acetic Acid Bact 2:6
Christensen CM (1980) Effects of taste quality and intensity on oral perception of viscosity. Percept Psychophys 28:315–320
Crank J (1979) The mathematics of diffusion. Oxford University Press, Oxford
Decourcelle N, Lubbers S, Vallet N, Rondeau P, Guichard E (2004) Effect of thickeners and sweeteners on the release of blended aroma compounds in fat-free stirred yoghurt during shear conditions. Int Dairy J 14:783–789
Falcone PM, Giudici P (2008) Molecular size and molecular size distribution affecting traditional balsamic vinegar aging. J Agric Food Chem 56:7057–7066. doi:10.1021/jf800706g
Falcone PM, Chillo S, Giudici P, Del Nobile MA (2007) Measuring rheological properties for applications in quality assessment of traditional balsamic vinegar: description and preliminary evaluation of a model. J Food Eng 80:234–240. doi:10.1016/j.jfoodeng.2006.05.023
Falcone PM, Verzelloni E, Tagliazucchi D, Giudici P (2008) A rheological approach to the quantitative assessment of traditional balsamic vinegar quality. J Food Eng 86:433–443
Falcone PM, Mozzon M, Frega NG (2012) Structure–composition relationships of the traditional balsamic vinegar of Modena close to jamming transition (part II): threshold control parameters. Food Res Int 45:75–84
Fan J, Zhang Y, Zhou L, Li Z, Zhang B, Saito M, Wang X (2011) Nutritional composition and. ALPHA.-glucosidase inhibitory activity of five Chinese vinegars. Jpn Agric Res Q JARQ 45:445–456
Godshall MA (1997) How carbohydrates influence food flavor. Food Technol 51:63–67
Liu D, Zhu Y, Beeftink R, Ooijkaas L, Rinzema A, Chen J, Tramper J (2004) Chinese vinegar and its solid-state fermentation process. Food Rev Int 20:407–424
Lubbers S, Butler E (2010) Effects of texture and temperature on the kinetic of aroma release from model dairy custards. Food Chem 123:345–350. doi:10.1016/j.foodchem.2010.04.041
Lubbers S, Decourcelle N, Vallet N, Guichard E (2004) Flavor release and rheology behavior of strawberry fatfree stirred yogurt during storage. J Agric Food Chem 52:3077–3082
Masino F, Chinnici F, Bendini A, Montevecchi G, Antonelli A (2008) A study on relationships among chemical, physical, and qualitative assessment in traditional balsamic vinegar. Food Chem 106:90–95
Mitropoulou A, Hatzidimitriou E, Paraskevopoulou A (2011) Aroma release of a model wine solution as influenced by the presence of non-volatile components. Effect of commercial tannin extracts, polysaccharides and artificial saliva. Food Res Int 44:1561–1570. doi:10.1016/j.foodres.2011.04.023
Muñoz-González C, Martín-Álvarez PJ, Moreno-Arribas MV, Pozo-Bayón MÁ (2014) Impact of the nonvolatile wine matrix composition on the in vivo aroma release from wines. J Agric Food Chem 62:66–73. doi:10.1021/jf405550y
Paula Barros E, Moreira N, Elias Pereira G, Leite SGF, Moraes Rezende C, Guedes de Pinho P (2012) Development and validation of automatic HS-SPME with a gas chromatography-ion trap/mass spectrometry method for analysis of volatiles in wines. Talanta 101:177–186. doi:10.1016/j.talanta.2012.08.028
Poinot P, Arvisenet G, Ledauphin J, Gaillard J-L, Prost C (2013) How can aroma–related cross–modal interactions be analysed? A review of current methodologies. Food Qual Prefer 28:304–316. doi:10.1016/j.foodqual.2012.10.007
Quek MC, Chin NL, Yusof YA (2013) Modelling of rheological behaviour of soursop juice concentrates using shear rate–temperature–concentration superposition. J Food Eng 118:380–386
Roberts DD, Elmore JS, Langley KR, Bakker J (1996) Effects of sucrose, guar gum, and carboxymethylcellulose on the release of volatile flavor compounds under dynamic conditions. J Agric Food Chem 44:1321–1326. doi:10.1021/jf950567c
Tabilo-Munizaga G, Barbosa-Cánovas GV (2005) Rheology for the food industry. J Food Eng 67:147–156. doi:10.1016/j.jfoodeng.2004.05.062
Wang A, Song H, Ren C, Li Z (2012a) Key aroma compounds in Shanxi aged tartary buckwheat vinegar and changes during its thermal processing. Flavour Fragr J 27:47–53
Wang A, Zhang J, Li Z (2012b) Correlation of volatile and nonvolatile components with the total antioxidant capacity of tartary buckwheat vinegar: influence of the thermal processing. Food Res Int 49:65–71
Wilke CR, Chang P (1955) Correlation of diffusion coefficients in dilute solutions. AIChE J 1:264–270. doi:10.1002/aic.690010222
Xiao Z, Dai S, Niu Y, Yu H, Zhu J, Tian H, Gu Y (2011) Discrimination of Chinese vinegars based on headspace solid-phase microextraction-gas chromatography mass spectrometry of volatile compounds and multivariate analysis. J Food Sci 76:C1125–C1135
Zhu H, Zhu J, Wang L, Li Z (2016) Development of a SPME-GC-MS method for the determination of volatile compounds in Shanxi aged vinegar and its analytical characterization by aroma wheel. J Food Sci Technol 53:171–183
Acknowledgments
This research was funded by the Chinese Agricultural Research System (grant number: CARS-08-D-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest to be declared.
Rights and permissions
About this article
Cite this article
Zhu, H., Qiu, J. & Li, Z. Determination of rheological property and its effect on key aroma release of Shanxi aged vinegar. J Food Sci Technol 53, 3304–3311 (2016). https://doi.org/10.1007/s13197-016-2305-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2305-x