Abstract
Biodegradable and active packaging based on cassava starch incorporated bixin nanocapsules with different concentrations were developed. The physical, mechanical, barrier properties and antioxidant activity of the active packaging were studieds. The films incorporated with bixin nanocapsules were found to be homogeneous and thermally stable. Films with higher concentrations of bixin nanocapsules exhibited a significant decrease in tensile strength, water solubility and increase in elongation at break and water vapour permeability, well as, significant improvement in protection against UV and visible light. The films were used to pack sunflower oil under accelerated oxidation conditions (65 % RH/35 °C). Sunflower oil packaged in films with bixin exhibited lower oxidation rates, thus maintaining its freshness according to Codex Alimentarius guidelines (<10 mEq kg−1). Films containing bixin nanocapsules are very promising materials for use as packaging with antioxidant properties for maintaining food safety and extending the shelf life.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The main objective of food packaging is to maintain the quality and safety of food during storage and distribution. However, the majority of packaging used by industry comes from non-degradable polymers, raising environmental concerns. Recent environmental regulations, societal concerns and growing environmental awareness throughout the world have triggered efforts in the plastic industry to develop new products and processes that are less harmful to the environment (Maran et al. 2013).
The use of biopolymers for the development of edible and/or biodegradable films could be an alternative to reduce or replace conventional non-biodegradable plastics (Tongdeesoontorn et al. 2012). Starch has been considered one of the most promising candidates for the future, primarily because of an attractive combination of its large availability and relatively low price (Souza et al. 2012). Cassava starch has been extensively used to produce films (Chen and Lai 2008; Maran et al. 2013; Souza et al. 2013). Starch is a polysaccharide primarily made up of amylose (20–30 %) and amylopectin (70–80 %). Amylose is water insoluble and the main source of our dietary fiber component whereas amylopectin is water-soluble. The presence of amylose makes starch insoluble while amylopectin allows starch to swell in aqueous phase to produce aqueous suspension which can be used to produce biodegradable environmental friendly films with several applications in food and pharmaceutical industries (Kaur et al. 2013)
In recent years, there have also been increased concerns about food quality and safety, which have motivated the development, in addition to biodegradable packaging, of active packaging that interact with food and assist in its conservation (Park et al. 2012; Silva-Weiss et al. 2013).
Oxidation processes are involved in most deterioration mechanisms present in nature, including in food products (López-de-Dicastillo et al. 2010). Active packages with antioxidant properties have received special attention because they are alternatives to traditional packaging, in which antioxidants are incorporated into or coated onto food packaging materials to reduce oxidation of the food, one of the main causes of food spoilage (López-de-Dicastillo et al. 2012).
Currently, the tendency to reduce the use of synthetic additives in packaging, such as BHA or BHT, has focused interest on their substitution by natural antioxidants, such as tocopherol, plant extracts, and essential oils from herbs, that are safer and in most cases offer multiple health benefits (Li et al. 2014; López-de-Dicastillo et al. 2012). Several studies have report the development of active packaging with antioxidants incorporated to improve food preservation, such as α-tocopherol (Gomez-Estaca et al. 2014; Martins et al. 2012), plant extracts (Li et al. 2014; Silva-Weiss et al. 2013), essences oils from herbs (Salarbashi et al. 2014; Silva-Weiss et al. 2013), curcumin (López-de-Dicastillo et al. 2012), eugenol (Burt 2004), among others. However, to date, there is limited work in the literature on the development of active films with the addition of antioxidants such as bixin.
The bixin is the primary pigment found in annatto seeds, a carotenoid consists of nine conjugated double bonds and two carboxylic groups; such a structure is responsible not only for its light absorption and antioxidant properties but also for its low solubility in water. Like other carotenoids, bixin is an efficient quencher of singlet oxygen and a scavenger of reactive species of oxygen and nitrogen (de Oliveira Rios et al. 2009).
Because of its low solubility and instability in the presence of oxygen, heat and light, its use may be limited for application in food packaging; however, nanoencapsulation technology provides a way to overcome these setbacks. In general, encapsulation improves the stability, solubility and bioavailability of encapsulated species and promotes its controlled release. The researchers de Lobato et al. (2013), have produced, characterized, and evaluated the stability of bixin nanocapsules, observing, in addition to increased solubility of the nanocapsules, increased stability, thus enabling their application in products with low lipid content or with antioxidant agents in active packaging. Therefore, it is important to evaluate the characteristics of the films after the incorporation of the antioxidant compound.
The mechanical properties of the films are also very important because these allow the handling, storage, and transport of the products without damage. The incorporation of nanostructures can affect the mechanical properties of the films, by different interactions with the polymer matrix (Bakshi et al. 2011), as observed for films of starch films containing gold (Au) and cadmium sulfide (CdS) nanoparticles (NPs) with significantly improved mechanical properties than pure starch films (Kaur et al. 2013); or protein films conjugated gold nanoparticles (Bakshi et al. 2011). However a more precise approach of this influence is necessary and this evaluation is of highest importance, since fragile packages may be inappropriate.
The aim of this study was to develop biodegradable cassava starch-films incorporated with bixin nanocapsules as an antioxidant for active packaging applications and to evaluate the effect of the addition of nanocapsules on the mechanical, physical, optical properties and the protector effect of active packaging against accelerated oxidation in Sunflower oil.
Materials and methods
Materials
For the preparation of the films were used cassava starch (CS) (Yoki Alimentos, São Paulo, Brazil) and glycerol (Merk) was used as a plasticizer. To obtain the bixin nanocapsules the polymer poly-ε-caprolactone (PCL) (Mw = 80,000) and sorbitan monostearate (Span 60) were obtained from Sigma (St. Louis, MO, USA). The capric/caprylic triglycerides (CCT) and polysorbate 80 (Tween 80) were purchased from Delaware (Porto Alegre, Brazil). All other chemicals and solvents were of analytical or pharmaceutical grade.
Obtaining bixin nanocapsules
The bixin nanocapsules were obtained by a technique of the interfacial deposition of preformed poly-ε-caprolactone (PCL) according to de Lobato et al. (2013). The PCL (250 mg), capric/caprylic triglyceride (CCT) (400 µL), sorbitan monostearate (Span 60) and bixin (0.4 mg) were dissolved in a mixture of acetone (60 mL) and ethanol (7.5 mL) under magnetic stirring at 40 °C. After solubilisation PCL, CCT and Span 60, bixin standard (98.7 %) was added and remained under magnetic stirring for 10 min (40 °C). This organic phase was added into an aqueous phase (130 mL) containing Tween 80 (195 mg) and remained under stirring for 10 min. The dispersion was concentrated under reduced pressure until it reached a final volume of 25 mL. The bixin concentration used in this work was 16.92 ± 0.16 g mL−1, which is the optimal concentration reported by the authors to ensure the stability of nanocapsules.
Preparation of films
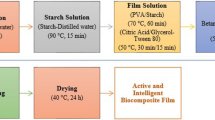
The films were produced by casting. The film-forming solution was prepared with a suspension of 4 % cassava starch (4 g 100 g−1 total film solution). The dispersion of starch was gelatinized at 82 °C for 15 min with constant stirring in a water bath (DeLeo B450). The glycerol was then added at a concentration of 0.25 g g−1 starch, thus obtaining the standard film. Different concentrations of solution containing bixin nanocapsules were added to the standard film with consideration for the total amount of starch in the film solution in grams, thus obtaining the films B2% (0.5 g/g), B5% (1.25 g/g), B8% (2 g/g) and B10% (2.5 g/g). The film-forming solution was then poured evenly onto acrylic plates in 0.24 g cm−2 batches. The films were dried in an oven with forced air circulation (DeLeo B5AFD) at 35 °C for 16 h.
Characterization of films
The starch films containing bixin nanocapsules were characterized by determining the solubility and morphological, thermal and optical properties (color, opacity), and conditioned in desiccators under 58 % RH at 25 °C for 48 h before being characterization of mechanical properties (Tensile strength and percent elongation at break). The acceleration of the antioxidant effects of the films was also tested.
Mechanical properties
The films were cut into strips (80 mm × 25 mm), and its thickness was measured using a micrometer (Precision 0.001 mm, resolution/0–25 mm), at five random positions on each strip. Tensile strength (TS) [MPa] and percent elongation at break (EB) [%] were evaluated by a tensile test performed on a texture analyzer (TA.XT2i e Stable Micro Systems, UK) with a load cell of 5 kg and using the A/TGT self-tightening roller grips fixture, according to ASTM D882-09, as described by Dick et al. (2015). Ten strips were cut, and each one was mounted between the grips of the equipment for testing with the initial distance between the grips and test speed set to 50 mm and 0.8 mm s−1, respectively.
Water vapor permeability (WVP)
The WVP was determined according to the method ASTM E-96-95, as described by Pagno et al. (2015). The samples were placed in permeation cells (inner diameter: 63 mm, height: 25 mm), filled with granular anhydrous calcium chloride and hermetically sealed. The permeation cells were placed in a glass chamber, with saturated sodium chloride solution, providing RH gradients of 0–75 % at 25 °C. The mass gain was monitored for 24 h by weighing the permeation cell on an analytical balance (AY 220, Shimadzu). The water vapor permeability of the samples was determined in triplicate using Eq. (1).
where w is the weight of water permeated through the film (g), L is the thickness of the film (mm), A is the permeation area (m2), t is the time of permeation (h), and Δp the water vapor pressure difference between the two sides of the film (KPa).
The WVP was expressed as g mm h−1 m−2 kPa−1.
Water solubility
The solubility was calculated as the percentage of dry matter of the film dissolved after immersion for 24 h in water at 25 °C. Discs of film (diameter: 2 cm) were cut, weighed, immersed in 30 mL of distilled water, and slowly and periodically agitated. The amount of dry matter in the initial and final samples was determined by drying the samples at 105 °C for 24 h (Pelissari et al. 2013). The solubility was calculated using Eq. (2):
where wi is the initial dry weight of the sample (g) and wf is the final dry weight of the sample (g).
Optical properties (color, opacity)
The opacity was determined by measuring the film absorbance at 210 and 500 nm using a UV spectrophotometer (Shimadzu UV-1800). The films were cut into rectangle pieces and directly placed in a spectrophotometer test cell. An empty test cell was used as the reference. The opacity of the films was calculated, dividing the values of absorbance (nm) by the thickness of the film (mm) (Wang et al. 2013).
The color of the films was determined with a colorimeter (Hunter Lab system, model Miniscan XE, USA) operating with D65 (day light) and using the CIELab color parameters. The parameters L* (luminosity), a* (red–green) and b* (yellow–blue) were determined. A white disk (L0: 97.5; a0: 0.13 and b0: 1.7) was used as a standard. The color difference (ΔE), compared to a white standard was calculated using the Eq. (3) (Rotta et al. 2009)
where ΔL* = L* − L0; Δa* = a* − a0, Δb* = b* − b0, where L0, a0, b0 are the values of color of standard and L*, a*, b* are the film color values.
Morphological properties
The morphological properties of the bixin nanocapsules were studied using transmission electron microscopy (TEM) and films containing bixin nanocapsules were studied using scanning electron microscopy (SEM). TEM images of were recorded using a JEOL JEM-1200 microscope, operated at an acceleration voltage of 80 kV. The nanocapsules samples were diluted in ultrapure water (1:10 v/v), where one drop of this dilution was deposited on a copper grid (Formvar-carbon support films mesh 400), and after 5 min, a drop of uranyl acetate (2 % w/v) was also deposited. SEM images were obtained using a scanning electron microscope JSM 5800 LV, JEOL (Tokyo, Japan), connected to a secondary electron detector with energy dispersive X-ray spectroscopy (EDS). The films were cut and pasted onto double sided conducting tape on an aluminum support and coated with a thin film of platinum using a Balted SCD 050 Sputter Coater (Scotia, New York, USA). The SEM analysis was performed in 5 kV.
Thermal stability
The thermal stability of the films was studied using thermogravimetric analysis. This was performed under argon flow on a Shimadzu Instrument model TGA-502, with a heating rate of 10 °C min−1 from room temperature up to 600 °C.
Testing acceleration of antioxidant effect of films
The method used to investigate the antioxidant effect has been described by Iahnke et al. (2015) where the films were cut to 5 × 10 cm2; with the aid of a sealing machine (Modelo F200, Fastvac, Brasil), bags were formed containing 7 g of sunflower oil. The liquid volume to surface area ratio was 0.07 g cm−2. After packing the oil, the bags were stored in a chamber at 35 ± 2 °C, exposed to fluorescent light at an average relative humidity of 75 %. Sunflower oil samples were collected at different times for 13 days to determine the peroxide value (PV), which quantifies the primary oxidation product using the method described for AOCS (1993). Sunflower oil samples encased in plastic packages (closed package—CP) and sunflower oil placed in a petri dish (without packaging—WP) were used as controls.
Three trials were carried out for each sample. The results were expressed as milliequivalents of oxygen per kilogram of oil (mEq kg−1). The Codex Alimentarius proposes that the PV for refined oils like sunflower oil should be up to 10 mEq kg−1 to be considered fresh (Codex Alimentarius 1999).
Statistical analysis
The results were evaluated by analysis of the variance (ANOVA) and Tukey test at a significance level of 0.05 using the software Statistica 12.0 (STATSOFT Inc., São Paul, Brazil).
Results and discussion
Thickness and mechanical properties
Table 1 shows the mechanical properties and thickness of the films containing bixin nanoparticles. The thickness of the developed films ranged from 97.2 ± 8.9 to 125.5 ± 14.3 µm.
The tensile strength (TS) is the measurement of maximum strength in the film under applied tensile stress. The analysis of the mechanical properties revealed that the film B2%, with 2 % bixin nanocapsules, exhibited a significant increase (p < 0.05) in TS (14.40 ± 1.69 MPa), when compared to the standard film (12.13 ± 0.95 MPa). However, with concentrations higher than 2 %, the films exhibited a significant decrease (p < 0.05) in TS to 1.94 ± 0.37 MPa (B10%). An inverse effect was observed in relation to elongation at break (EB) (Table 1), where the film containing the bixin nanocapsules at a concentration of 2 % (B2%), exhibited a significant decrease (p < 0.05) in EB from 6.05 ± 0.72 % (film standard) to 2.19 ± 0.349 %. As for the films with concentrations higher than 2 %, the films exhibited a significant increase (p < 0.05) in EB, with a maximum value of up to 34.34 ± 3.40 % for B10%.
A similar effect was observed earlier Norajit et al. (2010), where the incorporation of ginseng extracts in alginate films caused a significant reduction in the TS from 22.20 ± 4.00 MPa (control) to 8.05 ± 1.46 MPa and increased EB from 19.32 ± 6.81 % (control) to 27.95 ± 7.63 %. When adding α-tocopherol in chitosan-based films Martins et al. (2012) observed a significant decrease (p < 0.05) in the TS was observed (from 34.06 MPa (0 %) to 16.24 MPa (0.2 %) with the increased concentration of antioxidant; however there was a similarly observed significant reduction (p < 0.05) of EB with the addition of α-tocopherol from 53.84 % (0 %) to 23.12 % (0.2 %).
In developing methylcellulose films incorporated with α-tocopherol nanocapsules, Noronha et al. (2014) observed a significant reduction (p < 0.05) in the TS from 57.82 ± 7.17 MPa (control film) to 23.19 ± 1.44 MPa, and EB significantly increased (p < 0.05) from 17.91 ± 1.78 (control film) to 30.03 ± 1.94 %; these results suggested that the addition of hydrophobic substances modified their interactions with film-forming agents, which increased the spacing between macromolecule chains, reducing ionic and hydrogen bonding between the chains and inducing the development of structural discontinuities in the films; and according to Kaur et al. (2013) the hydrogen bonding is responsible for the superhelix arrangement among starch macromolecules. Greater hydrogen bonding generates stronger interhelical interactions with less flexibility and vice versa.
Water vapor permeability (WVP) and water solubility
The water vapor permeability (WVP) of films is shown in Table 1. It can be observed that the addition of bixin nanocapsules caused a gradual increase in WVP. However, this increase was significant (p < 0.05) in the film with higher bixin concentration, increasing from 0.207 ± 0.014 (standard film) to 0.273 ± 0.018 (B10%). This could be due to the influence of adding bixin nanocapsules over the cohesive forces of the film network, as observed in the mechanical properties results; this may have improved water vapor transport through the film matrix. A similar effect was observed by Martins et al. (2012) with a significant increase in WVP in chitosan-based films when adding α-tocopherol, from 6.02 ± 0.40 × 10−11 (g m−1 s−1 Pa−1) (0 % α-tocopherol) to 7.38 ± 0.03 × 10−11 (g m−1 s−1 Pa−1) (0.2 % α-tocopherol). Another factor suggesting that an increase in bixin nanocapsules concentration causes an increase in WVP, is the appearances of cracks observed by SEM in the films B8% and B10%.
It was observed (Table 1) that an increase in the concentration of bixin nanocapsules caused a decrease in the solubility of films when compared with the standard (40.18 ± 1.29 %); however, the films B2% (39.27 ± 2.48 %) and B5% (36.53 ± 2.16 %) showed no significant difference when compared with each other. In a study on cassava starch-based films, in varying the concentration of gelatin to improve the mechanical properties, the original solubility of the standard film decreased from 73 % with increasing concentration of gelatin (Tongdeesoontorn et al. 2012). Another study developing cassava starch-based films, by varying the concentration of glycerol, agar and Span 80, obtained values of solubility ranging from 24 to 27 % (Maran et al. 2013).
Lower solubility values were obtained for the films B8% (18.93 ± 0.71 %) and B10% (16.44 ± 1.28 %), which were significantly different when compared with the standard biofilm. The bixin is naturally insoluble in aqueous solution; the nanoencapsulation employed by these authors contributed to increasing its solubility, thus making it promising for applications in active food packaging (de Lobato et al. 2013). However, it should be noted that higher concentrations of bixin nanocapsules caused a decrease in solubility of the films. This was seen as a positive point when the objective of the film is food preservation in the presence of large amounts of water and with the release of antimicrobials or antioxidants (Ozdemir and Floros 2008).
In α-tocopherol-chitosan-based films development by Martins et al. (2012) the authors found no significant difference (p > 0.05) in films solubility with the increasing antioxidant concentration, ranging 31.6 ± 0.9 % to 29.1 ± 1.5 %. The water solubility of films based on cassava starch and carnauba wax, developed by Chiumarelli and Hubinger (2012) ranged from 27.86 to 51.09 % which, according to the authors, were suitable for applications on fresh-cut fruits. This way, the films B2% and B5% with respect to solubility have favorable properties for possible applications with fresh-cut fruits.
Optical properties (color, opacity)
The color of the films (Table 2), showed significant increase (p < 0.05) in the values of ΔE* with increasing concentration of bixin nanocapsules, 1.72 ± 0.02 for standard film up to 4131.65 ± 10.13 in B10%. Higher values of ΔE indicate films with a higher color intensity (Rotta et al. 2009).
The increase in ΔE* is mainly due to increased in b* CIELAB, corresponding to yellow, because of the presence of bixin nanocapsules. The nanocapsules developed by de Lobato et al. (2013) exhibited yellow with the following CIELAB coordinates of L* = 73.67 ± 0.34, a* = 6.01 ± 0.24 and b* = 48.60 ± 0.95. Compared to the pure bixin solution, prepared in hydrous ethanol (20 % ethanol and 80 % water), with parameters L* = 42.10 ± 0.35, a* = 13.54 ± 0.98 and b* = 25.50 ± 2.2, the bixin nanocapsules suspension presented an increase in luminosity and yellow, which was coupled with a decrease in red r, which was directly reflected in the color of the films.
In the analysis of opacity (Table 2), high absorbance values indicate less transparency and a high degree of opacity. The addition of bixin nanocapsules led to an improvement in blocking UV and visible light. In the visible region (500 nm), the absorbance increased significantly (p < 0.05) with increasing bixin nanocapsules concentration, from 12.76 ± 1.04 A mm−1 for standard film to 21.87 ± 2.27 A mm−1 for B10% film. Wang et al. (2013) developed films of chitosan using polyphenols as the antioxidants and noted an increase in opacity with increasing concentrations of the antioxidants. These researchers obtained values for the absorbance at 600 nm ranging from 0.489 ± 0.045 A mm−1 for the standard biofilm to 2.898 ± 0.158 A mm−1 for the maximum concentration of antioxidants.
Similar behavior was observed in the UV region (210 nm), with increasing absorbance values (Table 2) from 14.35 ± 0.78 A mm−1 for the standard film up to 41.55 ± 1.6 A mm−1 for the B10% film. Martins et al. (2012) reported that an increase of α-tocopherol concentration in chitosan-based films led to an improvement of the film barrier to UV and visible light. Films with 0.2 % α-tocopherol and chitosan presented the lowest level of transmittance in the UV range (especially between 250 and 300 nm). Due to the high absorbance in the UV region, the films with the addition of bixin nanocapsules could be excellent barriers to prevent UV light-induced lipid oxidation when applied in food systems.
Morphological properties
Figure 1 shows the TEM images of the nanocapsule formulations, where these presented a spherical shape and diameters lower than 200 nm. This spherical shape was also observed in nanocapsules obtained from a blend of β-carotene, α-carotene, and lutein and nanocapsules of synthetic β-carotene produced by da Silva et al. (2016), and hydrophobic phytochemicals (Campomanesia xanthocarpa O. Berg) nanoencapsulated produced by dos Santos et al. (2016) using the same method.
The scanning electron microscopy (SEM) results, showed in Figure 1A of supplementary material, show that, (1000×), the films containing bixin nanoparticles B2% (B), B5% (C) and the standard biofilm (A) presented compact and uniform structures without the presence of cracks or blistering. These results show that the addition of the nanocapsules at these concentrations did not alter the morphological properties of the starch films as far as we could observe at the magnification used. However, in the films B8% (D) and B10% (E), cracks appeared with increasing concentration of nanocapsules, which may explain the reduced strength of these films. According to Araujo-Farro et al. (2010) films with compact and uniform structures indicate good interaction between the components of the film solution.
Thermal stability
The films were subjected to thermogravimetric analysis. The obtained curves are presented in Fig. 2. Weight loss was observed from room temperature to 150 °C, which was attributed to water desorption. The estimated amount of water desorbed was nearly 10 %. The major weight loss occurred between 250 and 350 °C for all samples, which is ascribed to the organic phase desorption. Therefore, all films showed high thermal stability at least up to 270 °C. It was also possible to observe that the residual weight was higher with the gradual increase in concentration of nanocapsules, demonstrated by a higher amount of inorganic residue or carbonaceous material possibly originating from decomposition of high molecular weight polymers used in the preparation of the nanocapsules.
Acceleration of oxidative rancidity in sunflower oil
Figure 3 presents the changes in the peroxide value (PV), of sunflower oil packaged in bags made with the films containing nanoencapsulated bixin and with the control film, the bags made with the films are shown in the Figure 1B of Supplementary Material. All films retarded the lipid oxidation of sunflower oil during storage however, the samples not protected with film (WP and CP) oxidized rapidly, mainly starting from the sixth day. The sunflower oil had an initial PV of 1.66 ± 0.1, increasing up to 93.6 ± 3.2 for the control without protection (WP) and up to 48.7 ± 3.2 for closed control (CP). The oils packaged with biodegradable films increased to 20.0 ± 0.7 (standard), 8.6 ± 0.1 (B2%), 6.2 ± 0.1 (B5%), 6.0 ± 0.2 (B8%) and 12.6 ± 0.5 (B10%) by the end of storage. The Codex Alimentarius proposes that the PV for refined oils like sunflower oil should be up to 10 mEq kg−1 to be considered fresh; it could be observed that PV was significantly lower (p < 0.05) in oils packed in the films B2%, B5% and B8% than oils packed with the control packaging (WP and CP), which, even after the 13th day, presented PV below the rates indicated by the Codex Alimentarius.
When light and a sensitizing agent, such as chlorophyll, are present, the activation of oxygen in singlet oxygen can act as an important factor in the induction of oxidative deterioration. The carotenoids may control the lipid oxidation by quenching singlet oxygen or inhibiting free radicals induced by lipid peroxidation (de Oliveira Rios et al. 2007). Like other carotenoids, bixin is an efficient quencher of singlet oxygen and a scavenger of reactive species of oxygen and nitrogen. In the form of nanocapsules, the bixin, in addition to increasing stability, can be gradually released, acting as a natural antioxidant for foods (de Lobato et al. 2013). Even for the highest concentration of bixin nanocapsules in B10%, the PV was higher than those defined by Codex Alimentarius. This may be due to higher presence of cracks that were observed in the structure of this film, detected by SEM (Figure 1A of supplementary material), which increased the exchange of oxygen between the external medium and the lipid. The spontaneous reaction of atmospheric oxygen with lipids, known as auto-oxidation, is the most common process leading to oxidative deterioration. When developing films of low-density polyethylene (LDPE) added with marigold (Tagetes erecta) extract, Colín–Chávez et al. (2014) observed that bags made with these films showed a positive effect on soybean oil stability when used as packaging: the films were able to delay the oxidation of soybean oil under commercial conditions by tripling the time to reach the limit established for freshness by the Codex Alimentarius. Reis et al. (2015) produced antioxidant food packaging films by incorporating mango pulp and yerba mate extract into a cassava starch matrix. The films were then used to pack palm oil (maintained for 90 days in storage) under accelerated oxidation conditions (63 % RH and 30 °C) in order to simulate a storage experiment. Palm oil packaged in these films exhibited a decreased oxidation rate, which was attributed to the yerba mate and mango pulp, depending on their concentrations. The evolution of PV of the contents indicated that, in general, the films with high concentrations of additives improved oil stability.
Conclusion
The present study demonstrates the potential application of bixin nanocapsules in the development of biodegradable films for use in active food packaging. This packing can be applied for packaging fats and oils to prevent their oxidation. Intact, homogeneous and thermally stable films were obtained; however, it was observed that higher concentrations of the bixin nanocapsules affected the mechanical characteristics of the films, significantly decreasing the tensile strength (TS), although improving the elongation at break (EB) and leading to an improvement of the film barrier to UV and visible light. The packaging made from the films was able to delay the oxidation of sunflower oil under commercial conditions to remain below the limit established for freshness by the Codex Alimentarius (PV < 10 mEq kg−1). Thus, these films could be an advantageous alternative for food preservation and shelf life extension, helping to prevent lipid oxidation of fatty foodstuffs.
References
AOCS (1993) Peroxide value acetic acid-chloroform method–AOCS Official Method Cd 8-53. In: AOCS, Official methods and recommended practices of the American Oil Chemists’ Society, 5th edn. AOCS Press, Champaign, Illinois
Araujo-Farro PC, Podadera G, Sobral PJA, Menegalli FC (2010) Development of films based on quinoa (Chenopodium quinoa, Willdenow) starch. Carbohydr Polym 81(4):839–848
Bakshi MS, Kaur H, Khullar P, Banipal TS, Kaur G, Singh N (2011) Protein films of bovine serum albumen conjugated gold nanoparticles: a synthetic route from bioconjugated nanoparticles to biodegradable protein films. J Phys Chem C 115(7):2982–2992
Burt S (2004) Essential oils: their antibacterial properties and potential applications in foods—a review. Int J Food Microbiol 94(3):223–253
Chen C-H, Lai L-S (2008) Mechanical and water vapor barrier properties of tapioca starch/decolorized hsian-tsao leaf gum films in the presence of plasticizer. Food Hydrocoll 22(8):1584–1595
Chiumarelli M, Hubinger MD (2012) Stability, solubility, mechanical and barrier properties of cassava starch—Carnauba wax edible coatings to preserve fresh-cut apples. Food Hydrocoll 28(1):59–67
Codex-Alimentarius (FAO, WHO) (1999) Codex standard for named vegetable oils. Codex-Stan 210, Rome
Colín-Chávez C, Vicente-Ramírez E, Soto-Valdez H, Peralta E, Auras R (2014) The release of carotenoids from a light-protected antioxidant active packaging designed to improve the stability of soybean oil. Food Bioprocess Technol 7(12):3504–3515
da Silva MM, Nora L, Cantillano RFF, Paese K, Guterres SS, Pohlmann AR, Costa TMH, de Oliverira Rios A (2016) The production, characterization, and the stability of carotenoids loaded in lipid-core nanocapsules. Food Bioprocess Technol 9(7):1148–1158
de Lobato KBS, Paese K, Forgearini JC, Guterres SS, Jablonski A, de Oliveira Rios A (2013) Characterisation and stability evaluation of bixin nanocapsules. Food Chem 141(4):3906–3912
de Oliveira Rios A, Mercadante AZ, Borsarelli CD (2007) Triplet state energy of the carotenoid bixin determined by photoacoustic calorimetry. Dyes Pigment 74(3):561–565
de Oliveira Rios A, Antunes LMG, de LP Bianchi M (2009) Bixin and lycopene modulation of free radical generation induced by cisplatin–DNA interaction. Food Chem 113(4):1113–1118
Dick M, Pagno CH, Costa TMH, Gomaa A, Subirade M, de Oliverira Rios A, Flôres SH (2015) Edible films based on chia flour: development and characterization. J Appl Polym Sci 42455:1–9
dos Santos PP, Paese K, Guterres SS, Pohlmann AR, Jablonski A, Flôres SH, de Oliveira Rios A (2016) Stability study of lycopene-loaded lipid-core nanocapsules under temperature and photosensitization. LWT Food Sci Technol 71:190–195
Gomez-Estaca J, Lopez-de-Dicastillo C, Hernandez-Munoz P, Catala R, Gavara R (2014) Advances in antioxidant active food packaging. Trends Food Sci Technol 35(1):42–51
Iahnke AOES, Costa TMH, de Oliveira Rios A, Flôres SH (2015) Residues of minimally processed carrot and gelatin capsules: potential materials for packaging films. Ind Crops Prod 76:1071–1078
Kaur H, Banipal TS, Thakur S, Bakshi MS, Kaur G, Singh N (2013) Novel biodegradable films with extraordinary tensile strength and flexibility provided by nanoparticles. ACS Sustain Chem Eng 1(1):127–136
Li J-H, Miao J, Wu J-L, Chen S-F, Zhang Q-Q (2014) Preparation and characterization of active gelatin-based films incorporated with natural antioxidants. Food Hydrocoll 37:166–173
López-de-Dicastillo C, Alonso JM, Catalá R, Gavara R, Hernández-Muñoz P (2010) Improving the antioxidant protection of packaged food by incorporating natural flavonoids into ethylene-vinyl alcohol copolymer (EVOH) films. J Agric Food Chem 58(20):10958–10964
López-de-Dicastillo C, Gómez-Estaca J, Catalá R, Gavara R, Hernández-Muñoz P (2012) Active antioxidant packaging films: Development and effect on lipid stability of brined sardines. Food Chem 131(4):1376–1384
Maran JP, Sivakumar V, Sridhar R, Thirugnanasambandham K (2013) Development of model for barrier and optical properties of tapioca starch based edible films. Carbohydr Polym 92(2):1335–1347
Martins JT, Cerqueira MA, Vicente AA (2012) Influence of α-tocopherol on physicochemical properties of chitosan-based films. Food Hydrocoll 27(1):220–227
Norajit K, Kim KM, Ryu GH (2010) Comparative studies on the characterization and antioxidant properties of biodegradable alginate films containing ginseng extract. J Food Eng 98(3):377–384
Noronha CM, de Carvalho SM, Lino RC, Barreto PLM (2014) Characterization of antioxidant methylcellulose film incorporated with α-tocopherol nanocapsules. Food Chem 159:529–535
Ozdemir M, Floros JD (2008) Optimization of edible whey protein films containing preservatives for mechanical and optical properties. J Food Eng 84(1):116–123
Pagno CH, Costa TMH, de Menezes EW, Benvenutti EV, Hertz PF, Matte CR, Tosati JV, Monteiro AR, de Oliveira Rios A, Flôres SH (2015) Development of active biofilms of quinoa (Chenopodium quinoa W.) starch containing gold nanoparticles and evaluation of antimicrobial activity. Food Chem 173:755–762
Park H-Y, Kim S-J, Kim KM, You Y-S, Kim SY, Han J (2012) Development of antioxidant packaging material by applying corn-zein to LLDPE film in combination with phenolic compounds. J Food Sci 77(10):E273–E279
Pelissari FM, Andrade-Mahecha MM, do Amaral Sobral PJ, Menegalli FC (2013) Comparative study on the properties of flour and starch films of plantain bananas (Musa paradisiaca). Food Hydrocoll 30(2):681–690
Reis LCB, de Souza CO, da Silva JBA, Martins AC, Nunes IL, Druzian JI (2015) Active biocomposites of cassava starch: The effect of yerba mate extract and mango pulp as antioxidant additives on the properties and the stability of a packaged product. Food Bioprod Process 94:382–391
Rotta J, Ozório RÁ, Kehrwald AM, de Oliveira Barra GM, de Melo Castanho Amboni RD, Barreto PLM (2009) Parameters of color, transparency, water solubility, wettability and surface free energy of chitosan/hydroxypropylmethylcellulose (HPMC) films plasticized with sorbitol. Mater Sci Eng, C 29(2):619–623
Salarbashi D, Tajik S, Shojaee-Aliabadi S, Ghasemlou M, Moayyed H, Khaksar R, Noghabi MS (2014) Development of new active packaging film made from a soluble soybean polysaccharide incorporated Zataria multiflora Boiss and Mentha pulegium essential oils. Food Chem 146:614–622
Silva-Weiss A, Ihl M, Sobral PJA, Gomez-Guillen MC, Bifani V (2013) Natural additives in bioactive edible films and coatings: functionality and applications in foods. Food Eng Rev 5(4):200–216
Souza AC, Benze R, Ferrão ES, Ditchfield C, Coelho ACV, Tadini CC (2012) Cassava starch biodegradable films: influence of glycerol and clay nanoparticles content on tensile and barrier properties and glass transition temperature. LWT Food Sci Technol 46(1):110–117
Souza AC, Goto GEO, Mainardi JA, Coelho ACV, Tadini CC (2013) Cassava starch composite films incorporated with cinnamon essential oil: antimicrobial activity, microstructure, mechanical and barrier properties. LWT Food Sci Technol 54(2):346–352
Tongdeesoontorn W, Mauer LJ, Wongruong S, Sriburi P, Rachtanapun P (2012) Mechanical and physical properties of cassava starch-gelatin composite films. Int J Polym Mater 61(10):778–792
Wang L, Dong Y, Men H, Tong J, Zhou J (2013) Preparation and characterization of active films based on chitosan incorporated tea polyphenols. Food Hydrocoll 32(1):35–41
Acknowledgments
The authors are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Apoio à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) for financial support, and Eletronic Microscope Center (CME) of Federal University of Rio Grande do Sul UFRGS for technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pagno, C.H., de Farias, Y.B., Costa, T.M.H. et al. Synthesis of biodegradable films with antioxidant properties based on cassava starch containing bixin nanocapsules. J Food Sci Technol 53, 3197–3205 (2016). https://doi.org/10.1007/s13197-016-2294-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-016-2294-9