Abstract
Effects of nano-kaolin incorporation into semolina films on the physical, mechanical, thermal, barrier and antimicrobial properties of the resulting bio-nanocomposite films were investigated. The properties included crystal structure (by X-ray diffraction), mechanical resistance, color, Fourier transform infrared spectra, decomposition temperature, water-vapor permeability (WVP), oxygen permeability (OP), and antimicrobial activity against Staphylococcus aureus and Escherichia coli. Kaolin was incorporated into biofilms at various amounts (1, 2, 3, 4, and 5 %, w/w total solid). All films were plasticized with 50 % (w/w total solid) combination of sorbitol/glycerol at 3:1 ratio. The incorporation of nanokaolin into semolina films decreased OP and WVP. The moisture content and water solubility of the films were found to decrease by nanokaolin reinforcement, and mechanical properties of films were improved by increasing nanokaolin concentration. Tensile strength and Young’s modulus increased from 3.41 to 5.44 MPa and from 63.12 to 136.18, respectively, and elongation-at-break decreased. The films did not exhibit UV absorption. In conclusion, nanokaolin incorporation enhanced the barrier and mechanical properties of semolina films, indicating the potential application of these bio-nanocomposites in food-product packaging.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pollution caused by packaging materials that are produced from petroleum derivatives, problems associated with different methods of decontamination, such as burying, burning, and recycling (Tharanathan 2003). and hazards related to petroleum-derived raw materials used for packaging, have driven numerous food researchers to discover and produce natural-based biopolymers that can be used in food packaging (Ghanbarzadeh and Almasi 2011; Sorrentino et al. 2007). The above mentioned challenges and concerns are irrelevant in the case of biopolymers, because biodegradation can occur in nature. Therefore, the development of biodegradable materials is an essential need in different societies, and research works on biodegradable materials with controlled properties present a compelling topic for any food specialist (Ghasemlou et al. 2011; Gonzalez-Gutierrez et al. 2010).
Among biopolymer materials, proteins have received considerable attention for its potential use as a biodegradable film, because proteins have been shown to form transparent films that can act as excellent oxygen barriers and provide certain mechanical properties (Sothornvit et al. 2009). Proteins films are main renewable products that are economical, readily available, biodegradable, and highly safe. These proteins possess special nutritional and/or health-protective functions, as well as good film-forming properties (Zhang et al. 2005). In addition, the unique capacities of proteins to form networks, and induce plasticity and elasticity are beneficial in preparing biopolymer-based packaging materials (Voon et al. 2012).
Although most biodegradable polymers possess excellent properties that are comparable with those of petroleum-based plastics, certain poor properties such as brittleness and high permeability limit their applications. For these reasons, research works on biopolymers have recently focused on modifying and improving their mechanical and vapor barrier properties. The introduction of nanotechnology is one of the most important progresses that are recently accomplished in this field. The addition of small amounts of nanoparticles to biopolymers improves their mechanical, thermal, and barrier properties, which can expand the use of the polymers to various applications, especially food packaging (Dean and Yu 2005; Rhim et al. 2005; Hedenqvist et al. 2006).
Nano fillers possess high surface energy and large specific surface area, engendering strong interfacial interactions among polymer bonds and the nano filler and causing a significant increment in polymer properties (Kovacevic et al. 2008). Nanoclay has been widely used as functional fillers in protein films because of the presence of the clay layer, which decreased WVP and increased TS (Sothornvit et al. 2009). Kaolin nanoclay, a natural mineral, is a hydrated aluminosilicate that is used in numerous industries because of its low cost, abundance, versatile uses, ready availability, and environmental friendliness (Schwartz and Goodman 1982; Ma and Bruckard 2010). Furthermore, improvements to thermal stability and mechanical properties were observed in thermoplastic amylose/kaolin composites (Huang et al. 2006).
Different types of proteins are used as biodegradable materials in packaging (Khwaldia et al. 2010). Among the proteins, wheat is considered to be a valuable candidate for packaging applications because of its low price, biodegradability, renewability, and good film forming and adhesive/cohesive properties (Türe et al. 2013). Among various flours, semolina flour originates from a type of wheat that contains the highest gluten content (Quaglia 1988) which improve the nutritional properties of edible films. Semolina is an extra-hard, translucent, light- colored grain that exhibits antioxidant activities (Onyeneho and Hettiarachchy 1992; Zielinski and Kozlowska 2000). In addition, semolina extracts have been shown to suppress radical-induced liposome lipid peroxidation, as well as radical cation scavenging activity (Zielinski and Kozlowska 2000; Onyeneho and Hettiarachchy 1992). Considering the excellent properties of semolina for use as edible film and the potential ability of nanokaolin in reinforcing its mechanical, physicochemical, and barrier properties, nanokaolin-reinforced semolina has not been investigated.
We hypothesized that the application of nanokaolin into semolina film will lead to improved mechanical, physicochemical, thermal and barrier properties of film. The proposed film can be applied in the food packaging industry, especially cheeses, because of its good antioxidant property. In this study, nanokaolin was used as fillers to prepare semolina film bio-nanocomposites. We characterized the films based on their physicochemical, mechanical, thermal, barrier, morphological and antimicrobial properties.
Materials and methods
Materials
Semolina flour (14.2 % protein, 18.5 % gluten) was sourced from a local market in Tehran, Iran and stored in a dry and cool place until use. Food-grade glycerol and food-grade liquid sorbitol from SIM Company Sdn. Bhd. (Penang, Malaysia) and Liangtraco Sdn. Bhd. (Penang, Malaysia) were used as received. Magnesium nitrate for humidity control was obtained from Sigma–Aldrich (Kuala Lumpur, Malaysia), and nanokaolin was obtained from Sigma Chemical Co (St. Louis, MO, USA).
Preparation of nanobiocomposite films
Four grams of semolina flour was dispersed in 80 ml of distilled water (based on water or water/ethanol) at room temperature by magnetic stirring, and the pH of the dispersion was adjusted to 10 using 1 M, NaOH. Different concentrations of kaolin powder (1, 2, 3, 4, and 5 %; w/w total solid) plus 2 g of a sorbitol and glycerol (3:1) mixture (previously reported by Sadegh-Hassani and Nafchi 2014) were likewise dispersed in 20 ml of distilled water for 30 min, followed by sonication in an ultrasonic bath (Marconi model, Unique USC 45 kHz, Piracicaba, Brazil). Dispersions of semolina flour and kaolin–plasticizer were then mixed by stirring for 1 h at a constant temperature of 90 °C to allow gelatinization. Upon gelatinization completion, the solution was cooled to 40 to 45 °C. A portion (90 g) of each suspension was cast on Perspex plates and fitted with rims to yield a (16 × 16) cm2 film-forming area. Then, films were dried in an oven at 40 °C for 24 h, peeled off, and kept at 25 °C and 58 % relative humidity (RH) until testing. Control films were similarly prepared, but without the addition of nanoparticles.
Characterization studies
Determination of film thickness
Film thickness was determined using a micrometer (Model No. 2046-08; Mitutoyo Tokyo, Japan). Thickness was measured at five random spots on the film.
Water solubility and moisture content
To determine of film solubility in water, the procedure proposed by (Jouki et al. 2013) was used. First, pieces of film (2 × 3 cm) were cut and kept in a desiccator with P2O5 (0 % RH) for 48 h to measure initial dry matter. Then, the dried nanocomposite films were weighed and immersed in 80 mL deionized water for 1 h at room temperature. The remaining sample pieces were separated using a Whatman No. 1 filter paper, followed by oven drying at 60 °C to a constant final dry weight. Values of water solubility (%) were measured in triplicates and calculated using the following equation:
For moisture content measurement, each film sample was cut into 3 × 3 cm pieces and dried at 105 °C for 1 d using a hot air oven. Before and after drying, the weight loss of each film sample was determined as water content and expressed as percent moisture content based on the initial weight of film.
Fourier-transform infrared spectroscopy (FTIR) analysis
The FTIR spectra of the films were measured by attenuated total reflection (ATR) method using a Smart iTR (Thermo Scientific, Madison, USA). The thin films were applied directly onto the ZnSe ATR cell. For each spectrum, 64 consecutive scans at 4 cm−1 resolutions were averaged to reduce spectral noise.
UV-vis absorbance spectroscopy
Absorbance spectra of semolina films reinforced with nanokaolin as a UV-absorbing compound were obtained using a UV-visible spectrophotometer model, UV-1650PC (Shimadzu, Japan), in absorbance mode. A piece of bionanocomposite film was placed in a cuvette, with air as the blank, and recorded at different wavelengths in the range of 200 to 800 nm (Park et al. 2004).
Color measurement
Film color were measured with a colorimeter (Minolta CM-3500D; Minolta Co. Ltd., Osa Osaka, Japan). The instrument was calibrated with transmittance zero calibration plate, CM-A100, and air was used to measure full transmittance prior to use. A large-size aperture was used. Measurements were conducted in the CIELAB scale, in which each measurement is indicated as (L*, a *, b *). The “L” value indicates the lightness, whereas “a” and “b” values correspond to psychometric chromaticity. A positive value denotes red direction and a negative value indicates green. A positive b value corresponds to yellow, whereas a negative value indicates blue. All color measurements were carried out for five times for each type of nanocomposite film.
Water vapor permeability
Water vapor permeability (WVP) tests of the films were carried out following of ASTM standard E96-05 (ASTM 2005).
Oxygen permeability
Oxygen permeability (OP) of the nanocomposite film was measured using a Mocon Oxtran 2/21 (Minneapolis, USA) Automatic Gas Permeability Tester at a continual condition (25 °C temperature, 50 % RH, and P = 1 atm). Films were fixed on an aluminum mask with an open testing area of 50 cm2. The measurements were obtained using the ASTM standard method (ASTM 2005). OP values were calculated on five different tests.
Mechanical properties
Tensile strength (TS), elongation-at-break (EB), and Young’s modulus (YM) of the films were measured following ASTM D882-10 method in standard conditions using a texture analyzer machine (TA.XT2, Stable Micro System, Surrey, UK). The films were cut to 10 cm long and 2 cm wide rectangular shapes, conditioned for a minimum of 2 d in 25 °C and 58 % RH, and then measured (ASTM 2010).
Decomposition temperature
Samples (~15 mg) were scanned applied a Thermo-gravimetric analysis (TGA-1, Perkin Elmer, Massachusetts, USA) from 40 to 800 Ċ at a rate of 10 °C/min in a nitrogen environment. (Nuthong et al. 2009).
Antimicrobial assay
Antimicrobial activity of the films was tested using agar diffusion method according to Maizura et al. (2007). Antimicrobial effects of the films were determined by the inhibition zone against Staphylococcus aureus and Escherichia coli on solid media.
Film morphology
The structural properties of films were observed using a scanning electron microscope (JSM–6460 LV). Crystalline phases were examined using a high-resolution X-ray diffractometer (PANalytical X’Pert PRO MED PW3040) with Cu Kα radiation (λ = 1.5406 Å) and a Phillips CM12 transmission electron microscope, and EDX measurement was conducted under 15 kV incident electron energy.
Statistical analysis
ANOVA and Tukey’s post hoc tests were used to compare the means of physical, mechanical and barrier properties of nanocomposite films at 5 % significance level. Statistical analysis was conducted using SPSS version 22.0.
Results and discussion
Thickness
Thickness of semolina films incorporated with nanokaolin at different amounts is shown in Table 1. Film thickness increased when kaolin levels increased. Films containing 5 % nanokaolin exhibited higher thickness in comparison with those without nanokaolin. This result is possibly due to the combined effect of kaolin, which inserts between the protein network, as well as the increased solid content in the resulting films. This behavior was also observed by Sothornvit et al. (2009) in whey protein isolate nanocomposite films.
Water susceptibility
The characteristic of water susceptibility of protein films, which is due to the hydrophilic nature of those macromolecules, could be considered as one of the most disadvantageous properties with respect to certain applications, and is a feature that most discriminates these biopolymers from the more frequently used synthetic polymers. Thus, an increased resistance to water in biopolymers represent one of the most sought-after modifications for their more widespread practical use (Echeverría et al. 2014). Table 1 shows the moisture content and solubility in water of films composed of semolina protein alone and of nanocomposites. Increasing amounts of kaolin in the film formulation was accompanied by a progressive decrease in the percent moisture content. This finding is likely due to the interaction among plasticizer, biopolymer matrix, and nanokaolin, which reduced the availability of the hydroxyl group to interact with water, consequently resulting to a less hygroscopic matrix (Müller et al. 2011). The solubility in water of these films was progressively reduced with increasing kaolin content in the formulation. The same effect has been reported by other authors (Rhim 2011; Almasi et al. 2010) with nanoclay added to films composed of agar, chitosan, and cotton–carboxymethylcellulose. This effect is mainly due to the formation of strong interaction through hydrogen bonds between the hydroxyl groups of the biopolymer matrix and nanoclays with high surface area, thereby improving the cohesiveness of the biopolymer matrix and decreasing the water sensitivity (Casariego et al. 2009).
UV-visible (UV-vis) and FTIR spectra
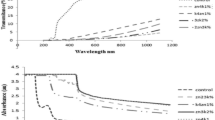
The optical properties of the semolina/kaolin nanocomposite films were determined by measuring the absorption spectra of films using a UV-vis spectrophotometer, and the results are shown in Fig. 1 The control film did not show any absorption peak, but semolina/kaolin nanocomposite films in the UV range (290 to 400 nm) indicated approximate good absorbance. This result was in agreement with those of Kanmani and Rhim (2014).
The image in Fig. 2 shows the FTIR spectra of the semolina film and semolina-based nanocomposite films. The spectrum of the semolina nanocomposite displayed various characteristic peaks in the range from 3287 to 753 cm−1. The broad absorption peak at 3287 cm−1 indicates the stretching vibration of hydroxyl groups (O-H) (Pandey et al. 2012). The spectrum of semolina film displayed relevant peaks that originate from C = O stretching at 1648 cm−1 (amide I), N-H stretching at 1549 cm−1 (amide II), and C-N and N-H stretching at 1246 cm−1 (amide III) (Gennadios 2002; Yin et al. 2005). This result indicates strong interaction between the nanoclay and polymer matrix through H-bonding (Deka and Karak 2009). Peaks at 900 and 1150 cm−1 were assigned to pyranose rings and amino groups, respectively (Lima et al. 2006). No new functional group is seen after nanokaolin application, showing that only physical interaction was involved between the kaolin and the film matrix. Similar results were seen for gelatin and chitosan films (Kanmani and Rhim 2014; Pereda et al. 2011).
Color measurement
The surface color of food packaging films is an important parameter because of its influence on the general appearance and consumer acceptance (Tharanathan 2003) The parameters of color characteristics for control and nanokaolin-incorporated protein films are summarized in Table 2. With increasing concentrations of kaolin, luminosity was seen to decrease (the parameter L declines), and parameters a and b became progressively increased by higher nanoparticles content, but at an insignificant level. The same effect had been reported by Kanmani and Rhim (2014) and Echeverría et al. (2014).
WVP
Given that one of the functions of a film for use in packaging is to minimize the transfer of moisture from the surrounding atmosphere to food or between two components of a heterogeneous product with differing moisture levels, the evaluation of a film’s permeability to water vapor is critical, and that parameter is always maintained at a lowest possible value. The WVP of the control semolina film was 8.61 × 10−7, decreasing significantly with the increase in the kaolin concentration to 4.58 × 10−7 with the incorporation of 5 % kaolin. Such phenomena of reduction in WVP with the incorporation of nanoclays have frequently been observed with different biopolymers, such as chitosan (Casariego et al. 2009; Rhim et al. 2006). (CMC)/starch (ST) (El Miri et al. 2015). whey protein (Sothornvit et al. 2010).sesame seed meal protein (Lee et al. 2014). and corn zein (Ozcalik and Tihminlioglu 2013). The WVP of polymer/clay nanocomposite films is generally known to exponentially decrease with the increase in clay content or increase in aspect ratio of the clay (Yano et al. 1997). The decrease in WVP of polymer/clay composite films is mainly attributed to the tortuous path for water vapor diffusion, which is due to the impermeable clay layers distributed in the polymer matrix with increasing effective diffusion path length (Cussler et al. 1998; Sun et al. 2008; Yano et al. 1997).
Permeability to oxygen
OP is the second most commonly studied transport property of edible polymer films after WVP. Films made from plant-based protein polymers present excellent OP because of the presence of polar interactions in their structure (Padua and Wang 2002; Tharanathan 2003; Hong and Krochta 2003, 2006; Rhim et al. 2006).
Among various protein flours, semolina flour possesses the highest gluten content. The cohesiveness and flexibility of gluten imparts integrity to facilitate film formation. In addition, gluten is composed of the following two main groups of proteins: gliadins, which consist of low-molecular-weight proteins, and glutenins, which contain high-molecular-weight proteins. In consequence, higher-purity gluten results in stronger films, and high-gluten films are effective oxygen barriers (Wittaya 2012).
The effect of kaolin content on oxygen barrier properties is shown in Table 2. As kaolin content increased, OP decreased significantly. The incorporation of nanoparticles into the matrix could likely introduce a tortuous pathway for oxygen molecules to pass through, and the reduction in permeability coefficients means that gases should travel this longer diffusive path (Zeppa et al. 2009; Nafchi et al. 2013). Also reported similar OP results with the incorporation of nanoparticles (Bae et al. 2009; Türe et al. 2013).
Mechanical properties
Tensile strength (TS), elongation-at-break (EB), and Young’s modulus (YM) of the bio-nanocomposite films are shown in Table 1. Obviously from different studies on biopolymer films reinforced by nanoparticles (Zolfi et al. 2014; Echeverría et al. 2014; Kanmani and Rhim 2014) significant increment was seen in TS and YM and a significant reduction was seen in EB by addition of nanokaolin.
Increment in TS is a vital criteria in food packaging applications, because high TS can allow packaging films to with stand ordinary stresses encountered during food handling, delivery, and transportation. The primary reason is related to the interfacial interaction between nanokaolin and the biopolymer matrix. The flexibility of films is related to interactions of macromolecules and can be decreased with the addition of plasticizers. Meanwhile, a decreasing trend was observed for elongation-at-break. Reduction in EB can be advantageous in food packaging, as this property is directly related to the biodegradability of the films (Voon et al. 2012). The mechanical properties of nanocomposite films have been reported to be strongly dependent on the interfacial interaction between the matrix and fillers (ASTM 2009) Wu and others demonstrated that, when used as a filling agent, nanoparticles enhance the wear performance and TS of starch films. Nanoparticles tend to bond with hydroxyl groups and other possible hydrogen or Van der Walls bonds of biopolymer macromolecules, thereby strengthening molecular forces between nanoparticles and starch (Wu et al. 2008).
Thermo-gravimetric analysis (TGA)
Thermal stability of control biopolymer with their nanocomposites films were measured by TGA and that result were shown in Figs. 3 and 4. The TGA thermograms curves indicate little difference between semolina films and reinforced with nano kaolin in decomposition temperature. As can be seen in Fig. 3 although the residue was around 18 and 24 % for control film and maximum percentage of nano kaolin (5 %), respectively, the differences in decomposition temperatures between the films were negligible. This can be probably relevant to the negligible level of nanoparticles added. In contrast, recent work by Zou and Yoshida exhibited that the decomposition temperature of polymeric films was increased by addition of nanoparticles and resulted in differences in the residue after decomposition in inert media (nitrogen) (Zou and Yoshida 2010).
Antimicrobial activity
The antimicrobial activity of nanoclay composite films depended on type of nanoclay and microorganisms tested (Sothornvit et al. 2009). Evidently, control semolina films showed no antimicrobial activity against both Gram-negative (Escherichia coli) and positive bacteria (S. aureus). Moreover, the incorporation of nanokaolin in semolina films did not exhibit any antimicrobial effect against Gram-negative and Gram-positive bacteria (Fig. 4). This result was in agreement with those of Rhim et al. (2006). which showed that WPI/Cloisite Na+ and WPI/Cloisite 20A films did not exhibit any antimicrobial activity. On the contrary, Rhim et al. (2006) found that a chitosan/Cloisite 30B nanocomposite film indicated bactericidal effects against the Gram-positive bacteria, S. aureus and L. monocytogenes, as well as bacteriostatic effects against Gram-negative bacteria, Salmonella typhimurium and E. coli. The group demonstrated that the antimicrobial activity of nanocomposite films may be attributed to the quaternary ammonium group in the silicate layer of Cloisite 30B, which disrupts bacterial cell membranes and causes cell lysis.
Film morphology
SEM micrographs of the semolina film surface in the absence and presence of 5 % nanokaolin are illustrated in Fig. 5. The control film showed a homogeneous surface, whereas a slightly rough surface was noticeable in films incorporated with kaolin. The increased roughness could be due to the distribution of nanokaolin droplets throughout the film matrix. Moreover, the roughness may also be associated with the coexistence of protruding film structures, as demonstrated by the increased thickness of films.
The images in Fig. 6(a) and (b) show the XRD curves of the semolina film and semolina incorporated with kaolin, respectively. The image in Fig. 6(b) shows that the semolina/nanokaolin film exhibits one broad reflection, indicating the nano size of kaolin, which matches TEM images. This may be additionally connected with the existing together of projected film structure as demonstrated by the expanded thickness of resulting films.
The TEM images of kaolin nanoparticles are shown in Fig. 7(a). We can see that the morphology of Kaolin is hexagonal, and its size is narrowly distributed at between 100 and 200 nm diameter. Meanwhile, Fig. 7(b) shows the EDX of the nanokaolin added in the biopolymer.
Conclusion
We incorporated nanokaolin into semolina protein to manufacture bio-nanocomposites. The incorporation of nanoparticles enhanced the mechanical properties of films made from semolina. The permeabilities to water vapor and oxygen, moisture content, and water solubility of nanocomposite films significantly decreased. These findings showed that under strict regulation, bio-nanocomposites based on nanokaolin may present potential applications in food-packaging industries.
References
Almasi H, Ghanbarzadeh B, Entezami AA (2010) Physicochemical properties of starch–CMC–nanoclay biodegradable films. Int J Biol Macromol 46(1):1–5
ASTM (2005) Annual book of ASTM standards. ASTM, Philadelphia
ASTM (2009) Standard test method for seal strength of flexible barrier materials F88/F88M 09, in: annual book of ASTM standards. Philadelphia, PA
ASTM (2010) Standard test method for tensile properties of thin plastic sheeting D882-10. In Annual book of ASTM standards. American Society for Testing and Materials, Philadelphia
Bae HJ, Park HJ, Hong SI, Byun YJ, Darby DO, Kimmel RM, Whiteside WS (2009) Effect of clay content, homogenization RPM, pH, and ultrasonication on mechanical and barrier properties of fish gelatin/montmorillonite nanocomposite films. LWT Food Sci Technol 42(6):1179–1186
Casariego A, Souza BWS, Cerqueira MA, Teixeira JA, Cruz L, Díaz R, Vicente AA (2009) Chitosan/clay films’ properties as affected by biopolymer and clay micro/nanoparticles’ concentrations. Food Hydrocoll 23(7):1895–1902
Cussler EL, Highes SE, Ward WJ, Aris R (1998) Barrier membranes. J Membr Sci 38:161–174
Dean K, Yu L (2005) Biodegradable protein-nanocomposites. In: Smith R (ed) Biodegradable polymers for industrial application. CRC Press, Boca Raton, pp. 289–309
Deka H, Karak N (2009) Vegetable oil-based hyperbranched thermosetting polyurethane/clay nanocomposites. Nanoscale Res Lett 4:758–765
Echeverría I, Eisenberg P, Mauri AN (2014) Nanocomposites films based on soy proteins and montmorillonite processed by casting. J Membr Sci 449:15–26
El Miri N, Abdelouahdi K, Barakat A, Zahouily M, Fihri A, Solhy A, El Achaby M (2015) Bio-nanocomposite films reinforced with cellulose nanocrystals: rheology of film-forming solutions, transparency, water vapor barrier and tensile properties of films. Carbohydr Polym 129:156–167
Gennadios A (2002) Soft gelatine capsules. In A Gennadios (Ed.), Protein-based films and coatings. 1era (pp. 1–41). CRC Press: Boca Raton
Ghanbarzadeh B, Almasi H (2011) Physical properties of edible emulsified films based on carboxymethyl cellulose and oleic acid. Int J Biol Macromol 48:44–49
Ghasemlou M, Khodaiyan F, Oromiehie A (2011) Physical, mechanical, barrier, and thermal properties of polyol-plasticized biodegradable edible film made from kefiran. Carbohydr Polym 84:477–483
Gonzalez-Gutierrez J, Partal P, Garcia-Morales M, Gallegos C (2010) Development of highly-transparent protein/starch-based bioplastics. Bioresour Technol 101:2007–2013
Hedenqvist MS, Backman A, Gallstedt M, Boyd RH, Gedde UW (2006) Morphology and diffusion properties of whey/montmorillonite nanocomposites. Compos Sci Technol 66:2350–2359
Hong SI, Krochta JM (2003) Oxygen barrier properties of whey protein isolate coatings on polypropylene films. J Food Sci 68(1):224–228
Hong SI, Krochta JM (2006) Oxygen barrier performance of whey-protein-coated plastic films as affected by temperature, relative humidity, base film and protein type. J Food Eng 77(3):739–745
Huang M, Wang H, Yu J (2006) Studies of biodegradable thermoplastic amylose/kaolin composites: fabrication, characterization, and properties. Polym Compos 27(3):309–314
Jouki M, Khazaei N, Ghasemlou M, HadiNezhad M (2013) Effect of glycerol concentration on edible film production from cress seed carbohydrate gum. Carbohydr Polym 96(1):39–46
Kanmani P, Rhim JW (2014) Physical, mechanical and antimicrobial properties of gelatin based active nanocomposite films containing AgNPs and nanoclay. Food Hydrocoll 35:644–652
Khwaldia K, Arab-Tehrany E, Desobry S (2010) Biopolymer coatings on paper packaging materials. Compr Rev Food Sci Food Saf 9(1):82–91
Kovacevic E, Stefanovic I, Berndt J, Godde C, Winter J, Boufendi L (2008) The nanoparticle formation in hydrocarbon plasmas. Publications de l’Observatoire Astronomique de Beograd 84:151–152
Lee JH, Song NB, Jo WS, Song KB (2014) Effects of nano-clay type and content on the physical properties of sesame seed meal protein composite films. Int J Food Sci Technol 49(8):1869–1875
Lima CGA, de Oliveira RS, Figueiro SD, Wehmann CF, Goes JC, Sombra ASB (2006) DC conductivity and dielectric permittivity of collagenechitosan films. Mater Chem Phys 99:284–288
Ma X, Bruckard WJ (2010) The effect of pH and ionic strength on starch–kaolinite interactions. Int J Miner Process 94:111–114
Maizura M, Fazilah A, Norziah MH, Karim AA (2007) Antibacterial activity and mechanical properties of partially hydrolyzed sago starchealginate edible film containing lemongrass oil. J Food Sci 72(6):C324–C330
Müller CM, Laurindo JB, Yamashita F (2011) Effect of nanoclay incorporation method on mechanical and water vapor barrier properties of starch-based films. Ind Crop Prod 33(3):605–610
Nafchi AM, Moradpour M, Saeidi M, Alias AK (2013) Thermoplastic starches: properties, challenges, and prospects. Starch-Stärke 65(1–2):61–72
Nuthong P, Benjakul S, Prodpran T (2009) Characterization of porcine plasma protein-based films as affected by pretreatment and cross-linking agents. Int J Biol Macromol 44(2):143–148
Onyeneho SN, & Hettiarachchy, NS (1992) Antioxidant activity of durum wheat bran. J Agric Food Chem 40(9):1496–1500
Ozcalik O, Tihminlioglu F (2013) Barrier properties of corn zein nanocomposite coated polypropylene films for food packaging applications. J Food Eng 114(4):505–513
Padua GW, Wang Q (2002) Formation and properties of corn zein films and coatings. In Gennadios A (Ed.). Protein-based films and coatings. CRC Press: Boca Raton, pp. 43–67.
Pandey S, Goswami GK, Nanda KK (2012) Green synthesis of biopolymere silver nanoparticle nanocomposite: an optical sensor for ammonia detection. Int J Biol Macromol 51:583–589
Park P, Je J, Kim S (2004) Free radical scavenging activities of differently deacetylated chitosans using an ESR spectrometer. Carbohydr Polym 55:17–22
Pereda M, Ponce AG, Marcovich NE, Ruseckaite RA, Martucci JF (2011) Chitosan-gelatin composites and bi-layer films with potential antimicrobial activity. Food Hydrocoll 25(5):1372–1381
Quaglia GB (1988) Other durum wheat products. In: Durum chemistry and technology, AACC International, St. Paul, MN, pp 263–282
Rhim JW (2011) Effect of clay contents on mechanical and water vapor barrier properties of agar-based nanocomposite films. Carbohydr Polym 86(2):691–699
Rhim JW, Lee JH, Hong SI (2006) Water resistance and mechanical properties of biopolymer (alginate and soy protein) coated paperboards. LWT Food Sci Technol 39(7):806–813
Rhim JW, Lee JH, Kwak HS (2005) Mechanical and barrier properties of soy protein and clay mineral composite films. Food Sci Biotechnol 14:112–116
Sadegh-Hassani F, Nafchi AM (2014) Preparation and characterization of bionanocomposite films based on potato starch/halloysite nanoclay. Int J Biol Macromol 67:458–462
Schwartz SS, Goodman SH (1982) Plastic materials and processes. Van Nostrand Reinhold, New York
Sorrentino A, Gorrasi G, Vittoria V (2007) Potential perspectives of bionanocomposites for food packaging applications. Trends Food Sci Technol 18:84–95
Sothornvit R, Rhim JW, Hong SI (2009) Effect of nano-clay type on the physical and antimicrobial properties of whey protein isolate/clay composite films. J Food Eng 91(3):468–473
Sothornvit R, Hong SI, An DJ, & Rhim JW. (2010) Effect of clay content on the physical and antimicrobial properties of whey protein isolate/organo-clay composite films. LWT- Food Sci Technol 43(2):279--284
Sun L, Boo WJ, Clearfield A, Sue HJ, Pham HQ (2008) Barrier properties of model epoxy nanocomposites. J Membr Sci 318:129–136
Tharanathan RN (2003) Biodegradable films and composite coatings: past, present and future. Trends Food Sci Technol 14:71–78
Türe H, Gällstedt M, Johansson E, Hedenqvist MS (2013) Wheat gluten/montmorillonite clay multilayer-coated paperboards with high barrier properties. Ind Crop Prod 51:1–6
Voon HC, Bhat R, Easa AM, Liong MT, Karim AA (2012) Effect of addition of halloysite nanoclay and SiO2 nanoparticles on barrier and mechanical properties of bovine gelatin films. Food Bioprocess Technol 5(5):1766–1774
Wittaya T (2012) Protein-based edible films: characteristics and improvement of properties. INTECH Open Access Publisher: Rijeka.
Wu M, Wang Y, Wang M, Ge M (2008) Effect of SiO2 nanoparticles on the wear resistance of starch films. Fibres Text East Eur 16: 96--99
Yano K, Usuki A, Okada A (1997) Synthesis and properties of polyimide-clay hybrid films. J Polym Sci A Polym Chem 35(11):2289–2294
Yin Y, Li Z, Sun Y and Yao K (2005) A preliminary study on chitosan/gelatine polyelectrolyte complex formation. J Mater Sci Lett 40:4649–4652
Zeppa C, Gouanvé F, Espuche E (2009) Effect of a plasticizer on the structure of biodegradable starch/clay nanocomposites: thermal, water-sorption, and oxygen-barrier properties. J Appl Polym Sci 112(4):2044–2056
Zhang Y, Xie B, Xin G (2005) Advance in the applications of konjac glucomannan and its derivatives. Carbohydr Polym 60:27–31
Zielinski H, Kozlowska H (2000) Antioxidant activity and total phenolics in selected cereal grains and their different morphological fractions. J Agric Food Chem 48(6):2008–2016
Zolfi M, Khodaiyan F, Mousavi M, Hashemi M (2014) The improvement of characteristics of biodegradable films made from kefiran–whey protein by nanoparticle incorporation. Carbohydr Polym 109:118–125
Zou DQ, Yoshida (2010) Size effect of silica nanoparticles on thermal decomposition of PMMA. J Therm Anal Calorim 99(1):21–26
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jafarzadeh, S., Alias, A.K., Ariffin, F. et al. Preparation and characterization of bionanocomposite films reinforced with nano kaolin. J Food Sci Technol 53, 1111–1119 (2016). https://doi.org/10.1007/s13197-015-2017-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-2017-7