Abstract
Probiotics are live microbes which when administered in adequate amounts as functional food ingredients confer a health benefit on the host. Their versatility is in terms of their usage which ranges from the humans to the ruminants, pigs and poultry, and also in aquaculture practices. In this review, the microorganisms frequently used as probiotics in human and animal welfare has been described, and also highlighted are the necessary criteria required to be fulfilled for their use in humans on the one hand and on the other as microbial feed additives in animal husbandry. Further elaborated in this article are the sources from where probiotics can be derived, the possible mechanisms by which they act, and their future potential role as antioxidants is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The concept of functional foods was introduced in Japan during the 1980s (De Sousa et al. 2011), and it is connected with considering food not only as a means of providing basic nutrition (Alissa and Ferns 2012) which is necessary for living but also as a source of mental and physical well-being, contributing to the prevention and reduction of risk factors responsible for several diseases or enhancing certain physiological functions (Lobo et al. 2010) like immuno-potentiation, the improvement of system circulation, and control of aging (Al-Sheraji et al. 2013). Thus, a food can be regarded as functional if it satisfactorily demonstrates to beneficially affect either one or more target functions in the body beyond adequate nutritional effects; in a way that is relevant to maintenance or promotion of a state of well being and health or reduction in the risk of a disease (Lobo et al. 2010).
“Health Canada defines functional foods as products that resemble traditional foods but possess demonstrated physiological benefits”. Improvement of heart health, enhancement of immune system, enhancement of gastrointestinal health, preservation of urinary tract health, anti-inflammatory influences, diminution of blood pressure, protection of vision, antibacterial and antiviral activities, decline of osteoporosis and anti-obese influences, being some of the desired physiological benefits. The functionality of functional foods is based on the bioactive components often contained naturally in the product but usually requiring the addition of a specific ingredient in order to optimize the beneficial properties. Included in the category of functional foods are: (i) usual foods with naturally occurring bioactive substances (e.g., dietary fiber), (ii) foods supplemented with bioactive substances (e.g., probiotics, antioxidants), and (iii) derived food ingredients introduced to conventional foods (e.g., prebiotics). Today, functional food ingredients consists of probiotics, prebiotics, vitamins and minerals; which are currently used for human consumption in the form of fermented milks and yogurts, sports drinks, baby foods, sugar-free confectionery and chewing gum (Figueroa-Gonzalez et al. 2011; Al-Sheraji et al. 2013).
This article summarizes the potential health benefits that probiotics can provide and the versatility of their applications ranging from the humans to the ruminants, pigs and poultry, and also in aquaculture practices.
Probiotics – the concept and definition
The concept of probiotics evolved at the turn of the 20th century from a hypothesis first proposed by the Russian scientist and Nobel Laureate, Elie Metchnikoff, who suggested that the long, healthy life of Bulgarian peasants; resulted from their consumption of fermented milk products. He believed that consumption of the fermenting Lactobacillus positively influenced the microflora of the gut, decreasing the toxic microbial activity of the pathogenic bacterial population (Figueroa-Gonzalez et al. 2011; Sharma et al. 2012).
The term probiotic was derived from a Greek word meaning “for life” and it was first coined by Lilly and Stillwell in 1965 to describe “substances secreted by one microorganism which stimulates the growth of another” and thus, was contrasted with the term antibiotic (Sharma et al. 2012). The definition of probiotics was later refined by Fuller in 1989 as “live microbial cultures which beneficially affect the host by improving its intestinal microbial balance” (Khan and Naz 2013). Then subsequently in 2001, a group convened by FAO/WHO Expert Consultation adopted the current definition of probiotics as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” (Singh et al. 2011).
Microorganisms frequently used as probiotics in human and animal welfare
To be used as probiotics, it is essential that the organisms must be considered as GRAS (generally recognized as safe); which is a status used to address the problem of pathogen colonization in different ecosystems (Bouchard et al. 2013). Some of the important microorganisms considered as probiotics have been listed in Table 1.
In human nutrition, the most frequently used probiotic microorganisms include Lactobacillus spp., Bifidobacterium spp., Enterococcus spp.; whereas yeast especially Saccharomyces cerevisiae plays a major role in ruminants; while Bacillus spp., Enterococcus spp. and Lactobacillus spp. are more likely to be efficient in pigs and poultry (Bernardeau and Vernoux 2013); and in aquaculture practices the commonly used being Lactobacillus, Lactococcus, Leuconostoc, Enterococcus, Carnobacterium, Shewanella, Bacillus, Aeromonas, Vibrio, Enterobacter, Pseudomonas, Clostridium, and Saccharomyces species (Nayak 2010). But, it is important to note that the health benefits provided by probiotics are strain specific and not species or genus specific (Figueroa-Gonzalez et al. 2011).
Furthermore, with regards to specific use in humans, probiotics should be able to provide health benefits; promote or maintain the state of well being; and safety assessment should integrate long-term effects and also consider possible chronic effects.
In contrast, microbial feed additives used in animal husbandry must produce a quick response; as because the typical industrial life-spans are for example 42–80 days for a broiler chicken, around 120–200 days for shrimps, 6 months for pigs, 18–24 months for fish, a few months for calves and a few years for beef cattle. This duration being <5 % of the entire life expectancies of the corresponding animal species, which are generally >10 years except for fish and shrimp which ranges between 5 and 7 years. Further, microbial feed additives should be authorized as ‘zootechnical additives’ which means that their use should affect favorably the performance of animals in good health and also affect favorably the environment. Then, microbial feed additives should also enhance the performance characteristics which include feed efficiency through improvement of feed conversion ratio, average daily weight gain through improvement of body weight gain (BWG), milk or egg production, improving carcass composition by increasing protein and muscle deposition while reducing fat deposition, and improving herd performance by bringing about improvement in the reproductive performance of the breeding females, lowering disease incidences, lowering the culling rates in case of dairy cattle, etc. (Bach et al. 2008; Jacela et al. 2009; Bernardeau and Vernoux 2013).
Sources of probiotics
The common sources of probiotics are yogurt, cultured buttermilk, and cheese. The other foods that are produced by bacterial fermentation are Japanese miso, tempeh, sauerkraut, beer, sour dough, bread, chocolate, kimchi, olives, and pickles. Another fermented dairy product is kefir. But among these, the dominant food vehicles for probiotics are still yogurts and fermented milks, both of which provide a relatively low pH environment in which the probiotic bacteria must survive (Anandharaj et al. 2014).
However, many studies have shown that probiotic strains are also found in nondairy fermented substrates which includes soy based products, cereals, legumes, cabbage, maize, pearl millet, sorghum, and so forth (Kechagia et al. 2013; Anandharaj et al. 2014).
The other sources of probiotics include breast milk, the human gastrointestinal tract, and the guts of several animal species, including pigs, rats and even poultry. Recently, L. johnsonii CRL 1647, isolated from the Apis mellifera L. bee gut, was shown to exhibit a beneficial effect on honeybee colonies. Additionally, probiotic strains have also been obtained from the intestinal tracts of marine and freshwater fish, such as Carassius auratus gibelio, rainbow trout and shrimp (Fontana et al. 2013).
Desirable probiotic characteristics
The important criteria which are used for selecting probiotic strains includes that the organism should be: non-pathogenic and non-toxic (Singh et al. 2011); isolated from the same species as its intended host (Maurya et al. 2014); able to survive during transit through the gastrointestinal tract by being acid and bile resistant (Khan and Naz 2013; Maurya et al. 2014); able to adhere and colonize the intestinal epithelium which is an important property for successful immune modulation and competitive exclusion of pathogens (Kechagia et al. 2013; Maurya et al. 2014); able to stabilize the normal intestinal microflora (Parvez et al. 2006); able to produce antimicrobial substances like the bacteriocins against the pathogens (Kral et al. 2012); having a demonstrated beneficial effect on the host (Fontana et al. 2013); durable enough to withstand the duress of commercial manufacturing, processing and distribution (Khan and Naz 2013); and also having good sensory characteristics by not providing unpleasant flavors or textures (Parracho et al. 2007; Mitropoulou et al. 2013). The essential criteria for the use of probiotics in humans has been listed in Table 2.
Mechanisms of probiotic action
Probiotics have various mechanisms of action although the exact manner in which they exert their effects is still not fully elucidated (Kechagia et al. 2013). Nonetheless, the possible modes of action include the following:
-
a.
Enhancement of epithelial barrier function
Intestinal barrier function is maintained by several interrelated systems including mucus secretion, chloride and water secretion, and binding together of epithelial cells at their apical junctions by tight junction proteins (Ng et al. 2009). Integral to the gut barrier defense is mucus which is composed of mucins (MUC 2 and MUC 3) which are secreted from the goblet cells. Mucin polymerization provides the structural foundation of the mucus, granting protection from pathogens, enzymes, toxins, dehydration and abrasion. Lactobacillus plantarum 299v and Lactobacillus rhamnosus GG have been shown to up-regulate production of intestinal mucins (MUC 2 and MUC 3) which subvert the adherence of the enteropathogenic bacterium Escherichia coli O157:H7 to intestinal epithelial cells, consequently preventing pathogenic bacterial translocation (Hardy et al. 2013).
Further, some probiotic bacteria have been found to limit chloride and water secretion, as is the case with Streptococcus thermophilus and Lactobacillus acidophilus which reversed the E. coli- induced chloride secretion by epithelial cells (Brown 2011).
On the other hand, intestinal barrier integrity may be increased by enhancing the expression of genes involved in tight junction signaling. On this count, some of the probiotics like lactobacilli for instance, have been shown to modulate the regulation of several genes encoding adherence junction proteins such as E-cadherin and β-catenin in T84 epithelial cells. Moreover, incubation of intestinal cells with lactobacilli modulates tight junction protein phosphorylation.
Probiotics have also been indicated to initiate repair of the barrier function after damage. For example, Escherichia coli Nissle 1917 not only counteracted the disruptive effects of enteropathogenic E. coli, but also restored the mucosal integrity in T84 and Caco-2 cells. This effect is achieved by increasing expression and repartition of tight junction proteins of the zonula-occludens (ZO-2) and altering protein kinase C signaling (Goudarzi et al. 2014).
-
b.
Increased adhesion to intestinal epithelial cells
The effective performance of a probiotic depends on their strong adhesion and colonization of the gut, which in turn improves the host immune system. Lactobacillus plantarum 299v has been shown to exhibit a mannose-specific adhesion by which it can adhere to human colonic cells. Once the probiotic adheres to the cell, various biological activities take place, which primarily include the release of cytokines and chemokines. These then exert their secondary activity such as stimulation of mucosal and systemic host immunity (Hemaiswarya et al. 2013).
-
c.
Competitive exclusion of pathogenic microorganisms
Probiotic bacteria are able to exclude or reduce the growth of pathogens by any one of the following ways which includes creation of a hostile microenvironment like the lowering of the pH of the gut below than what is essential for the survival of pathogenic bacteria such as E. coli and Salmonella, by producing organic acids like acetic acid and lactic acid (Brown 2011; Bermudez-Brito et al. 2012; Goudarzi et al. 2014). The others include physical blocking of available bacterial receptor sites (Goudarzi et al. 2014); compete with pathogenic bacteria for essential nutrients and energy source (Brown 2011); secretion of antimicrobial substances and release of selective gut-protective metabolites like arginine, glutamine, short-chain fatty acids and conjugated linoleic acids (Bermudez-Brito et al. 2012; Hemaiswarya et al. 2013).
-
d.
Production of antimicrobial peptides
Many lactic acid bacteria produce well characterized inhibitory peptides which include, but are not limited to lantibiotics (class I), peptide bacteriocins (class II), and bacteriolysins (class III) (Saulnier et al. 2009). Bacteriocins are antimicrobial compounds with a molecular weight of >1,000 Dalton. Bacteriocins produced by gram-positive bacteria usually the lactic acid bacteria include lactacin B from L. acidophilus, plantaricin from L. plantarum and nisin from Lactococcus lactis. These have a narrow activity spectrum and act only against closely related bacteria, but some bacteriocins are also active against food-borne pathogens. The common mechanisms of bacteriocin-mediated killing include the destruction of target cells by pore formation and/or inhibition of cell wall synthesis (Bermudez-Brito et al. 2012).
The probiotic bacteria Lactobacillus reuteri produces an antimicrobial agent reuterin which has broad-spectrum activity against a variety of pathogens including bacteria, fungi, protozoa and viruses, and can be differentially expressed by various L. reuteri strains (Saulnier et al. 2009).
Defensins are a family of highly conserved small cysteine-rich antimicrobial peptides particularly abundant at mucosal sites where they contribute to the host defense by disrupting the cytoplasmic membrane of susceptible microorganisms (Hardy et al. 2013). The probiotic E. coli Nissle strain has been shown to induce expression of human beta- defensin 2 in Caco-2 intestinal epithelial cells and this type of effect may contribute to an improved mucosal barrier and provide a means of limiting access of enteric pathogens (Ng et al. 2009).
-
e.
Modulation of the immune system
There are growing evidences that probiotics have immunomodulatory properties and that these properties of probiotics are strain-dependent. Some of these probiotic immunomodulatory properties are as listed below:
-
i.
Increasing the phagocytic capacity of macrophages
Many probiotic strains have been shown to influence innate defense mechanisms such as phagocytosis. It has been demonstrated that L. acidophilus La1 increased the phagocytic capacity of leucocytes isolated from the blood of humans who had consumed probiotics and this was consistent with the adhesion potential of this bacterium. But it has also been found that even Bifidobacterium lactis Bb12 which exhibits slightly less adhesion is also able to increase phagocytosis substantially (Delcenserie et al. 2008).
-
ii.
Enhancing natural killer cell activity
Consistent evidences point to the fact that a number of probiotics enhance natural killer (NK) cell activity in vitro and there is some supportive evidence that this also occurs in vivo. Probiotic strains whose cell walls are resistant to digestion appear to be particularly potent enhancers of NK cell activity. It is suggested that monocytes phagocytose the probiotic bacteria and the insoluble cell wall components induce the production of interleukin-12 (IL-12), which augments NK cell activity. In a particular study, it was found that when neonatal and infant mice were administered with Lactobacillus casei Shirota by stomach tube for 3 weeks prior to infection with influenza; the mice demonstrated a lower rate of accumulated symptoms, a greater survival rate and lower titres of influenza in nasal washings taken a few days after infection. These correlated with an increase in NK cell activity and production of IL-12 (Yaqoob 2014).
-
iii.
Stimulating IgA production
Many probiotic strains are apparently able to stimulate the production of IgA by B cells, which help maintain intestinal humoral immunity by binding to antigens, thereby limiting their access to the epithelium. Study subjects who consumed fermented milk containing Bifidobacterium bifidum and L. acidophilus La1 following vaccination against Salmonella typhi Ty21 showed a significant increase in IgA serum concentration.
In addition, children who were 2 to 5 years old and who received L. rhamnosus GG concomitantly with a rotavirus vaccination showed an increased number of IgA secreting cells.
A study conducted to examine IgA production by intestinal mucosal lymphoid cells in mice showed that B. bifidum significantly induced IgA production in Peyer’s patches and mesenteric lymph nodes, and that optimal secretion was obtained with probiotics encapsulated in alginate microparticles. Surprisingly, rather than inducing a specific humoral immune response, B. bifidum apparently had a more systemic immune effect.
Another study demonstrated that a peptide fraction derived from Lactobacillus helveticus- fermented milk contributed to induce local mucosal and systemic IgA immune responses in mice that were infected with E. coli O157:H7 (Delcenserie et al. 2008).
-
iv.
Modulation of cytokine production
Probiotics have also been demonstrated to modulate cytokine production in a strain-dependent manner. These immunomodulatory capacities of probiotic strains have been extensively studied using various in vitro co-culture assays that employ different types of immune cells such as human monocyte-derived dendritic cells, human peripheral blood mononuclear cells and mouse bone-marrow-derived dendritic cells and use cytokine production profiles as a functional read-out. Interleukin-10 (IL-10) which is associated with T helper 2 (TH2) cell or T regulatory (TReg) cell stimulation is typically used as a marker for anti-inflammatory effects, whereas the p70 IL-12 heterodimer and tumor necrosis factor (TNF), which are associated with both TH1 cell stimulation and the induction of interferon-γ (IFN- γ) production by T cells and natural killer (NK) cells, are commonly measured as markers for pro-inflammatory responses (Bron et al. 2012).
It has been proposed that probiotics fall into two main categories: those which are ‘immunostimulatory’ characterized by their ability to induce IL-12 and therefore augment host defense via enhancement of NK cell activity and TH1 pathways, and those which are ‘immunoregulatory’ characterized by their ability to induce IL-10 and the T regulatory pathway. In general, lactobacilli tend to fall in the immunostimulatory category, whereas bifidobacterium tend to fall in the immunoregulatory category (Yaqoob 2014).
It has been revealed that lactobacilli, such as Lactobacillus casei Shirota or Lactobacillus reuteri ATCC 23272, induce TH1 cells via the induced production of IL-12 generated by macrophages and dendritic cells, and Bifidobacterium bifidum W23 and Bifidobacterium longum W52 inhibit the production of cytokines generated by TH2 cells via the production of IL-10 generated by monocytes (Chiba et al. 2009). Another study also demonstrated that T cell-derived IL-10 suppresses T cell-dependent intestinal inflammation in Bifidobacterium breve-treated severe combined immunodeficient (SCID) mice (Jeon et al. 2012).
-
i.
-
f.
Interference with quorum sensing signaling molecules
Bacteria communicate with each other as well as with their surrounding environment through chemical signaling molecules called auto-inducers. This phenomenon of communication is known as quorum sensing and one of its characteristics is that it can control the gene expression of the entire community in response to changes in cell number.
Probiotic bacteria such as lactobacillus, bifidobacterium and Bacillus cereus strains degrade the auto-inducers of pathogenic bacteria by enzymatic secretion or production of auto-inducer antagonists and thereby control the virulence gene expression in pathogenic bacteria. L. acidophilus secretes a compound that inhibits the quorum sensing signaling or interferes with the bacterial transcription of the E. coli O157 gene which is involved in colonization and thus helps prevent bacterial toxicity. Similar results have also been reported from studies using B. cereus and Bacillus toyoi probiotic bacteria (Brown 2011; Goudarzi et al. 2014).
Probiotics in human health and disease
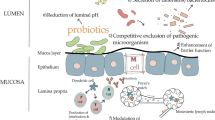
Probiotics have been shown to promote a variety of biological effects in a number of physiological conditions and pathologies, and the overall health benefits of probiotic microorganisms has been depicted in Fig. 1.
Overall benefits of probiotic bacteria on human health (Anandharaj et al. 2014)
-
a.
Intestinal-related diseases
-
i.
Antibiotic-associated diarrhea
Mild or severe episodes of diarrhea are common side effects of antibiotic therapy as the normal microflora tends to be suppressed, encouraging the overgrowth of opportunistic or pathogenic strains. Treatments with Bacillus spp., Bifidobacterium spp., Lactobacillus spp., Lactococcus spp., Leuconostoc cremoris, Saccharomyces spp., or Streptococcus spp., individually or in combination were shown to have a protective effect in preventing antibiotic-associated diarrhea (Fontana et al. 2013; Kechagia et al. 2013). A meta-analysis consisting of 34 studies which included 4138 patients reported that the pooled relative risk (RR) for antibiotic-associated diarrhea (ADD) in the probiotic group versus placebo was 0.53 [95 % CI: 0.44-0.63] which in terms of the risk reduction corresponded to a number needed to treat (NNT) of 8 [95 % CI: 7–11]. Further, the pooled RR from 6 studies during Helicobacter pylori treatment was 0.37 [95 % CI: 0.20-0.69] corresponding to a NNT of 5 [95 % CI: 4–10], while the pooled RR excluding studies during H. pylori treatment was 0.56 [95 % CI: 0.46-0.67] corresponding to a NNT of 9 [95 % CI: 7–12], which clearly indicates a greater risk reduction in the pooled analysis of studies having H. pylori eradication regimens (Videlock and Cremonini 2012).
-
ii.
Rotavirus diarrhea
Rotavirus is the most common cause of acute infantile diarrhea in the world and a significant cause of infant mortality. Clinical studies have shown that probiotics such as Lactobacillus rhamnosus GG, Lactobacillus reuteri, Lactobacillus casei Shirota, Bifidobacterium animalis Bb12 can shorten the duration of acute rotavirus diarrhea with the strongest evidence pointing to the effectiveness of L. rhamnosus GG and B. animalis Bb12 (Kechagia et al. 2013).
-
iii.
Travellers’ diarrhea
People traveling to warmer climates and less-developed countries experience a high incidence of diarrhea, often in the 50 % range (Goldin and Gorbach 2008). With regard to this, a meta-analysis by Guarino et al. (2008) revealed evidence of a protective effect by Saccharomyces boulardii and by a mixture of Lactobacillus acidophilus and Bifidobacterium bifidum.
-
iv.
Irritable bowel syndrome
Irritable bowel syndrome (IBS) is a chronic condition affecting 3-25 % of the population for which no curative treatment is available. Accordingly, therapy is aimed at reducing the symptoms (Fontana et al. 2013). A study by Drouault-Holowacz et al. (2008) reported an increase in patients with satisfactory relief of overall IBS symptoms after receiving a probiotic mix of Bifidobacterium longum LA101, Lactobacillus acidophilus LA102, Lactococcus lactis LA103, Streptococcus thermophilus LA104. Another study by Enck et al. (2008) reported that the use of the bacterial lysate of Enterococcus faecalis and Escherichia coli as being effective in reducing the typical symptoms of IBS. The combined data from a meta-analysis of 14 randomized placebo controlled trials suggested a modest improvement in the overall symptoms of IBS after several weeks of probiotics treatment with the overall odds ratio (OR) of 1.6 [95 % CI: 1.2-2.2] for the dichotomous data from seven trials (895 participants); and for continuous data from six trials (657 participants), the standardized mean difference (SMD) was 0.23 [95 % CI: 0.07-0.38] (Hoveyda et al. 2009).
-
v.
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is an umbrella term comprising different conditions of the gut, of which the two main types are ulcerative colitis and Crohn’s disease; that are characterized by chronic or recurrent mucosal inflammation. The nonpathogenic strain E. coli Nissle 1917 has proved to be effective in preventing relapse in Crohn’s disease patients and S. boulardii has shown some success in relieving the symptoms of active Crohn’s disease and also in reducing the risk of a relapse (Maurya et al. 2014).
-
vi.
Lactose intolerance
Lactose intolerance is a genetically determined beta-galactosidase (lactase) deficiency resulting in the inability to hydrolyze lactose into the monosaccharides glucose and galactose. Lactose intolerant individuals develop diarrhea, abdominal discomfort, and flatulence after consumption of milk or milk products. The use of Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus has been effective in this direction, partly because of higher beta-galactosidase (lactase) activity (Kechagia et al. 2013).
In general, probiotics are beneficial in the treatment and prevention of gastrointestinal diseases; but before choosing a probiotic for the purpose, the type of disease and probiotic species (strain) are the most important factors to be taken into consideration. This is according to the conclusion from a meta-analysis which included randomized controlled trials in humans that used a specified probiotic in the treatment or prevention of pouchitis, infectious diarrhea, irritable bowel syndrome, Helicobacter pylori, Clostridium difficile disease, antibiotic associated diarrhea, traveler’s diarrhea or necrotizing enterocolitis. This meta-analysis which contained 74 studies, 84 trials and 10,351 patients showed that in general probiotics were found to have a beneficial effect on the treatment and prevention of all eight gastrointestinal diseases with a relative risk of 0.58 [95 % CI: 0.51-0.65]. But out of the eight diseases, only six showed positive significant effects; whereas in the case of traveler’s diarrhea and necrotizing enterocolitis, probiotics showed no efficacy. This effect may be due to the low number of studies on these diseases, or in the case of traveler’s diarrhea where the underlying mechanism of the disease is often not bacterial. Further, out of the eleven species and species mixtures which were analyzed, majority showed positive significant effects except for Lactobacillus acidophilus, Lactobacillus plantarum, and Bifidobacterium infantis (Ritchie and Romanuk 2012).
-
i.
-
b.
Allergy
A limited number of strains have been tested for their efficiency in the treatment and prevention of allergy in infants (Kechagia et al. 2013). A study on breast fed infants suffering from atopic eczema reported that Bifidobacterium lactis and L. rhamnosus GG were found to be effective in decreasing the eczema severity. Furthermore, L. rhamnosus GG was found successful in preventing the occurrence of atopic eczema in high risk infants (Isolauri et al. 2000). Probiotics however have not been very successful in alleviating symptoms of asthma (Kechagia et al. 2013). This is in agreement with a meta-analysis observation which included seventeen studies reporting data from 4755 children (2381 in the probiotic group and 2374 in the control group) and which reported that infants treated with probiotics had a significantly lower risk ratio (RR) for eczema compared to controls (RR 0.78 [95 % CI: 0.69-0.89], p = 0.0003), especially those supplemented with a mixture of probiotics (RR 0.54 [95 % CI: 0.43-0.68], p < 0.00001). But no significant difference in terms of prevention of asthma (RR 0.99 [95 % CI: 0.77-1.27], p = 0.95), wheezing (RR 1.02 [95 % CI: 0.89-1.17], p = 0.76) or rhino-conjunctivitis (RR 0.91 [95 % CI: 0.67-1.23], p = 0.53) was documented. The results of this meta-analysis indicate that probiotic supplementation prevents infantile eczema; thus suggesting a new potential indication for probiotic use in pregnancy and infancy (Zuccotti et al. 2015).
As far as food allergy is concerned, it is described as an immunologically mediated adverse reaction against dietary antigens leading to secondary intestinal inflammation and disturbances. The mechanism of the immune modulating effect of L. rhamnosus GG are not entirely understood but it seems to be related with the antigen’s transport across the intestinal mucosa (Kechagia et al. 2013).
-
c.
Reduction in serum cholesterol
An in vivo study on the cholesterol-lowering potential of Lactobacillus casei subsp. casei in rats reported that the cholesterol levels were lower in the plasma of groups fed fermented milk by 2 – 11 % and by 15 – 25 % in groups fed lyophilized culture as compared to the group fed skim milk (Mishra et al. 2015).
On this aspect, a number of cholesterol lowering mechanisms by Lactobacillus strains have been proposed including assimilation of cholesterol by growing cells, binding of cholesterol to cellular surface, incorporation of cholesterol into the cellular membrane, deconjugation of bile via bile salt hydrolase, and coprecipitation of cholesterol with deconjugated bile. But the exact mechanisms still remain unclear and controversial (Anandharaj et al. 2014).
A meta-analysis of 13 randomized controlled trials which included 485 participants with high, borderline high and normal cholesterol levels reported that the pooled mean net change in total cholesterol for those treated with probiotics compared to controls was −6.40 mg dl−1 [95 % CI: −9.93 to −2.87], the mean net change in low-density lipoprotein (LDL) cholesterol was −4.90 mg dl−1 [95 % CI: −7.91 to −1.90], the mean net change in high-density lipoprotein (HDL) cholesterol was −0.11 mg dl−1 [95 % CI: −1.90 to −1.69] and the mean net change in triglycerides was −3.95 mg dl−1 [95 % CI: −10.32 to −2.42]. These results indicate that a diet rich in probiotics decreases total cholesterol and LDL cholesterol concentration in plasma for participants with high, borderline high and normal cholesterol levels (Guo et al. 2011).
-
d.
Prevention of dental caries formation
Streptococcus mutans is the main microorganism involved in causation of dental caries. The use of probiotics like L. rhamnosus GG has been reported to inhibit oral colonization by cariogenic pathogens and thereby reduce tooth decay incidence in children (Goel et al. 2014). Twetman and Keller (2012) reported that a search for retrieving papers containing relevant clinical trials on the use of probiotic bacteria as a potential and clinically applicable anti-caries measure yielded 2 animal and 19 human studies. Out of the 19 papers reviewed, 12 papers reported a significant reduction of Streptococcus mutans in saliva or plaque following daily intake of probiotic lactobacilli or bifidobacteria; whereas 3 papers reported an increase of lactobacilli. But it was also outlined that a high degree of heterogeneity among the included investigations hampered the analysis. There were further three caries trials in preschool children and the elderly which demonstrated prevention of caries formation between 21 % and 75 % following regular intakes of milk supplemented with L. rhamnosus. But in their conclusion the authors noted that large-scale clinical studies with orally derived specific anti-caries candidates are still lacking.
-
e.
Prevention of respiratory infections
A meta-analysis that included ten randomized controlled trial (RCT) comparing probiotics with placebo to prevent acute upper respiratory tract infections reported that probiotics were more effective than the placebo in reducing the number of participants experiencing episodes of acute respiratory infections, the rate ratio of episodes of acute upper respiratory tract infections and reducing antibiotic use.
Another meta-analysis consisting of five randomized controlled trial (RCT) showed that the administration of probiotics is associated with lower incidence of ventilator-associated pneumonia compared with the placebo (Fontana et al. 2013).
-
f.
Urinary tract infections
Urinary tract infections (UTI) are common among women and frequently have a tendency to recur. The causative organisms include E. coli, Proteus spp., Klebsiella spp., Staphylococcus spp., which are mainly intestinal uropathogens. The depletion of vaginal Lactobacilli is associated with UTI risk, which suggests that repletion might be beneficial (Fontana et al. 2013; Maurya et al. 2014). It has been found that high level vaginal colonization with Lactobacillus crispatus probiotic was associated with a significant reduction in recurrent UTI (Stapleton et al. 2011). Data from a random-effects model meta-analysis comparing incidence of recurrent UTI in 294 adult women patients across five studies showed no statistically significant difference in the risk for recurrent UTI in patients receiving Lactobacillus versus controls, as indicated by the pooled risk ratio of 0.85 [95 % CI: 0.58-1.25, p = 0.41]. But when a sensitivity analysis was performed which excluded studies using ineffective strains and studies testing for safety; and included data from 127 patients in two studies, observed a statistically significant decrease in recurrent UTI in patients given Lactobacillus denoted by the pooled risk ratio of 0.51 [95 % CI: 0.26-0.99, p = 0.05] with no statistical heterogeneity (I2 = 0 %). These results indicate that probiotic strains of Lactobacillus are safe and effective in preventing recurrent UTI in adult women. But the authors in their conclusion stated that more randomized clinical trials (RCTs) are required before a definitive recommendation can be made; since the population contributing data to this meta-analysis was small (Grin et al. 2013).
-
g.
Prevention of osteoporosis
One of the main bone diseases which is associated with aging and postmenopausal condition is osteoporosis; with one of the most serious problems among women over 50 years of age being hip fracture due to osteoporosis. The possibility of wrist, hip, or spine fracture is estimated to be parallel to the risk of heart disease (approximately 15 %). In this regard, a few animal studies and a particular study on humans demonstrated a positive effect of probiotics on bone metabolism and bone mass density. In the human study, twenty postmenopausal women with a mean age of 65 years and a mean body mass index (BMI) of 26 were assessed for the effects of probiotics on bone. The study was a double-blind randomized crossover study with the subjects being segregated into two groups; in which the group consuming Lactobacillus helveticus fermented milk was compared to the control milk consumption group. The results from this study confirmed the reduction of parathyroid hormone (PTH) followed by an increase in serum calcium levels in the group that consumed Lactobacillus fermented milk and as a consequence of which there was reduced bone resorption. Further, the ionized serum calcium, total calcium, phosphate, and urinary calcium were higher in the group that consumed L. helveticus fermented milk as compared to the control group.
Although the exact mechanisms by which probiotic bacteria exert their effects on bone health in humans is still unclear; nonetheless the principal mechanisms by which probiotic bacteria increase the bioavailability of minerals include (i) increasing mineral solubility by the production of short chain fatty acids; (ii) producing phytase enzyme that overcomes the effects of depressed mineral availability due to phytate; (iii) reducing intestinal inflammation which in-turn increases bone mass density; and (iv) hydrolyzing the glycosidic bonds of estrogenic food in the intestines (Parvaneh et al. 2014).
-
h.
Anticancer effects
Several animal studies have shown that supplementation with specific strains of lactic acid bacteria (probiotics) could prevent the establishment, growth, and metastasis of transplantable and chemically induced tumors. Studies in human subjects have also revealed that probiotic therapy may reduce the risk of colon cancer by inhibiting transformation of procarcinogens to active carcinogens, binding/inactivating mutagenic compounds, producing antimutagenic compounds, suppressing the growth of pro-carcinogenic bacteria, reducing the absorption of mutagens from the intestine, enhancing immune function, have anti-proliferative effects via regulation of apoptosis and cell differentiation, fermentation of undigested food which helps generate short-chain fatty acids (SCFA), and inhibition of tyrosine kinase signaling pathways (Gill and Guarner 2004; Uccello et al. 2012). An inverse relationship between the consumption of fermented dairy products, containing lactobacilli or bifidobacteria, and the incidence of colon and breast cancer has also been reported in epidemiological and population based case–control studies.
However, there is little “direct experimental evidence” regarding the anticancer effectiveness (tumor suppression) of probiotic therapy in humans (Gill and Guarner 2004). But as of the moment, there is a completed phase 2 trial assessing the role of probiotics on gut microbiota and colorectal cancer but the results have not been published yet. Furthermore, the role of the VSL#3 probiotic combinations in rectal cancer is being investigated in a phase 3 clinical trial and the results are also due (Whyand and Caplin 2014).
Probiotics for ruminants
In adult ruminants, probiotics have mostly been selected to target the rumen compartment, which is the main site of feed digestion, and the most common probiotics for ruminants being the live yeast (Saccharomyces cerevisiae). In dairy ruminants, live yeasts have been shown to improve performance with respect to increasing the dry matter intake and milk production. Further in beef cattle, growth parameters (average daily gain, final weight, intake, feed to gain ratio) has been reported to be improved by daily live yeast supplementation.
There is also an increasing amount of evidence through in vitro studies that live yeast stabilizes ruminal pH and decreases the risk of acidosis by limiting lactate production by Streptococcus bovis and favoring lactate uptake by Megasphaera elsdenii (Chaucheyras-Durand and Durand 2010). Regarding the use of lactate-producing bacteria like Enterococcus and Lactobacillus, the underlying principle behind it is that by providing a constant supply of lactate in the rumen, lactate-utilizing bacteria are stimulated and the overall microflora can adapt to the presence of a higher concentration of lactate (Chiquette 2009). Alternately, lactate users like Megasphaera elsdenii or Propionibacterium spp., could be administered as direct-fed microbials to avoid ruminal lactate accumulation.
Regarding the strategy to reduce digestive carriage by adult ruminants of human pathogens, certain strains of Lactobacillus acidophilus have been shown to decrease the numbers of E. coli O157 and also appear to reduce shedding of Salmonella enterica.
In young pre-ruminants, bacterial probiotics such as Lactobacillus spp., Bifidobacterium spp., Enterococcus spp., Propionibacterium spp., or Bacillus spores, represent an interesting means of stabilizing the gut microbiota and limiting the risk of pathogen colonization by generally targeting the small intestine as the rumen is not yet developed (Chaucheyras-Durand and Durand 2010).
Probiotics for pigs
The most common probiotics for monogastric animals are the yeasts (Saccharomyces boulardii), and bacteria (Lactobacillus spp, Enterococcus spp., Pediococcus spp., Bacillus spp.) which target the hindgut (cecum, colon) that harbors an abundant and very diverse microbial population mainly composed of bacteria and archaea (Chaucheyras-Durand and Durand 2010).
Studies suggest that certain microbial supplements are useful in protecting the young pigs particularly from intestinal infections around weaning. This period and other stressful mixing events during their lives is probably important because it is at these points that pigs pick up important zoonotic pathogens like Salmonella enterica and Streptococcus suis (Kenny et al. 2011). It is in this context that performance benefits have been reported after weaning by using S. boulardii. Similar findings have been reported with Pediococcus acidilactici - based probiotic supplementation. The benefits of intestinal IgA secretion and reduction of translocation of enterotoxigenic E. coli have also been observed with S. boulardii or P. acidilactici given to piglets (Chaucheyras-Durand and Durand 2010).
Furthermore in a recent study, Mishra et al. (2014) indicated that dietary supplementation with S. cerevisiae or L. acidophilus had a positive effect on the performance of weaned piglets.
Probiotics for poultry
Probiotic species belonging to Lactobacillus, Streptococcus, Bacillus, Bifidobacterium, Enterococcus, Aspergillus, Candida and Saccharomyces have been shown to have a beneficial effect on broiler performance with evidences of increased resistance of chickens to Salmonella, E. coli or Clostridium perfringens infections (Chaucheyras-Durand and Durand 2010; Kral et al. 2012). La Ragione et al. (2001) reported that oral inoculation of Bacillus subtilis spores could reduce intestinal colonization of E. coli O78:K80 in chickens. Probiotics have also been reported to increase feed efficiency and productivity of laying hens with an improvement in egg quality (decreased yolk cholesterol level, improved shell thickness, egg weight) (Chaucheyras-Durand and Durand 2010).
Probiotics in aquaculture practices
The gastrointestinal microbiota of aquatic species is particularly dependent on the external environment due to the flow of water which passes through the digestive tract. Thus, the majority of bacteria are transient in the intestine due to constant intake of water and food, together with the microorganisms present in them. Apart from the potentially pathogenic bacteria such as Salmonella, Listeria, and E. coli, probiotic bacteria and other microorganisms have also been identified in the gastrointestinal tract of aquatic animals which include the gram-positive bacteria such as Bacillus, Carnobacterium, Enterococcus, and several species of Lactobacillus; gram-negative, facultative anaerobes such as Vibrio and Pseudomonas, as well as certain fungi, yeasts, and algae of the genera Debaryomyces, Saccharomyces, and Tetraselmis, respectively.
The use of probiotics in aquaculture is relatively recent and the initial application had been to test their ability to increase growth of hydrobionts (organisms that live in water). Then subsequently probiotics were used for the purpose of improving water quality and control of bacterial infections. Furthermore, there are also documented evidences that probiotics can improve the digestibility of nutrients, increase tolerance to stress, and encourage reproduction.
Currently, there are commercial probiotic products prepared from various bacterial species such as Bacillus spp., Lactobacillus spp., Enterococcus spp., Carnobacterium spp., and the yeast S. cerevisiae among others, and their use is regulated by careful management recommendations (Cruz et al. 2012).
Regarding the role of probiotics as growth promoters, Lara et al. (2003) reported that when the diet of Nile tilapia (Oreochromis niloticus) was amended with a probiotic Streptococcus strain, there was a significant increase in crude protein, crude lipid content, together with an increase in the weight of the fish. Further, Balcazar (2003) demonstrated that the administration of a mixture of bacterial strains (Bacillus and Vibrio spp.) positively influenced the growth and survival of white shrimp juveniles.
In the context of disease control, Rengpipat et al. (2000) had reported that the use of Bacillus spp. (strain S11) provided disease protection by activating both cellular and humoral immune defenses in tiger shrimp (Penaeus monodon). Further, it has also been demonstrated that oral administration of Clostridium butyricum bacteria to rainbow trout enhanced the resistance of fish to vibriosis by increasing the phagocytic activity of leucocytes (Pandiyan et al. 2013).
In relation to improvement of water quality, it had been observed that when Streptomyces was applied as a probiotic in the laboratory culture of Penaeus monodon, there was a marked improvement in the water quality parameters accompanied by an increase in the length and weight of the tiger shrimp (Lakshmi et al. 2013).
As agents for improving nutrient digestion, Tovar et al. (2002) reported that the probiotic yeast Debaryomyces hansenii HF1 has the ability to produce spermine and spermidine, the two polyamines involved in the differentiation and maturation of the gastrointestinal tract in mammals. In addition, this yeast secretes amylase and trypsin enzymes that aid digestion in sea bass larvae. Probiotics have also been used in the case of European bass larvae (Dicentrarchus labrax) (Cruz et al. 2012).
With regard to increasing the level of stress tolerance, Carnevali et al. (2006) reported that supplementation of Lactobacillus delbrueckii subsp. delbrueckii in the diet of European sea bass (Dicentrarchus labrax) significantly lowered the levels of cortisol in fish tissues.
Regarding the effect on the reproductive performance of fishes, Ghosh et al. (2007) reported that using a strain of Bacillus subtilis isolated from the intestine of Cirrhinus mrigala and its subsequent incorporation at different concentrations to four species of ornamental fishes: Poecilia reticulata, Poecilia sphenops, Xiphophorus helleri, and Xiphophorus maculates, led to an increase in the gonadosomatic index, fecundity, viability, and production of fry from the females of all four species. It was further proposed that the complex B vitamins synthesized by the probiotic, especially thiamine (vitamin B1) and vitamin B12, contributed to reduce the number of dead or deformed alevins.
Future prospects of probiotics as antioxidants
Various studies during the recent times have focused on the antioxidative property of probiotics and a few patents on the use of Bifidobacterium lactis BS05, Lactobacillus acidophilus LA06, and Lactobacillus brevis LBR01, have been recently granted in this area. There are also strong evidences emerging for the antioxidative activity of Lactobacillus plantarum, L. helveticus, L. acidophilus, Lactobacillus fermentum, L. casei, L. rhamnosus GG, and a few bifidobacteria and foods containing these organisms. Although the mechanisms of antioxidative action has not been properly understood; but the production of bioactive peptides has been considered an effective mode of antioxidative activity in foods containing probiotic bacteria. Peptides obtained from hydrolyzed food proteins have been shown to possess antioxidative activities, which can impart protection against the peroxidation of lipids or fatty acids. It has been observed that the peptic digest of casein liberates small peptides with radical scavenging activity. The antioxidant ability was considered due to the presence of histidine and some hydrophobic amino acids in high concentrations. Increased antioxidant activity of milk during fermentation with commonly used dairy starter cultures which include Leuconostoc mesenteroides subsp. cremoris strain, L. jensenii, and L. acidophilus has also been observed. Further, L. rhamnosus GG was reported to have potent antioxidative activity by down-modulating the reactive oxygen species (ROS) production and phagocytic capacity of neutrophils. It has also been reported that some probiotics increased the activity of antioxidative enzymes like glutathione S-transferase, glutathione reductase, glutathione peroxidase, superoxide dismutase, and catalase or modulate the circulatory oxidative stress which in turn protects cells against carcinogen-induced damage. However, it should be noted that the antioxidative property of probiotics is strain specific.
The manifestations that probiotics have antioxidant attributes is significant in the sense that this can help diversify the potential sources of antioxidants which can be safely used; which until now has been the property of substances mostly available in plant material only; including garlic, broccoli, green tea, soybeans, tomatoes, carrots, Brussels sprouts, kale, cabbages, onions, cauliflower, red beets, cranberries, cocoa, blackberries, blueberries, red grapes, prunes, and citrus fruits. This is highly desirable as because reactive oxygen species (ROS) - mediated oxidative stress is known to play a vital role in the development of chronic diseases such as cancer, diabetes, heart disease, stroke, Alzheimer’s disease, rheumatoid arthritis, cataract, and aging (Mishra et al. 2015).
Conclusion
There are numerous scientific evidences supporting the incorporation of probiotics in nutrition as a means of derivation of health benefits. In humans, the best documented effects are for bowel disorders such as lactose intolerance, antibiotic-associated diarrhea and infectious diarrhea; allergy; and a large number of evidences are still emerging concerning their potential role in various other conditions (Kechagia et al. 2013). Even in animal health and nutrition, probiotics can expect a promising future because of the fact that they offer a viable alternative for the generation of a higher-quality livestock product in terms of size, production time, and health (Chaucheyras-Durand and Durand 2010; Cruz et al. 2012). All these become more important since there is a worldwide concern over the present state of antimicrobial resistance among zoonotic bacteria that potentially circulate among food-producing animals including poultry, beef and dairy cattle, goats, sheep and aquaculture. This has resulted in the general public perception that antibiotic use by humans and in food animals, selects for the development of antimicrobial resistance among food-borne bacteria that could complicate public health therapies. Consequently, there is a need for developing novel intervention methods to deal with this situation and, on this count a potentially efficient and versatile option being the use of probiotics to selectively target pathogenic organisms while avoiding killing of beneficial organisms (Seal et al. 2013).
References
Alissa EM, Ferns GA (2012) Functional foods and nutraceuticals in the primary prevention of cardiovascular diseases. J Nutr Metab Article ID 569486
Al-Sheraji SH, Ismail A, Manap MY, Mustafa S, Yusof RM, Hassan FA (2013) Prebiotics as functional foods: a review. J Funct Food 5:1542–1553
Anandharaj M, Sivasankari B, Rani RP (2014) Effects of probiotics, prebiotics, and synbiotics on hypercholesterolemia: a review. Chin J Biol Article ID 572754
Bach A, Valls N, Solans A, Torrent T (2008) Associations between nondietary factors and dairy herd performance. J Dairy Sci 91:3259–3267
Balcazar JL (2003) Evaluation of probiotic bacterial strains in Litopenaeus vannamei: final report. National Center for Marine and Aquaculture Research, Guayaquil, Ecuador
Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gomez-Llorente C, Gil A (2012) Probiotic mechanisms of action. Ann Nutr Metab 61:160–174
Bernardeau M, Vernoux JP (2013) Overview of differences between microbial feed additives and probiotics for food regarding regulation, growth promotion effects and health properties and consequences for extrapolation of farm animal results to humans. Clin Microbiol Infect 19:321–330
Borchers AT, Selmi C, Meyers FJ, Keen CL, Gershwin ME (2009) Probiotics and immunity. J Gastroenterol 44:26–46
Bouchard DS, Rault L, Berkova N, Loir YL, Even S (2013) Inhibition of Staphylococcus aureus invasion into bovine mammary epithelial cells by contact with live Lactobacillus casei. Appl Environ Microbiol 79(3):877–885
Bron PA, Van Baarlen P, Kleerebezem M (2012) Emerging molecular insights into the interaction between probiotics and the host intestinal mucosa. Nat Rev Microbiol 10:66–78
Brown M (2011) Modes of action of probiotics: recent developments. J Anim Vet Adv 10(14):1895–1900
Carnevali O, de Vivo L, Sulpizio R et al (2006) Growth improvement by probiotic in European sea bass juveniles (Dicentrarchus labrax, L.), with particular attention to IGF-1, myostatin and cortisol gene expression. Aquac 258(1–4):430–438
Chaucheyras-Durand F, Durand H (2010) Probiotics in animal nutrition and health. Benef Microbes 1(1):3–9
Chiba Y, Shida K, Nagata S, Wada M, Bian L, Wang C et al (2009) Well-controlled proinflammatory cytokine responses of Peyer’s patch cells to probiotic Lactobacillus casei. Immunol 130:352–362
Chiquette J (2009) The role of probiotics in promoting dairy production. WCDS Adv Dairy Technol 21:143–157
Cruz PM, Ibanez AL, Hermosillo OAM, Saad HCR (2012) Use of probiotics in aquaculture. ISRN Microbiol Article ID 916845
De Sousa VMC, dos Santos EF, Sgarbieri VC (2011) The importance of prebiotics in functional foods and clinical practice. Food Nutr Sci 2(2):133–144
Delcenserie V, Martel D, Lamoureux M, Amiot J, Boutin Y, Roy D (2008) Immunomodulatory effects of probiotics in the intestinal tract. Curr Issues Mol Biol 10:37–54
Drouault-Holowacz S, Bieuvelet S, Burckel A (2008) A double blind randomized controlled trial of a probiotic combination in 100 patients with irritable bowel syndrome. Gastroenterol Clin Biol 32:147–152
Enck P, Zimmermann K, Menke G et al (2008) A mixture of Escherichia coli (DSM 17252) and Enterococcus faecalis (DSM 16440) for treatment of the irritable bowel syndrome- a randomized controlled trial with primary care physicians. Neurogastroenterol Motil 20:1103–1109
Figueroa-Gonzalez I, Quijano G, Ramirez G, Cruz-Guerrero A (2011) Probiotics and prebiotics-perspectives and challenges. J Sci Food Agric 91:1341–1348
Fontana L, Bermudez-Brito M, Plaza-Diaz J, Munoz-Quezada S, Gil A (2013) Sources, isolation, characterization and evaluation of probiotics. Br J Nutr 109:S35–S50
Ghosh S, Sinha A, Sahu C (2007) Effect of probiotic on reproductive performance in female live bearing ornamental fish. Aquac Res 38(5):518–526
Gill HS, Guarner F (2004) Probiotics and human health: a clinical perspective. Postgrad Med J 80:516–526
Goel R, Vedi A, Goyal P (2014) Probiotics: contribution to oral health. J Orofac Health Sci 5(1):12–16
Goldin BR, Gorbach SL (2008) Clinical indications for probiotics: an overview. Clin Infect Dis 46:S96–S100
Goudarzi M, Goudarzi H, Rashidan M (2014) Probiotics: an update on mechanisms of action and clinical applications. Novel Biomed 2:22–30
Grin PM, Kowalewska PM, Alhazzani W, Fox-Robichaud AE (2013) Lactobacillus for preventing recurrent urinary tract infections in women: meta-analysis. Can J Urol 20(1):6607–6614
Guarino A, Vecchio AL, Canani RB (2008) Probiotics as prevention and treatment for diarrhea. Curr Opin Gastroenterol 25:18–23
Guo Z, Liu XM, Zhang QX, Shen Z, Tian FW, Zhang H et al (2011) Influence of consumption of probiotics on the plasma lipid profile: a meta-analysis of randomized controlled trials. Nutr Metab Cardiovasc Dis 21(11):844–850
Hardy H, Harris J, Lyon E, Beal J, Foey AD (2013) Probiotics, prebiotics and immunomodulation of gut mucosal defences: homeostasis and immunopathology. Nutr 5:1869–1912
Hemaiswarya S, Raja R, Ravikumar R, Carvalho IS (2013) Mechanism of action of probiotics. Braz Arch Biol Technol 56(1):113–119
Hoveyda N, Heneghan C, Mahtani KR, Perera R, Roberts N, Glasziou P (2009) A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. BMC Gastroenterol 9:15
Isolauri E, Arvola T, Sutas Y, Moilanen E, Salminen S (2000) Probiotics in the management of atopic eczema. Clin Exp Allerg 30(11):1604–1610
Jacela JY, De Rouchey JM, Tokach MD, Goodband RD, Nelssen JL, Renter DG et al (2009) Feed additives for swine: fact sheets-carcass modifiers, carbohydrate-degrading enzymes and proteases, and anthelmintics. J Swine Health Prod 17(6):325–332
Jeon SG, Kayama H, Ueda Y, Takahashi T, Asahara T, Tsuji H et al (2012) Probiotic Bifidobacterium breve induces IL-10-producing Tr 1 cells in the colon. PLoS Pathog 8(5), e1002714
Kechagia M, Basoulis D, Konstantopoulou S, Dimitriadi D, Gyftopoulou K, Skarmoutsou N, Fakiri EM (2013) Health benefits of probiotics: a review. ISRN Nutr Article ID 481651
Kenny M, Smidt H, Mengheri E, Miller B (2011) Probiotics- do they have a role in the pig industry? Anim 5(3):462–470
Khan RU, Naz S (2013) The applications of probiotics in poultry production. World’s Poult Sci J 69:621–632
Kral M, Angelovicova M, Mrazova L (2012) Application of probiotics in poultry production. Anim Sci Biotechnol 45(1):55–57
La Ragione RM, Casula G, Cutting SM, Woodward MJ (2001) Bacillus subtilis spores competitively exclude Escherichia coli O78:K80 in poultry. Vet Microbiol 79:133–142
Lakshmi B, Viswanath B, Sai Gopal DVR (2013) Probiotics as antiviral agents in shrimp aquaculture. J Pathog Article ID 424123
Lara F, Olvera N, Guzman M, Lopez M (2003) Use of the bacteria Streptococcus faecium and Lactobacillus acidophilus, and the yeast Saccharomyces cerevisiae as growth promoters in Nile tilapia (Oreochromis niloticus). Aquac 216(1–4):193–201
Lobo V, Patil A, Phatak A, Chandra N (2010) Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev 4(8):118–126
Maurya P, Mogra R, Bajpai P (2014) Probiotics: an approach towards health and disease. Trends Biosci 7(20):3107–3113
Mishra DK, Verma AK, Agarwal N, Mondal SK, Singh P (2014) Effect of dietary supplementation of probiotics on growth performance, nutrients digestibility and fecal microbiology in weaned piglets. Anim Nutr Feed Technol 14:283–290
Mishra V, Shah C, Mokashe N, Chavan R, Yadav H, Prajapati J (2015) Probiotics as potential antioxidants: a systematic review. J Agric Food Chem. doi:10.1021/jf506326t
Mitropoulou G, Nedovic V, Goyal A, Kourkoutas Y (2013) Immobilization technologies in probiotic food production. J Nutr Metab Article ID 716861
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 9(1):2–14
Ng SC, Hart AL, Kamm MA, Stagg AJ, Knight SC (2009) Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15(2):300–310
Pandiyan P, Balaraman D, Thirunavukkarasu R, George EGJ, Subaramaniyan K, Manikkam S, Sadayappan B (2013) Probiotics in aquaculture. Drug Invent Today 5:55–59
Parracho H, Mc Cartney AL, Gibson GR (2007) Probiotics and prebiotics in infant nutrition. Proc Nutr Soc 66:405–411
Parvaneh K, Jamaluddin R, Karimi G, Erfani R (2014) Effect of probiotics supplementation on bone mineral content and bone mass density. Sci World J Article ID 595962
Parvez S, Malik KA, Ah Kang S, Kim HY (2006) Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 100:1171–1185
Rastogi P, Saini H, Dixit J, Singhal R (2011) Probiotics and oral health. Natl J Maxillofac Surg 2(1):6–9
Rengpipat S, Rukpratanporn S, Piyatiratitivorakul S, Menasaveta P (2000) Immunity enhancement in black tiger shrimp (Penaeus monodon) by probiotic bacteria (Bacillus S11). Aquac 191:271–288
Ritchie ML, Romanuk TN (2012) A meta-analysis of probiotic efficacy for gastrointestinal diseases. PLoS ONE 7(4), e34938
Saulnier DMA, Spinler JK, Gibson GR, Versalovic J (2009) Mechanisms of probiosis and prebiosis: considerations for enhanced functional foods. Curr Opin Biotechnol 20(2):135–141
Seal BS, Lillehoj HS, Donovan DM, Gay CG (2013) Alternatives to antibiotics: a symposium on the challenges and solutions for animal production. Anim Health Res Rev 14(1):78–87
Sharma SK, Joshi VK, Sharma S (2012) Probiotics: concepts and applications in food. In: Joshi VK, Singh RS (eds) Food biotechnology: principles and practices. IK International Publishing House Pvt Ltd, India, pp 781–798
Singh K, Kallali B, Kumar A, Thaker V (2011) Probiotics: a review. Asian Pac J Trop Biomed S287-S290
Stapleton AE, Au-Yeung M, Hooton TM et al (2011) Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis 52:1212–1217
Tovar D, Zambonino J, Cahu C, Gatesoupe F, Vazquez R, Lesel R (2002) Effect of live yeast incorporation in compound diet on digestive enzyme activity in sea bass (Dicentrarchus labrax) larvae. Aquac 204(1–2):113–123
Twetman S, Keller MK (2012) Probiotics for caries prevention and control. Adv Dent Res 24(2):98–102
Uccello M, Malaguarnera G, Basile F, D'agata V, Malaguarnera M, Bertino G et al (2012) Potential role of probiotics on colorectal cancer prevention. BMC Surg 12(Suppl 1):S35
Videlock EJ, Cremonini F (2012) Meta-analysis: probiotics in antibiotic-associated diarrhea. Aliment Pharmacol Ther 35:1355–1369
Whyand TL, Caplin ME (2014) Review of the evidence for the use of probiotics in gastrointestinal disorders. J Gastroenterol Pancreatol Liver Disord 1(4):1–9
Yaqoob P (2014) Ageing, immunity and influenza: a role for probiotics? Proc Nutr Soc 73:309–317
Zuccotti G, Meneghin F, Aceti A, Barone G, Callegari ML, Di Mauro A et al (2015) Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allerg. doi:10.1111/all.12700
Acknowledgments
The authors express their gratitude to the Principal, Lady Keane College, Shillong and the DBT-Institutional Biotech Hub, Lady Keane College, Shillong for the generosity in providing the facilities during the preparation of this manuscript. The authors are also thankful to Kitriphar Tongper and Ananya Barman for some of the resources provided.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Syngai, G.G., Gopi, R., Bharali, R. et al. Probiotics - the versatile functional food ingredients. J Food Sci Technol 53, 921–933 (2016). https://doi.org/10.1007/s13197-015-2011-0
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-015-2011-0