Abstract
In the present study sweet potato starch was treated with hydrochloric acid or citric acid at 1 or 5 % concentration and its properties were investigated. Citric acid treatment resulted higher starch yield. Water holding capacity and water absorption index was increased with increased acid concentration. Emulsion properties improved at 5 % acid concentration. The DE value of acid-thinned sweet potato starches was ranged between 1.93 and 3.76 %. Hydrochloric acid treated starches displayed a higher fraction of amylose. X-ray diffraction (XRD) study revealed that all the starches displayed C-type crystalline pattern with varied crystallinity. FT-IR spectra perceived a slight change in percentage intensity of C-H stretch of citric acid modified starches. Starch granules tended to appear less smooth than the native starch granules after acid treatment in Scanning Electron Micrographs (SEM) with granule size ranging between 8.00 and 8.90 μm. A drastic decrease in the pasting profile was noticed in hydrochloric acid (5 %) treated starch. While 5 % citric acid treated starch exhibited higher pasting profile. Differential Scanning Calorimeter (DSC) showed that peak and conclusion gelatinisation temperatures increased with increase in hydrochloric acid or citric acid concentration. Hence citric acid was found to mimic the hydrochloric acid with some variation which suggests that it may have promising scope in acid modification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Starches are commonly modified by physical, chemical or enzymatic methods. Acid treatment is one type of chemical modification (BeMiller and Whistler 2009). Acid modified starches are prepared industrially by treating the starch slurry (40 %) with mineral acids at 25–55 °C for a range of time periods (Hoover 2000). Acid hydrolysis changes the physicochemical properties of starch without destroying granular structure. The amorphous regions of the starch granules are more susceptible to acid hydrolysis than the crystalline regions. Acid treatment increases the gelatinization parameters (gelatinization temperature and enthalpy). The retrogradation tendency of acid-thinned starch also increases due to increase in the concentration of the linear chains (Shi and Seib 1992). Acid modification allows the starch to be used at higher solids concentration for quick gelling and provides gum or jelly with shorter texture and flexible properties (Zallie 1988). They can also be employed as fat mimetic or stabilizers in sausages and dressings (Omojola et al. 2011). Hydrochloric acid and sulphuric acid are the generally used mineral/inorganic acids (Pomeranz 1991). Hydrochloric acid is industrially used inorganic acid for acid treatment of starch but it generates toxic fumes. After neutralization, high cost ion exchange resins are required for desalinating the syrup (Fontana et al. 2008). Inorganic acids may depart a lot of side products that slow down the progress of hydrolysis (Peppler and Perlman 1980) and indeed they cause some health concern among the consumers. As far as industries are concern the situation is far more difficult because in spite of extensive washing some residual protein and fat of bounded starch react with HCl to produce coloured products like bitter millard compounds which carries additional cost for its removal (Fontana et al. 2008). Hence in this scenario, we certainly required a natural alternative for the modification of starch with low cost. Citric acid is renowned as nutritionally safe compared to other substance used for modification of starch (Xie and Liu 2004).
Sweet potato (Ipomoea batatas (L.)) belongs to family Convolvulaceae (IPC 2010). Sweet potato is one of the worlds most significant and under-exploited tubers (Sathe and Salunkhe 1999). Sweet potato starch can be extracted simply by the combination of grinding and wet separation techniques. Starch granule size varies from less than 1 μm to more than 100 μm. Sweet potato starch granules have been reported as round, oval, and polygonal shapes (Hoover 2001). Industrial usage of this tuber is very low causing negative growth in production (Srivastava et al. 2012). Modification of sweet potato starch by physical and chemical methods may make it more suitable for use in traditional products that normally use other type of starches. Hence the present study was aimed to compare the effect of citric acid or hydrochloric acid modification on sweet potato starch properties.
Materials and methods
Preparation of acid modified starches
Sweet potato tubers were obtained from local market in Salem, Tamil Nadu, India and starch was isolated by the method of Wickramasinghe et al. (2009). Sweet potato starch was dispersed in 1 % (0.27 mol/L) or 5 % (1.38 mol/L) hydrochloric acid or 1 % (0.05 mol/L) or 5 % (0.26 mol/L) citric acid and allowed to proceed for 1 h at 45 °C in a water bath with constant stirring. After hydrolysis, the solution was immediately neutralised initially with 1 M sodium hydroxide and finally adjusted to pH 7.0 with 0.1 M sodium hydroxide. Starch was washed three times with the deionised water prior to filtration and dried in a convection oven at 45 °C for 48 h (Zambrano and Camargo 2002). The dried starch was made into powder and recovery yield was calculated by the following formula.
Starches were marked as A and B for 1 and 5 % Hydrochloric acid treated starches whereas C and D for 1 and 5 % of citric acid treated starches respectively.
Chemical properties of native and acid treated starches
Dextrose equivalent (DE)
The reducing sugar value was measured using the dinitrosalicylic acid method Miller (1959) to determine its dextrose equivalent (DE).
Amylose content
Amylose content determination was carried out using a colorimetric iodine affinity procedure (Williams et al. 1958), briefly a mixture of 0.1 g of the starch sample, 1 ml of ethanol and 9 ml 1 N sodium hydroxide were boiled for 10 min in a boiling water bath and allowed to cool. To a portion (5 ml) of the mixture, 1 ml of 1 N acetic acid and 2 ml of iodine solution were added and absorbance (A) was read using a Spectrophotometer at 620 nm. The amylose content was calculated as:
Functional properties of native and acid treated starches
Water Holding Capacity (WHC) and Water Absorption Index (WAI)
Water holding capacity and water absorption index were determined by the method described by Niba et al. (2002). Starch samples (1 g) were suspended in 5 mL water in a centrifuge tube. The slurry was shaken on a platform tube rocker for 1 min at room temperature and centrifuged at 3000×g for 10 min. The supernatant was poured carefully into a tared evaporating dish.
Water holding capacity was calculated as follows (g/g):
Water absorption index was calculated as follows (g/g):
Emulsifying properties
The emulsifying activity and stability of starch samples were determined by the methods of Neto et al. (2001). Starch dispersion (10 mg/mL) was homogenized (1 min) with 5 mL oil (refined sunflower oil). The emulsions were centrifuged (1100 g, 5 min) and the height of the emulsified layer and the total contents in the tube was determined. The emulsifying activity was calculated.
Emulsion stability was determined by heating the emulsion (80 °C, 30 min) before centrifuging (1100 g, 5 min)
Pasting properties of native and acid modified starches
Pasting properties of starch samples were evaluated with a Rapid Visco Analyzer (RVA) (RVA Tech Master, Perten Instruments, Japan). The viscosity profiles were recorded using starch suspensions (12 % w/v). The Std1 profile of Perten Instruments was used, where the samples were held at 50 °C for 1 min, heated from 50 to 95 °C at 12.16 °C/min, held at 95 °C for 2.30 min, cooled from 95 to 50 °C at 11.84 °C/min, and held at 50 °C for 2 min. The peak viscosity (PV), break down (BD) Trough Viscosity (TV), Set Back (SB), Final Viscosity (FV), Pasting Time (PT), Pasting Temperature (PT) were recorded.
Structural properties of native and acid modified starches
Powder X-ray Diffraction (XRD)
X-ray diffraction patterns of starch samples were obtained using a Powder X-ray Diffractometer (Rigaku Mini Hex-II, Japan). The scanning range was 2–60° of 2θ values with a scan speed of 8° 2θ/min. The percentage crystallinity of the starches was calculated by using the method of Nara and Komiya (1983) with the Origin - version 6.0 software (Microcal Software, Inc., Northampton, MA 01060 USA).
Scanning Electron Microscope (SEM)
Starch granules were observed using a Scanning Electron Microscope (SEM) (JEOL-Model 6390, Japan) operating at an acceleration voltage of 5–10 kV. Granule size was determined by using ImageJ 1.46r (National Institute of Health, USA) software.
FT-IR spectroscopy
The infra-red spectra for all the starch samples were attained with a FT-IR spectrometer (Brucker, Tensor-27). The equipment was operated with a resolution of 2.0 cm−1 and scanning range of 4000–370 cm−1.
Thermal analysis of native and acid modified starches
The thermal properties of starches were measured using a Differential Scanning Calorimeter (DSC 6000- PerkinElmer, USA). A 4.5 mg sample (dry basis) was weighed in an aluminium pan and 10 mL of deionised water was added. The pan was sealed tightly and then it was allowed to stand for 1 h before carrying out the analysis. An empty aluminium pan was used as reference. The sample was subjected to a heating program over a range of temperature from 10 to 125 °C and a heating rate of 5 °C/min. The onset, peak, and final temperatures (To, Tp, and Tc, respectively) and transition enthalpy (∆H) were determined.
where ∆H is the enthalpy change of reaction, m is the mass of the sample at the beginning of the experiment, K is the calibration coefficient, and A is the area under the peak (Hoffman and Pan 1991).
Statistical analysis
All data obtained were subjected to Two Way Analysis of Variance (ANOVA) using MS Excel 2007 and means were compared by Critical Difference (CD). Difference at P < 0.05 was considered to be significant.
Results and discussion
Recovery yield of citric acid and hydrochloric acid modified sweet potato starch was above 93 % (Table 1) as reported in the study of Lin et al. (2003). With increase in acid concentration yield was reduced as starches might be hydrolyzed more rapidly at higher acid concentration (5 %).
Chemical properties of native and acid modified starches
Dextrose Equivalent (DE) value is an indication of extent of acid hydrolysis. The DE value of acid-thinned sweet potato starches (Table 1) was ranged between 1.93 and 3.76 %. Since A and B starches were treated with HCl (0.27 mol/L and 1.38 mol/L) they found to exhibit a higher DE value of 2.56 and 3.76 % respectively compared to 1.93 and 2.21 % of citric acid modified C (0.05 mol/L) and D (0.26 mol/L) starches. Thys and Aires (2013) reported a DE value of 6.5 for pinhão starch treated with HCl (3.20 mol/L).
Amylose content of sweet potato native starch was 18.56 % which was on par with Tsakama et al. (2011). Acid treated starches found to display a higher fraction of amylose which was significant with native starch. The highest amylose (31.05 %) content was noticed for B starch. Amylose content was increased with increased acid concentration for both kind of acid hydrolysis. The possible reason may be due to the de-polymerization of amylopectin fractions at a higher acid concentration. Increase in degree of acid hydrolysis can lead to an increase in the amylose content of starch (Betancur and Chel 1997) hence D starch had higher amylose than its counter ones.
Functional properties of native and acid modified starches
WHC of native and 1 % acid treated starches (A & C) was in the similar range varying from 0.78 to 0.80 g/g (Table 2). However samples B and D treated with 5 % acid concentration displayed a higher WHC (1.42 g/g). High acid concentration probably increased the low molecular weight starch fraction with hydroxyl groups which may hold water molecules forming hydrogen bonds consequently increasing the WHC. This high water holding capacity of citric acid treated starch may find a significant role as fat replacer. No significant difference in WAI among native, A, B and C samples was noticed. Sample D displayed a higher WAI of 2.46 g/g similar trend was observed by Amaya-Llano et al. (2008) in case of Jicama starch. The higher water absorption index (WAI) of starch sample D was could be due to higher disruption of crystalline structure after acid treatment. Hence disrupting the crystalline structure of the starch tends to pay a way for the interaction of hydrogen bonding between starch and water, thereby increasing the WAI. This result was confirmed by the lower crystallinity of D starch (34.35 %).
Emulsion activity and emulsion stability of starch samples were displayed in Tables 2. Hydrochloric acid (5 %) treated B starch exhibited a higher emulsion activity (70.76 %) and emulsion stability (44.55 %) followed by A starch (66.93 & 43.18 %), citric acid treated D (67.03 & 44.51 %) and C starches (64.99 & 41.60 %) compared to native starch (54.44 & 42.99 %). Starches (B & D) with higher amylose found to exhibit superior emulsion properties. The present study revealed that higher amount of linear amylose fraction would contribute to emulsion activity. The high amylose starch may function as the interface between oil and water during which linear amylose chains of starch granules might be more favoured in stabilizing the emulsion system than branched amylopectin chains. The linear amylose fractions might be capable of film formation which would enhance the emulsion capacity and stability of the starch. Hence B starch having higher amylose (31.05 %) showed superior emulsion properties than other starches.
Pasting profile of native and acid modified starches
Peak viscosity of native sweet potato starch was 3421.50 cP (Table 4). A drastic reduction in peak viscosity up on 5 % hydrochloric acid treatment was noticed which may be due to break down of the glycoside linkages of the long amylopectin chains. Starches treated with HCL found to have a reduced viscosity; this trend was likely due to an extensive disruption of amorphous region in starch granule and conversion of amylose to low molecular weight chains (Abdorreza et al. 2012). D starch exhibited the highest peak viscosity of 5396.50 cP than their counter parts. Trough viscosity of hydrochloric acid or citric acid treated starches ranged from 15.50–1583.50 cP to 1917.50–3127 cP respectively. Acid modified starch showed high peak and trough viscosity than native starch similar pattern was reported by Balasubramanian et al. (2014). D starch displayed the highest break down (2269.50 cP) value while B starch showed the lowest break down (19 cP). The difference in susceptibility of starch granule to heat and variation in the amylose leeching might attribute to the difference in break down value among the starches. The final viscosity (3991.50–4201.50 cP) and set back (1074.50–2074 cP) of C and D starches were greater than native, A and B starches. D starch experienced high peak time (4.76 min) to reach its peak viscosity than other starches. Citric acid modified starch showed low pasting temperature compared to native and hydrochloric acid modified starch. Since citric acid is a weaker acid compared to hydrochloric acid mild hydrolysis might occur at the amorphous region of starch. When citric acid is heated it dehydrates to form an anhydride on further heating may result in dehydration with forming cross-linking with other starch molecules (Wing 1996). The formation of citrate complex probably strengthens starch-starch interactions that require most heat for disruption of starch and it results in higher pasting characteristics of C and D starches predominantly D starch.
Structural properties of native and acid treated starches
X-ray diffractogram of the native and acid modified sweet potato starches are presented in Fig. 1. Sweet potato starches showed peaks at the 2θ diffraction angles around 10, 11, 15, 17, 20 and 23 (Table 2). These patterns were considered as characteristic feature of sweet potato starch, which is C-type pattern that is a combination of A and B type crystalline structures. Previous literature (Osundhahunsi et al. 2003) showed that sweet potato starch had 2θ values at 15.4°, 17.2°, 18.3° and 23.4°. X-ray diffraction pattern of sweet potato starch was not altered after treatment of sweet potato starch with different acids with increased concentration. Nevertheless, the peak intensities and 2θ values showed distinct variation between native and acid modified starches. Citric acid treatment slightly increased the 2θ values of sweet potato starch (Table 3). Crystallinity of native, A, B, C and D starches was 35.46 %, 38.85 %, 39.04 %, 38.18 % and 34.35 % respectively. Acid treatment increased the crystallinity of sweet potato starch. Increase in crystallinity was noticed for acid treated jackfruit starch by Dutta et al. (2011). With increase in hydrochloric acid concentration crystallinity of sweet potato starch was increased, this pattern wasn’t noticed in case of citric acid. This may be due to rapid amylose hydrolysis and the concentration of amylopectin in the B starch.
Scanning Electron Micrograph (SEM) of the sweet potato native starch was illustrated in Fig. 2. Illustration of starch sample showed the presence of starch granules from small to large sizes. Granule surface of starches appeared to be smooth with no sign of any fissure. Zhu et al. (2011) also observed smooth granule surface of sweet potato starches without cracks. Most of the sweet potato starch granules were polygonal in shape, however round and irregular shapes were also noted. After acid modifications, starch granules appear less smooth than the native starch granules (depicted by arrows). Granules appeared to be similar with no obvious change in both HCl and citric acid treated starches. The size of both the native and acid modified starch granules was ranged between 8.00 and 8.90 μm. Similar range of granular size was reported by Singh et al. (2005) for acid modified sweet potato starch. No significant difference was observed in the granule size among the starches.
The FT-IR spectra of native and acid treated sweet potato starches were illustrated in the Fig. 3. In the present study all the characteristic peaks observed in native starch was noticed in both hydrochloric acid treated (A & B) and citric acid (C & D) treated starches. Peak at 900–950 cm−1 in the infrared spectrum of all the starch samples is an evidence for the vibration of the glycosidic linkage. A distinctive peak was aroused at 1648 cm-1, which may be due to the presence of the water molecules bounded to the starch (Fang et al. 2002). A strong, broad band between 3000 and 3700 cm−1 was noticed which corresponds to the O-H stretch. The sharp band at 2932 cm−1 is characteristic of C-H stretches associated with the ring hydrogen atoms. The percentage intensity of C-H stretch of acid modified starches was decreased slightly compared to the native sweet potato starch. This trend was more pronounced in C and D starch samples than A and B samples. Intensity changes in C-H stretch range may be attributed to the change in the amylose and amylopectin content of starch molecule (Young 1984). Dutta et al. (2011) indicated gradual changes in the positions of the peak between 2970 and 2910 for acid treated jackfruit seed starch.
Thermal analysis of native and acid treated starches
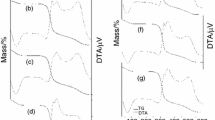
Thermal properties of starches determined by the DSC are represented in Table 5. Results showed variations in onset (To), peak (Tp), conclusion (Tc) temperatures and enthalphy (ΔH) among native and acid treated starches. Native starch has higher onset temperature of 42.31 °C while hydrochloric acid treated starches
A (41.08 °C) and B (35.04 °C) and citric acid treated starches C (35.81 °C) and D (35.35 °C) recorded a reduced onset temperature. This was in agreement with the result reported for acid modified maize starch which showed a decreased To value (Lin et al. 2003). The Tp and Tc of native starch were 81.25 °C and 124.96 °C respectively conversely the acid modified starches displayed a higher Tp and Tc temperatures. However this trend was more pronounced in case of hydrochloric acid treated starches ranging from 84.50 to 86.50 °C of Tp and 125.95 to 126.75 of Tc. Singh et al. (2005) stated that higher gelatinisation temperatures in acid modified starches was due to higher degree of hydrolysis that might occur in starches at amorphous region, thus resulting in an increase in relative crystallinity and subsequently an increase in the gelatinisation temperature. This might be the possible reason for the high Tp and Tc values of acid treated starches. Similar results were reported for acid modified potato starch (Wang and Wang 2001) and sweet potato starch (Singh et al. 2005). On the other hand ∆H of acid modified starches were lower than their counter part native starches. A and B starches (11.14 J/g & 10.78 J/g) showed lower enthalphy than C and D starches (12.74 J/g & 12.13 J/g). The ΔH gives an overall measure of crystallinity and is an indicator of the loss of molecular order within the granule during gelatinisation (Hoover and Vasanthan 1994). The higher concentration of hydrochloric acid may be resulted in greater loss of ordered structure of starch than citric acid treated starch.
Conclusion
The present study concluded the properties of acid modified starches depend on the acid concentration and type of acid used for starch hydrolysis. The properties of acid modified sweet potato starches were significantly different from native starch. The B starch showed a higher DE of 3.76 % indicating a higher degree of acid hydrolysis. Citric acid modified starch had higher water holding capacity (1.42 ml/g), water absorption index (2.46 g/g) and superior emulsion properties than hydrochloric treated starches. Acid hydrolysis increased amylose content. A & B starches showed lower pasting properties while C & D starches exhibited higher viscosity profile than native starch. Native and modified starches showed a C-type crystalline pattern. After acid modifications, starch granules tended to appear less smooth than the native starch granules. Thermal analysis showed that increased acid concentration raised the gelatinisation temperatures (Tp & Tc) and decreased the enthalpy. The DE value can also be controlled in case of citric acid as it a weak acid than hydrochloric acid. The study concludes that citric acid can be potentially applicable for acid treatment of starch as citric acid treated starch showed enhanced functional and pasting properties with less structural damage to the starch granule.
References
Abdorreza MN, Robal M, Cheng LH, Tajul AY, Karim AA (2012) Physicochemical, thermal, and rheological properties of acid-hydrolyzed sago (Metroxylon sagu) starch. LWT-Food Sci Technol 46:135–141
Amaya-Llano SL, Martinez-Alegr AL, Zazueta-Morales JJ, Martinez-Bustos F (2008) Acid thinned jicama and maize starches as fat substitute in stirred yogurt. LWT–Food Sci Technol 41:1274–1281
Balasubramanian S, Sharma R, Kaur J, Bhardwaj N (2014) Characterization of modified pearl millet (Pennisetum typhoides) starch. J Food Sci Technol 51:294–300
BeMiller JN, Whistler RL (2009) Starch: chemistry and technology. In: Taylor SL (ed) Food science and technology international series, 3rd edn. Academic, New York, p 879
Betancur AD, Chel GL (1997) Acid hydrolysis and characterization of Canavalia ensiformis starch. J Agric Food Chem 45:4237–4241
Dutta H, Paul SK, Kalita D, Mahanta CL (2011) Effect of acid concentration and treatment time on acid—alcohol modified Jackfruit seed starch properties. Food Chem 128:284–291
Fang JM, Fowler PA, Tomkinson J, Hill CAS (2002) The preparation and characterization of series of chemically modified potato starches. Carbohydr Polym 47:245–252
Fontana JD, Mitchell DA, Molina OE, Gaitan A, Bonfim TMB, Adelmann J, Grzybowski A, Passos M (2008) Starch depolymerization with diluted phosphoric acid and application of the hydrolysate in Astaxanthin fermentation. Food Technol Biotechnol 46:305–310
Hoffman R, Pan W-P (1991) Measuring ∆H using TG-DTA and incorporating mass change into the result. Thermochim Acta 19:2135–2146
Hoover R (2000) Acid-treated starches. Food Rev Int 16:369–392
Hoover R (2001) Composition, molecular structure, and physicochemical properties of tuber and root starches: a review. Carbohydr Polym 45:253–267
Hoover R, Vasanthan T (1994) Effect of heat moisture treatment on the structure and physicochemical properties of cereal, legume and tuber starches. Carbohydr Res 252:33–53
International Potato Center. http://www.cipotato.org/sweetpotato/.Assessed on November 29th, 2010
Lin JH, Lee SY, Chang YH (2003) Effect of acid—alcohol treatment on the molecular structure and physicochemical properties of maize and potato starches. Carbohydr Polym 53:475–482
Miller GL (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal Chem 31:426–428
Nara S, Komiya T (1983) Studies on the relationship between water—saturated state and crystallinity by the diffraction method for moistened potato starch. Starch-Starke 35:407–410
Neto VQ, Narain N, Silvia JB, Bora PS (2001) Functional properties of raw and heat-processed cashew nut (Anacardium occidentale L.) kernel protein isolate. Nahrung 45:258–262
Niba LL, Bokanga MM, Jackson FL, Schlimme DS, Li BW (2002) Physicochemical properties and starch granular characteristics of flour from various Manihot Esculenta (Cassava) genotypes. J Food Sci 67:1701–1705
Omojola MO, Manu N, Thomas SA (2011) Effect of acid hydrolysis on the physicochemical properties of cola starch. Afr J Pure Appl Chem 5:307–315
Osundhahunsi OF, Fagbemi TN, Kesselman E, Shimoni E (2003) Comparison of the physico-chemical properties and pasting characteristics of flour and starch from red and white sweet potato cultivars. J Agric Food Chem 51:2232–2236
Peppler HJ, Perlman D (1980) Microbial Processes, Vol 1, 2nd edn. Academic, New York, pp 30–64
Pomeranz Y (1991) Functional properties of food components. Academic, New York, pp 24–77
Sathe SK, Salunkhe DK (1999) Functional properties of the great northern bean protein: emulsion, foaming, viscosity and gelation properties. J Food Sci 46:71–76
Shi Y-C, Seib PA (1992) The structure of four waxy starches related to gelatinization and retrogradation. Carbohydr Res 227(131):145
Singh S, Raina CS, Bawa AS, Saxena DC (2005) Effect of heat-moisture treatment and acid modification on rheological, textural, and differential scanning calorimetry characteristics of sweet potato starch. J Food Sci 70:E373–E378
Srivastava S, Genitha TR, Yadav V (2012) Preparation and quality evaluation of flour and biscuit from sweet potato. J Food Process Technol 3:1–5
Thys RCS, Aires AG (2013) The effect of acid hydrolysis on the technological functional properties of pinhão (Araucaria brasiliensis) starch. Ciênc Tecnol Aliment Campinas 33:89–94
Tsakama M, Mwangwela AM, Manani TA, Mahungu NM (2011) Effect of heat moisture treatment on physicochemical and pasting properties of starch extracted from 11 sweet potato varieties. Int Res J Agric Sci Soil Sci 1:254–260
Wang L, Wang YJ (2001) Structure and physicochemical properties of acid- thinned corn, potato and rice starches. Starch-Starke 53:570–576
Wickramasinghe HAM, Takigawa S, Matsura-Endo G, Yamauchi H, Noda T (2009) Comparative analysis of starch properties of different root and tuber crops Sri Lanka. Food Chem 112:98–103
Williams VR, Wu W, Tsai HY, Bates HG (1958) Varietal differences in amylose content of rice starch. J Agric Food Chem 6:47–48
Wing RE (1996) Starch citrate preparation and ion exchange properties. Starch-Starke 48:275–279
Xie XJ, Liu Q (2004) Development of new resistant starch-citrate starch as a functional food ingredient. Starch-Starke 56:364–370
Young AH (1984) Fractionation of starch. In: Whistler RL, BeMiller JN, Paschall EF (eds) Starch: chemistry and technology. Academic, Orlando, pp 249–283
Zallie J (1988) Benefits of quick setting starches. Manuf Confect 66:41–43
Zambrano F, Camargo CRO (2002) Otimizacao das condicoes dehidrolise acida do amido de mandioca para obtencao de substituto degordura. Braz J Food Technol 4:147–154
Zhu F, Yang X, Cai Y, Bertoft E, Corke H (2011) Physicochemical properties of sweet potato starch. Starch-Starke 63:249–259
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Babu, A.S., Parimalavalli, R., Jagannadham, K. et al. Chemical and structural properties of sweet potato starch treated with organic and inorganic acid. J Food Sci Technol 52, 5745–5753 (2015). https://doi.org/10.1007/s13197-014-1650-x
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1650-x