Abstract
During the microwave treatment process of the milk, response surface methodology (RSM) based on three-level three-factorial Box-Behnken design was used. The response vitamin C concentration was studied. The predicted value of model (11.84 μg/mL) was in excellent accordance with experimental value (11.83 μg/mL). Milk layer thickness was the most significant factor that affects the measured responses, and the effects of microwave time and microwave power were dependent on milk layer thickness levels. The variables microwave time,milk layer thickness and microwave power have the opposite effect on vitamin C concentration in milk treated by microwave. Synergistic interactions between milk layer thickness and microwave power was highly significant (p < 0.0001).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitamin C (ascorbic acid) is on of the most important biologically active substances which humans should receive in a sufficient amount together with food. Vitamin C also has some impact upon the activity of ferments and other vitamins,stimulates regeneration of tissues and enhances immunity to infections. Vitamin C is also necessary for the absorption of iron (Giese 1995). Therefore, vitamin C is widely used as an additive to improve the quality of food products. It is known that vitamin C inhibits oxidation processes and can be used as an antioxidant (Padayatty et al. 2003; Giese 1995). Milk is one of the basic food products. However,the amount of vitamin C naturally available in milk is rather small. Vitamin C is among the least stable vitamins. That vitamin is hghly susceptible to oxidation. Metal ions,heat and light accelerate that process (Gil et al. 1999). Therefore,technological processes used for manufacturing milk products have an impact on the amount of vitamin C in the finished product. It is considered that the biggest amount of vitamin C is lost during sterilization process (Gliguem and Birlouez-Aragon 2005).
Microwave processing of milk has been proposed as an alternative to conventional heating methods (Sieber et al. 1989; Vasavada 1990; Young and Jolly 1990). To the effectiveness of microwave sterilization,milk layer thickness, microwave time and microwave power are major effect factors. Several works on microbiological and nutritional aspects to ascertain the safety and nutritional value of microwave-treated milk (Choi et al. 1993a; 1993b; Galuska et al. 1989; Khalil and Villota 1988; LopezFandino et al. 1996; Merin and Rosenthal 1984; Stearns and Vasavada 1986; Villamiel et al. 1996a; 1996b).
It is well known that one of the most serious threats to the nutritional value of milk is the destruction of very heat-sensitive vitamins,like vitamin C.In this sense,some studies on ascorbic acid changes during conventional and microwave processes have been carried out recently (Wernery et al. 2005; Donglin and Yasunori 2004).
Response surface methodology (RSM) can be used to evaluate the relative significance of several factors in the presence of complex interactions. It is a powerful technique for testing multiple-process variables because fewer experimental trials are needed as compared with the study of one variable at a time. RSM answers the question of how to select the levels for the applied factors to obtain the desirable, smallest or largest, value of the response function in a reduced number of experiments (Hanrahan and Lu 2006). This technique includes primary screening of variables by the application of “one variable at a time” method or Plackett-Burman design (Xin et al. 2005; Preetha et al. 2007) as well as using a design for fitting the chosen model (Myers and Montgomery 1995). The most popular approach is based on full factorial central composite design (CCD), which enables one to estimate the coefficients of a second-order model.
In this study, RSM combined with Box-Behnken design was used to study the vitamin C concentrations in microwave treated milk. By the use of three-level three-factorial Box-Behnken design and a full range of RSM, the following parameters were optimized: microwave power, microwave time, milk layer thickness. A second-order polynomial regression model was used to generate three-dimensional response surfaces of vitamin C concentrations. The regression model would provide a good explanation of the relationship between the independent variables and the response.
Materials and methods
Materials
Raw cow’s milk used in this research was obtained from the Experimental Farm of the Inner Mongolia Agricultural University. Milk was kept refrigerated at 4 ± 0.5 °C until it was processed. Methanol (chromatographic pure, Fisher Chemicals, USA),vitamin C (chromatographic pure, Sigma, USA). Other reagents were of analytical grade.
Experimental design
The three-level, three-factorial Box-Behnken experimental design with categoric factor of 0 was employed to study the effect of microwave based on the concentration of the vitamin C concentrations in milk (response). The design was composed of three levels (low, medium and high, being coded as −1, 0 and +1) and a total of 17 runs were carried out in duplicate to optimize the level of chosen variables, such as microwave power, microwave time, milk layer thickness. For the purpose of statistical computations,the three independent variables were denoted as x1, x 2,and x3, respectively. According to the preliminary experiments, the range and levels used in the experiments are selected and listed in Table 1. The experimental design matrix by the Box-Behnken design is tabulated in Table 2 and corresponding experiments were performed.
The results were analyzed by applying the coefficient of determination (R 2), response plots and analysis of variance (ANOVA). Central composite design was chosen to estimate the response calculated according to the following form of a second degree polynomial Eq. (1):
where Y is the estimated response, β0, βi, βii, andβij are the regression coefficients for the intercept, linearity, square, and interaction, respectively, X i and X j (i = 1–3, j = 1–3 and i ≠ j) are the different interaction coefficients between input factors. The equation expresses the relationship between the predicted response and independent variables in coded values according to Tables 1 and 2.
Vitamin C assay
The milk vitamin C concentration was measured by High Performance Liquid Chromatography (Agilent 1100, Agilent, USA) using the following procedure (Wernery et al. 2005).
Each milk sample was piptted into a 100 ml cylinder to which 1 g of citric acid was added. This solution was carefully mixed,and 50 ml of meta-phosphoric acid (1.5 g meta-phosphric acid in 100 ml of sterile distilled water,adjusted to pH3.5 with a 60%KOH solution)added. After mixing gently, distilled water was added to the 100 ml mark. This solution was incubated for 5 min at room temperature and filtered through 0.45 μm filter. The milk filtrate was then treated with Chromsystems reagents.
The setting of HPLC for vitamin C was as follows:
Column: Zorbax SB-C18 5 μm, 4.6 mm × 250 mm,Agilent, USA;
Mobile Phase: 0.015 mol/L HAc-NaAc buffer solution (pH3.5)/Methanol (95/5,V/V);
Column Temp.: 30 °C;
Injection Volume: 10 μL
Flow rate: 1.0 ml/min;
UVdetector wavelength: 254 nm.
Results and discussion
Statistical analysis
The effect experiments are conducted according to the design matrix and corresponding results are listed in Table 2. The quadratic equation for predicting the optimum point was obtained according to the Box-Behnken design and input variables, and then the empirical relationship between the response and the independent variables in the coded units was presented on the basis of the experimental results as follows:
where Y is the vitamin C concentration, X 1 , X 2 and X 3 is milk layer thickness, microwave time and microwave power, respectively.
The results of the analysis of variance (ANOVA) for the quadratic equation are tabulated in Table 3. The ANOVA indicates the equation and actual relationship between the response and significant variables represented by the equation are accurate. The significance of the coefficient term is determined by the values of F and p, and the larger the value of F and the smaller the value of p, the more significant is (Amini et al. 2008; Kalavathy et al. 2009). The p is lower than 0.05, suggesting the model is considered to be statistically significant (Kim et al. 2003). For the treated milk system, the ANOVA results indicated the F-value for the model was 45.83, suggesting that only a 0.01 % chance of a“Model F-value”so large could occur due to noise and the most of the variation in the response could be explained by the regression equation and that the model was significant. In addition, the probability p < 0.0001 also validated the model was significant.
In the present investigation, X 1 X 3 and X 2 2 were highly significant parameters, while X 1 , X 3 , X 1 X 2 , X 1 2and X 3 2 were significant factors. The other model terms were not significant (Table 3). Based on the analysis of Eq. (2) depicted that the variables microwave time,milk layer thickness and microwave power have the opposite effect on vitamin C concentration in milk treated by microwave. Synergistic interactions between milk layer thickness and microwave power was highly significant (p < 0.0001).On checking the R 2 values, the“Predicted R 2”of 0.7984 was in reasonable agreement with the “Adjusted R 2” of 0.9619. “Adequacy Precision” measures the signal to noise ratio. It is reported that a ratio greater than 4 is desirable (Muthukumar et al. 2003). The ratio of 22.486 suggested an adequate signal. As analyzed above, this model can be used to navigate the design space.
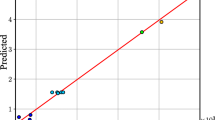
The data were analyzed to examine the correlation between the experimental (Y experimental ) and predicted responses, as given in Fig. 1. As can be seen that the data points were well distributed close to a straight line (R 2 = 0.9833), which suggested an excellent relationship between the experimental and predicted values of the response, and the underlying assumptions of the above analysis were appropriate. The results also indicated that the selected quadratic model was adequate in assuming the response variables for the experimental data.
Three-dimensional response surface plot
Vitamin C concentration in milk as the response, the threedimensional response surface plots are shown in Fig. 2. It was obvious that vitamin C concentration was sensitive to milk layer thickness and microwave power, which was consistent with those analyzed about Table 3.
Figure 2a depicted the three-dimensional response surface relationship between microwave power and microwave time on the vitamin C concentration in milk treated by microwave at milk layer thickness of 4 cm. The vitamin C concentration increased from 11.68 to 11.76 μg/mL and then decreased to 11.69 μg/mL at microwave time of 60s when microwave power increased from 160 to 480w. The vitamin C concentration increased from 11.59 to 11.76 μg/mL and then decreased to 11.58 μg/mL at microwave power of 320 W as microwave time increased from 40 to 80s. The effect of microwave time-milk layer thickness was such that, the vitamin C concentration increased from 9.8 to 11.76 μg/mL as milk layer thickness increased from 3 to 5 cm (Fig. 2b, at microwave time of 60s) indicating high milk layer thickness was favorable for the enhancement in the vitamin C concentration in the milk treated microwave. The effects of microwave power and milk layer thickness (Fig. 2c at milk layer thickness of 4 cm) are same to that in Fig. 2a and b. The information indicated that appropriate milk layer thickness, microwave time and microwave power would render treated milk with higher vitamin C concentration.
Conclusions
The use of RSM has facilitated the evaluation of the vitamin C concentrations in microwave treated milk in these studies. Three-dimensional and contour-response surface plots clearly demonstrate the impact of the different levels of milk layer thickness, microwave time and microwave power on the vitamin C concentration in the milk treated microwave. The response surface plots show that milk layer thickness was the most significant factor that affects the measured responses, and the effects of microwave time and microwave power were dependent on milk layer thickness levels. The variables microwave time,milk layer thickness and microwave power have the opposite effect on vitamin C concentration in milk treated by microwave. The use of this approach has permitted the identification of significant factors for the effects of microwave studied in this research and provides a framework for further investigation.
References
Amini M, Younesi H, Bahramifar N, Lorestani AAZ, Ghorbani F, Daneshi A, Sharifzadeh M (2008) Application of response surface methodology for optimization of lead biosorption in an aqueous solution by aspergillus niger. J Hazard Mater 154:694–702
Choi HK, Marth EH, Vasavada PC (1993a) Use of microwave energy to inactive Listeria monocytogenes in milk. Milchwissenschaft 48:200–203
Choi HK, Marth EH, Vasavada PC (1993b) Use of microwave energy to inactive Yersinia enterocolitica and campilobacter jejuni in milk. Milchwissenschaft 48:134–136
Donglin Z, Yasunori H (2004) Phenolics, ascorbic acid, carotenoids and antioxidant activity of broccoli and their changes during conventional and microwave cooking. Food Chem 88(4):503–509
Galuska PJ, Kolarik RW, Vasavada PC, Marth EH (1989) Inactivation of Listeria monocytogenes by microwave treatment. Dairy Sci 72:139
Giese J (1995) Vitamins and mineral fortification of foods. Food Technol 49(5):110–122
Gil MI, Fereres F, Tomas-Barberan FA (1999) Effect of post-harvest storage and processing on the antioxidant constituents (flavonoids and vitamin C) of fresh-cut spinach. J Agricult Food Chem 47(6):2213–2217
Gliguem H, Birlouez-Aragon I (2005) Effect of sterilization, packaging, and storage on vitamin C degradation, protein denaturation, and glycation in fortified milk. J Dairy Sci 88(3):891–899
Hanrahan G, Lu K (2006) Application of factorial designs and response surface methodology in modern experimental design and optimization. Rev Anal Chem 36:141–151
Kalavathy MH, Regupathi I, Pillai MG, Miranda LR (2009) Modelling, analysis and optimization of adsorption parameters for H3PO4 activated rubber wood sawdust using response surface methodology (RSM). Colloids Surf B: 7035–45
Khalil H, Villota R (1988) Comparative study on injury and recovery of staphylococcus aureus using microwaves and conventional heating. J Food Protect 51(3):181–186
Kim HK, Kim JG, Cho JD, Hong JW (2003) Optimization and characterization of UV-curable adhesives for optical communications by response surface methodology. Polym Test 22:899–906
LopezFandino R, Villamiel M, Corzo N, Olano A (1996) Assessment of the thermal-treatment of milk during continuous microwave and conventional heating. J Food Protect 59(8):889–892
Merin U, Rosenthal I (1984) Pasteurisation of milk by microwave irradiation. Milchwissenschaft 39(11):643–644
Muthukumar M, Mohan D, Rajendran M (2003) Optimization of mix proportions of mineral aggregates using Box Behnken design of experiments, cement concrete compos. Cem Concr Compos 25:751–758
Myers RH, Montgomery DC (1995) Response surface methodology. Process and product optimization using designed experiments. John Wiley & Sons Inc, New York, pp 297–340
Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M (2003) Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr 22(1):18–35
Preetha R, Jayaprakash SN, Rosamma P, Bright Singh IS (2007) Optimization of carbon and nitrogen sources and growth factors for the production of an aquaculture probiotic (Pseudomonas MCCB 103) using response surface methodology. J Appl Microbiol 102:1043–1051
Sieber R, Eberhard P, Rüegg M (1989) Mikrowellenbehandlung von lebensmitteln, insbesondere von milch und milchprodukten-eine literaturübersicht. Lebensm-Techn 22:198–203
Stearns G, Vasavada PC (1986) Effect of microwave processing on quality of milk. J Food Protect 49:853–858
Vasavada P (1990) Microwave processing for the dairy industry. Food Aust 42:562–564
Villamiel M, López-Fandiňo R, Corzo N, Martinez-Castro I, Olano A (1996a) Effects of continuous-flow microwave treatment on chemical and microbiological characteristics of milk. Z-Lebensm Unters Forch 201:15–18
Villamiel M, López-Fandiňo R, Olano A (1996b) Microwave pasteurisation in a continuous flow unit. Shelf life of cow’s milk. Milchwissenschaft 51:674–677
Wernery U, Johnson B, Abarhm A (2005) The effect of short-term heat treatment on vitamin C concentrations in camel milk. Milchwissenschaft 60(3):266–267
Xin C, Yin L, Guocheng D, Jian C (2005) Application of response surface methodology in medium optimization for spore production of coniothyrium minitans in solid-state fermentation. World J Microbiol Biotechnol 21:593–599
Young GS, Jolly PG (1990) Microwave:the potential for use in dairy processing. Aust J Dairy Technol 45:34–37
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bai, Y., Saren, G. & Huo, W. Response surface methodology (RSM) in evaluation of the vitamin C concentrations in microwave treated milk. J Food Sci Technol 52, 4647–4651 (2015). https://doi.org/10.1007/s13197-014-1505-5
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-014-1505-5