Abstract
In this study, gelatin was extracted from bighead carp (Hypophthalmichthys nobilis) scales by water bath (WB) and ultrasound bath (UB) at 60 °C for 1 h, 3 h and 5 h, named WB1, WB3, WB5, UB1, UB3 and UB5, respectively. The physicochemical properties of gelatin were investigated. The result indicated that gelatin extracted from bighead carp scales had a high protein content (84.15 ~ 91.85 %) with moisture (7.11 ~ 13.65 %), low ash content (0.31 ~ 0.97 %). All extracted gelatin contained α-and β-chains as the predominant components. Gelatin extracted by UB obtained much higher yield (30.94–46.67 %) than that of WB (19.15–36.39 %). More voids and less sheets of gelatin structure were observed, when the gelatin was extracted by UB for longer time. Gelatin of UB-assisted extraction normally exhibited lower gel strength and melting points than that of WB, which may be resulted from the protein degradation reflected by the FTIR spectra and higher free amino group content. However, there was no significant difference between WB1 and UB1. Therefore, the ultrasound assisted extraction in a short time was a promising method to enhance the yield and obtain gelatin with high quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gelatin, derived from partial hydrolysis of fibrous protein collagen, is a compound mixture water-soluble protein (Karim and Bhat 2009). It has been widely used both in food and pharmaceutical industry due to its excellent functional properties. It has been reported that the annual growth rate of gelatin production in the past seven years was about 3–4 % (Gómez-Guillén et al. 2009). At present, mammalian gelatins, such as pig and cow gelatin are most widely used in the world. It had been reported that the worldwide production of gelatin was 326,000 t, with gelatin extraction of pig skin (46 %), bovine hide (29.4 %), pork and cattle bones (23.1 %) and other sources (1.5 %) (Gómez-Guillén et al. 2009). However, it is significant to explore new resource for gelatin due to some health-related and social-culture problems.
Fish gelatin has been popularly studied since the 1950s, in recent decades, some studies on the food properties of fish gelatin have been conducted (Norland 1987; Gudmundsson and Hafsteinsson 1997; Jamilah and Harvinder 2002). Physicochemical and functional properties of fish gelatin have been also researched, such as rheological (Gilsenan and Ross-Murphy 2000; Jamilah and Harvinder 2002; Haug et al. 2004; Badii and Howell 2006), emulsifying and foaming (Dickinson and Lopez 2001; Surh et al. 2006), film forming (Avena Bustillos et al. 2006; Jongjareonrak et al. 2006a; Zhang et al. 2007), and sensory properties (Choi and Regenstein 2000). Most of the studies have been carried out on fish gelatin extracted from skins or bones of many fish species (Jamilah and Harvinder 2002; Muyonga et al. 2004a). The aquatic production is forecast at 55,300,000 t in China in 2012, and fish scales accounted for 3 %–5 % of the fish weight (Melinda Meador et al. 2011). Therefore, it is significant to extract gelatin from fish scales.
Ultrasound-assisted extraction is an efficient way of extraction in food and pharmaceutical industry (Nascentes et al. 2001; Rostagno et al. 2003; Li et al. 2004; Rodrigues and Pinto 2007; Zhang et al. 2008; Zhu et al. 2009). The enhancement of yield induced by ultrasound is attributed to the disruption of the cell walls and the enhancement on the mass transfer of the cell content to the solvent, allowing more intracellular product released by some mechanical effects and acoustic cavitation (Zhang et al. 2008). It could not only enhance the yield of the products, but also improve some functional property (Jambrak et al. 2009; Arzeni et al. 2012). Fish scales are biocomposites of highly ordered type I collagen fibers and hydroxyapatite Ca10(OH)2(PO4)6 (Onozato and Watabe 1979). Therefore, it is not easy to extract gelatin from scales. Previous research has focused on gelatin extracted from fish scales by water bath heating, such as Lizardfish (Harpodontidae) scales (Wangtueai and Noomhorm 2009), grass carp (Ctenopharyngodon) fish scales (Zhang et al. 2011), while ultrasound-assisted extraction has not been paid attention to.
Bighead carp (Hypophthalmichthys nobilis) is one of the“four major cultured fish species”, which inhabits fresh warm water in China. It has been widely consumed in more than 70 countries around the world (Liu et al. 2012). Therefore, in this study, gelatin was extracted from bighead carp scales by ultrasound-assisted extraction. The yield, physicochemical properties were studied and compared with that of water bath extraction.
Material and methods
Collection and preparation of bighead carp scales
Fresh bighead carp (Hypophthalmichthys nobilis) scales were collected from a fish market in Nanchang (JiangXi, China). The diameter of the fish scales were from 1.5 to 2.0 cm. The fish scales were washed by water to remove the impurities adhering to the surface. The fish scales were minced by a tissue mashing machine (HR-2094, PHILIPS) for 2 min, and then they were washed for four times with distilled water. Fish scales were decalcified with 0.5 M HCl at ratio of 1:25(w/v) for 1 h. Fish scales were rinsed again to remove residual acid and the fish scales solution was adjusted to pH 6.0 using 0.1 M NaOH after decalcification
Extraction of gelatin from bighead carp scales
The decalcified bighead carp scales were added with distilled water at ratio of 1:3 w/v (wet decalcified scales: distilled water). The gelatin was extracted from bighead carp scales with ultrasound purifier instrument (KQ5200DE, Kunshan ultrasound instrument, China) upon 200 w at 60 °Cfor 1 h, 3 h and 5 h, named UB1, UB3 and UB5, respectively. The control extracted from bighead carp scales at 60 °C in a temperature-controlled water bath (HH-6, Guohua electrical equipment, Changzhou, China) for 1 h, 3 h and 5 h, referred as WB1, WB3 and WB5, respectively. The mixture was then filtered with two layers of filter paper (NO. GB/T1914-2007), and gelatin solution was then freeze-dried using a LBJ-1freeze dryer (Yataikelong Apparatus,Beijing, China). All dried fish scales gelatin samples were weighed and subjected to further analysis. The extraction yield was calculated using the following equation:
Where M: the weight of dried bighead carp scales gelatin (g), M 0 : the weight of the dried decalcified bighead carp scales (g), w: the dry weight of decalcified fish scales (1 g).
Determination of proximate composition
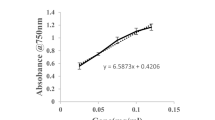
The moisture and ash content of the extracted gelatin were determined according to the methods of AOAC (2000). Protein content was determined by biuret reaction (Gornall et al. 1949). Fish gelatin (0.1 g) was dissolved in 10 mL 0.9 % of NaCl at 50 °C. One milliliter of the solution was mixed with 4 mL of biuret and reacting for 30 min at room temperature, and then was measured at 540 nm using spectrophotometer (ModelUV-3200, Mapada, Shanghai, China), each sample was measured for three times and the protein content was expressed as percentage of protein in fish gelatin.
Gel strength
Gel strength was determined using a Texture analyzer(CT3, Brookfield, Amercian)according to Wangtueai et al.(2010). The dried gelatin was dissolved in distilled water at 50 °C to obtain the final concentration of 6.67 % (w/v). Gelatin solution was placed in a 25 mL glass beaker, and then cooled in a refrigerator at 10 °C and matured for 16–18 h. The gel strength was determined on a texture analyzer with a cell load of 100 N, cross-head speed of 1 mm/s, equipped with a 1.27 cm-diameter flat-faced cylindrical Teflon. The dimensions of the gel samples were approximately 33 mm in diameter and 22 mm in height. Gel strength was expressed as maximum force (in g) when the plunger had penetrated 4 mm into the gelatin gels, each sample was tested for three times.
Melting point
Melting point of gelatin was determined by the method of Muyonga et al. (2004a) using cryostat (CXDC-0510, Shunma, Nanjing, China). Gelatin solution 6.67 % (w/v) was prepared by dissolving dry sample in distilled water at 50 °C, and then transferred in screw cap test tubes((12 mm × 75 mm) with some headspace, closed and put at inverted position in a 4 °C refrigeration for 16–18 h. The samples were transferred into a cold-water bath (10 °C). The water bath was warmed gradually at a rate of 0.5 °C/min, and the gel melting temperature was recorded as gas started to move up to the headspace, each sample was tested for three times.
SDS–polyacrylamide gel electrophoresis (SDS–PAGE)
Gelatin samples (5 mg) were dissolved in 1.0 mL of distilled water at 50 °C, and then 3 × −concentrated loading buffer containing β-mercaptoethanol was added as described earlier (Gómez-Guillén et al. 2002). Gelatin samples were heated–denatured for 10 min at 100 °C water bath and analyzed by SDS-PAGE according to Laemmli (1970). Samples using 7.5 % running gels and 5 % stacking gels subjected to electrophoresis at a constant current of 15 mA/gel using a Mini Protean II unit (Bio-RadLaboratories, Hercules, CA). The loading volume was 10 μL. Protein bands were stained with 0.05 % (w/v) Coomassie brilliant Blue R250 in 15 % (v/v) methanol and 5 % (v/v) acetic acid for about 1 h, and then distained with 30 % (v/v) methanol and 10 % (v/v) acetic acid. High molecular weight markers at the range of 10 kDa–200 kDa (Thermo, Lithuania) were used to estimate the molecular weight of the proteins.
Free amino group determination
The free amino groups were determined using 2,4,6-trinitrobenzenesulfonic acid(TNBS), following the method of Fields (1972). Briefly, fish gelatin (0.2 g) was dissolved in 10 mL of phosphate buffer (pH 8.2). One hundred microliter of gelatin solution was mixed with 900 μL of phosphate buffer and 1.0 mL of 0.01 % TNBS solution. The reaction mixture was vortexed and incubated in a temperature-controlled water bath at 50 °C for 30 min in the dark. The reaction was terminated by adding 2.0 mL of 0.1 M sodium sulfite solution. The mixture was cooled down at room temperature for 15 min, and then the absorbance was measured at 420 nm using spectrophotometer (Model UV-3200, Mapada, Shanghai, China). The absorbance was converted into free amino concentrations (expressed as millimolar leucine equivalent) using a calibration curve obtained with L–leucine, each sample was measured for three times.
Fourier transform infrared (FTIR) spectroscopic analysis
The spectra of freeze-dried fish gelatin samples were recorded using a FTIR-Spectrum One (Perkin Elmer, American), dried gelatin (4 mg) samples and KBr powder (120 mg) were mixed uniformly and pressed into slice. Slice was placed on the crystal cell and the cell was clamped into the mount of the FTIR spectrometer. Spectra were acquired in the IR range of 4,000–500 cm−1(mid-IR region) at 25 °C. Automatic signals gained were collected in 32 scans at a resolution of 4 cm−1 against a background spectrum recorded from the clean empty cell at 25 °C. The spectral data was analyzed using the Nicolet 380 data collection software program. Prior to data analysis, the spectra were baseline corrected and normalised.
Scanning electron microscopy (SEM)
In order to investigate the influence of ultrasound on the microstructure of gelatin, dried fish scales gelatin was mounted on conducting resin with 2–3 mm thickness respectively. The specimens were observed with an environmental scanning electron microscope Quanta200F (FEI Deutschland GmbH, Germany) at an acceleration voltage of 20 kV. All micrographs were produced at 1,000×.
Statistical analyses
A completely randomized design was used and all experiments were performed in triplicate. Data were compared using analysis of variance (ANOVA) using Statistic program (Statsoft Inc.) and the probability value of P < 0.05 was considered significant. Analysis was performed using SPSS 17.0(SPSS Inc, Chicago, IL, USA).
Results and discussion
Yield and proximate composition
The yield of the gelatin obtained by the WB and UB was shown in Table 1. It indicated that, the yield was 19.15 %, 25.57 %, 36.39 % for WB1, WB3 and WB5, respectively. While the yield of 30.94 %, 42.97 %, 46.67 % was gained for UB1, UB3 and UB5, respectively. Increasing yield was obtained when extraction time increased (p < 0.05). The result was in accordance with Arnesen and Gildberg (2007), who reported that longer extraction time was associated with the higher yield of gelatin extracted from Atlantic salmon skin. The increasing time directly provided more energy to disrupt bonds stabilizing the collagen structures as well as peptide bonds of α-chains, leading to helix-to-coil transition (Nagarajan et al. 2012). This resulted in larger amount of the conversion of collagen to soluble gelatin. Result also indicated the yield of UB was higher than that of control, due to mechanical and cavitation effect of ultrasound. The mechanical effect of ultrasound allowed greater penetration of water into the fish scales matrix, and provided a greater agitation of the water used for extraction (Mason et al. 1996). The cavitation released large amounts of energy to disrupt fish scales and order type I collagen fibers and accelerated the washing out of the gelatin. As time increased, more and more fish scales were broken, and gelatin was released gradually (Zhu et al. 2009). Therefore, the mechanical effects and acoustic cavitation of ultrasound provided a greater penetration of solvent into cellular materials and improved mass transfer (Chemat and Khan 2011).
Proximate compositions of fish gelatin extracted from bighead carp scales were summarized in Table 1. Gelatin extracted from bighead carp scales contained high protein content (84.15 ~ 91.85 %) with moisture (7.11 ~ 13.65 %), and low ash content (0.31 ~ 0.97 %). The gelatin extracted by UB had higher protein and slightly higher ash content than that of WB. In general, recommended moisture and ash content of edible gelatin is less than 15 %, 2 % respectively (GME 2005). Therefore, gelatin extracted from bighead carp scales revealed the efficient process in gelatin extraction for high contents of protein and low contents of ash.
Gel strength
Gel strength is the major physical property normally measured for gelatin. From Table 1, the gelatin extracted from bighead carp scales showed high gel strength ranged from 490.6 to 656.5 g. This was sharply higher than red tilapia skin gelatin (384.9 g) (Jamilah et al. 2011), bovine gelatin(322 g), lizard fish scales gelatin (268 g) (Wangtueai and Noomhorm 2009), porcine skin gelatin (180 g), grass carp fish scales gelatin (276 g) (Zhang et al. 2011), siliver carp scales gelatin (152 g) (Wang and Regenstein 2009). These differences between species were possibly due to differences in sample composition, size of the protein chains, as well as complex interactions determined by the amino acids composition (Muyonga et al. 2004a). Gel strength of gelatin was presented in Table 1. Gel strength of gelatin decreased as the extraction time increasing, this was in accordance with the observation by Ahmad and Benjakul (2011). Gelatin extracted by UB normally had lower gel strength than that of WB. WB1 showed the highest gel strength, while UB5 showed lowest gel strength (p < 0.05). When it reached 3 h and 5 h, ultrasound had more significant effect on gel strength than traditional water bath heating. For instance, compared with gel strength of WB1 and WB5, the latter was decreased 13.89 %, however, compared with UB1 and UB5, the latter was decreased 22.7 %. Gel strength is largely dependent on the molecular weight distribution, which is resulted mainly from processing conditions (Gómez-Guillén et al. 2011). Longer time of ultrasound contributed to more protein degradation during gelatin extraction, which might reduce the ability of α-chain to anneal correctly by hindering the growth of the existing nucleation sites (Clark et al. 1986; Jongjareonrak et al. 2006b). So longer time of ultrasound provided more energy to disrupt long chains into short chains, which led to gelatin with lower gel strength,α-chain and β -chain at higher amount in gelatin extracted by WB might form stronger gel network than α-chain at the lower amount found in that of UB. However, there was no difference between WB1 and UB1, indicating ultrasound had no effect on gel strength in a short time. It was attributed to ultrasound lead to high mount of aggregation of protein (Chandrapala et al. 2011). There was no difference between WB5 and UB3, which indicated ultrasound-assisted extraction could shorten extraction time and guarantee the quality of the gelatin.
Melting point
Gel melting point is one of the major physical properties of gelatin gels. Melting points of the gelatins extracted from bighead carp scales were ranged from 27.25 to 28.65 °C as shown in Table 1. This was significantly higher than that of the gelatin extracted from black tilapia (Jamilah and Harvinder 2002) and was also higher than that of hake (14 °C), sole (19.4 °C), megrim (18.8 °C) (Gómez-Guillén et al. 2002), grass carp (19.5 °C) (Kasankala et al. 2007). These melting points were much higher than those reported for cod skin which were in the range 8–10 °C (Gudmundsson and Hafsteinsson 1997). This difference was closely related to the fish species (Yudi et al. 2011). In this work, gelatin extracted from bighead carp scales presented relatively high melting points and gel strength. Therefore, bighead carp scales can be potentially used as a good gelatin source.
Melting point is governed by complicated interactions determined by the amino acid composition and the ratio of α/β-chains, as well as molecular weight of the gelatin (Cho et al. 2004). As it was shown in Table 1, gelatin usually exhibited lower melting points when extracted by UB and longer time. This was due to ultrasound and longer time providing more power to facilitate more high-molecular weight fragments degradation, resulting in weakening the gel network. However, there was no significant difference between WB1 and UB1, this might be because ultrasound produce protein aggregation at low temperature for short time (Chandrapala et al. 2011).
Protein patterns and free amino group content
The molecular weight of gelatin was analyzed by SDS-PAGE (Fig. 1a). The molecular weight distribution including the α1/α2 molar ratio and β chain amount, which determine the properties of gelatin, such as gel strength and melting point (Karim and Bhat 2009). Gelatin extracted by WB possessed higher content of α-chain, which represented stronger gel strength and normally higher melting points than that of gelatin extracted by UB. This was accordance with Gómez-Guillén et al. (2002), who reported gelatin possess better functional properties including gel strength and melting point due to higher content of α-chain. WB1 contained the least β-chain but highest α-chain, possessing the highest gel strength. The decreasing α-chain band intensity was observed in UB3 and UB5, which had lower gel strength. It was also noticed that gelatin extracted by UB showed slightly lower content of protein at the range of 70 KDa–85 KDa, for ultrasound had great effects on hydrolysis of gelatin. However, the β-chain was still observed in UB5, due to the large shear forces generated from cavitation breaking inter and intramolecular bonds, which in turn may lead to fragmentation of clusters and aggregates (Chandrapala et al. 2011).
Free amino groups of six samples are shown in Fig. 1b. Gelatin extracted by UB presented higher content of free amino group than that of WB. The highest degradation of gelatin was observed at UB5. This was in agreement with the highest degradation of α–chain and β-chain in UB5 (Fig. 1a). Therefore, the result reconfirmed that ultrasound and longer time caused the hydrolysis of gelatin proteins, leading to increasing free amino group during the extraction of gelatin.
FTIR spectra analysis
FTIR spectra of gelatin extracted from bighead carp scales were displayed in Fig. 2, FTIR spectroscopy has been usually used to monitor the functional groups and secondary structure of gelatin samples (Muyonga et al. 2004b). The amide I band, between 1,600 and 1,700 cm−1, is the most important for infrared spectroscopic analysis of the secondary structure of proteins (Muyonga et al. 2004b). Amide-I represents C = O stretching vibration hydrogen bonding coupled with COO, coupled to contributions from the CN stretch, CCN deformation and in-plane NH bending mode (Bandekar 1992). The different absorption peaks at amide I were observed as characteristic of the coiled structure of gelatin (1,633 cm−1, 1634.73 cm−1) (Yakimets et al. 2005). From Fig. 2, UB1, UB3 and UB5 exhibited the amide I bands at the wavenumbers of 1653.61, 1654.57 and 1653.81, respectively, however WB1, WB3 and WB5 exhibited amide I were 1651.78, 1652.19 and 1652.76, respectively. The spectral differences in amide I of different gelatin samples were largely due to different conformation of polypeptide chains. It has been proved amide I areas were useful in studying the nature and the extent of protein conformational changes (Surewicz and Mantsch 1988). Gelatin extracted by UB had slight higher amide I than that of WB, indicating the greater loss of molecular order due to thermal uncoupling of inter-molecular cross-link as ultrasound used (Ahmad and Benjakul 2011). According to Muyonga et al. (2004b) reported, lower temperature had lower amide I with more content of random coils and β-turns, it indicated that gelatin extracted by WB had higher content of random coils and β-turns. The amide II band in the infrared spectrum of dry collagen is in the range of 1,530–1,540 cm−1, and often has minor bands at lower frequencies (Doyle et al. 1975). The characteristic absorption bands of gelatin extracted by UB in amide-II region were noticeable at the wavenumbers of 1535.13, 1541.67 and 1541.24, respectively. However, the wavenumbers of control samples were 1539.39, 1539.42 and 1540.43, respectively. The amide II vibration modes primarily represent an out-of-phase combination of CN stretch and in-plane NH deformation modes of the peptide group (Lavialle et al. 1982; Ahmad and Benjakul 2011). In addition, the wavenumbers of amide III was detected around 1237.27, 1237.93 and 1236.77 for UB1, UB3 and UB5, respectively. However, control samples were 1235.51, 1239.04 and 1236.98, respectively. The amide III, a complex vibrational mode having combination peaks between C-N stretching vibrations and N-H in plane bending from amide linkages as well as significant absorptions arising from wagging vibrations from CH2 groups from the glycine back bone and proline side chains (Jackson et al. 1995).
Moreover, the wavenumbers of amide A, arising from the stretching vibrations of N-H group, appeared at 3303.61, 3298.95 and 3309.2 for UB1, UB3 and UB5, respectively. However WB1, WB3 and WB5, were 3318.17, 3306.23 and 3307.64, respectively. Amide–A, which represents NH-stretching coupled with hydrogen bonding. In general, a free NH-stretching vibration occurs in the range of 3,400–3,440 cm−1. When the N-H group of a peptide is involved in a hydrogen bond, amide A is shifted to lower wavenumbers, usually near the 3,300 cm−1. It has also reported that the amide A band of the infrared spectra of these triple-helical polymer is observed at an unusually high frequency (3,315–3,360 cm−1), as compared with the amide A frequencies of other polypeptides and proteins (Doyle et al. 1975; Nagarajan et al. 2012). Compared with gelatin extracted by WB, gelatin extracted by UB had lower wavenumbers, providing greater free amino groups and this might be due to ultrasound produced more degradation of gelatin. These degraded gelatin might undergo hydrogen bonding interaction, resulting in decreased wavenumbers of amide-A (Nagarajan et al. 2012). The amide B was observed at 3073.12, 3072.44 and 3072.35 for UB1, UB3 and UB5, respectively. However the control samples were at 3075.39, 3071.43 and 3078.57, respectively. Amide-B was corresponding to the asymmetric stretching vibration of = C-H as well as NH+ 3. Among all samples, UB5 and WB3 showed the lowest wavenumbers of amide- B peak, suggesting the interaction of- NH3 group between peptide chains (Ahmad and Benjakul 2011). Thus, it can be concluded that the secondary structure and functional group of gelatins obtained from the fish scales of bighead fish was affected by ultrasound and extraction time.
Microstructure of fish scales gelatin
The microstructure of gelatin extracted from bighead carp sales was visualized by SEM as shown in Fig. 3. In general, the SEM photographs of fish scale gelatin show sheet morphology with pores. The severity pores improved progressively with time increasing. This phenomenon properly accounted for decreasing gel strength accompanied with time increasing. However, at the same time, gelatin extracted by WB exhibited the finest gel network with more sheets and very small voids. The finer structure of gelatin was in accordance with the higher gel strength (Table 1), compared with that of UB, which possessed the coarser gel structure. This was similar with Wangtueai et al. (2010), who reported gelatin with higher gel strength had finer and denser gel network. When extraction time was longer, the sheets were decreasing but voids were increasing. SEM images also revealed longer time of ultrasound significantly reduced protein agglomeration (Narducci et al. 2011).
Conclusions
The bighead carp scales gelatin with high protein component and strong gel strength could be obtained and used in food applications to replace mammalian gelatin. Ultrasound-assisted extraction could significantly increased the yield and free amino group contents of gelatin from bighead carp scales. However longer time of ultrasound could decrease gel strength and melting points by weakening gel network structure, accompanying with more voids in its surface. Additionally, without ultrasound, the extraction time had a significant effect on extraction yield, gel strength, melting points, free amino group contents and microstructure of gelatin. Therefore, short time of ultrasound-assisted extraction could be used as an appropriate method to obtain gelatin from bighead carp scales with high yield and high quality .
References
Ahmad M, Benjakul S (2011) Characteristics of gelatin from the skin of unicorn leatherjacket (Aluterus monoceros) as influenced by acid pretreatment and extraction time. Food Hydrocoll 25(3):381–388
AOAC (2000) Official methods of analysis, 17th edn. Association of Official Analytical Chemists, Washington
Arnesen JA, Gildberg A (2007) Extraction and characterisation of gelatine from Atlantic salmon (Salmo salar) skin. Bioresour Technol 98(1):53–57
Arzeni C, Martinez K, Zema P, Arisa A, Perez OE, Pilosof AMR (2012) Comparative study of high intensity ultrasound effects on food proteins functionality. J Food Eng 108(3):463–472
Avena Bustillos R, Olsen C, Olson D, Chiou B, Yee E, Bechtel P, McHugh T (2006) Water vapor permeability of mammalian and fish gelatin films. J Food Sci 71(4):E202–E207
Badii F, Howell NK (2006) Fish gelatin: structure, gelling properties and interaction with egg albumen proteins. Food Hydrocoll 20(5):630–640
Bandekar J (1992) Amide modes and protein conformation. BBA-Protein Struct Mol 1120(2):123–143
Chandrapala J, Zisu B, Palmer M, Kentish S, Ashokkumar M (2011) Effects of ultrasound on the thermal and structural characteristics of proteins in reconstituted whey protein concentrate. Ultrason Sonochem 18(5):951–957
Chemat F, Khan MK (2011) Applications of ultrasound in food technology: processing, preservation and extraction. Ultrason Sonochem 18(4):813–835
Cho S, Kwak K, Park D, Gu Y, Ji C, Jang D, Lee T, Kim S (2004) Processing optimization and functional properties of gelatin from shark (Isurus oxyrinchus) cartilage. Food Hydrocoll 18(4):573–579
Choi SS, Regenstein J (2000) Physicochemical and sensory characteristics of fish gelatin. J Food Sci 65(2):194–199
Clark A, Lee Tuffnell C, Mitchell J, Ledward D (1986) Functional properties of food macromolecules. Mitchell, JR
Dickinson E, Lopez G (2001) Comparison of the emulsifying properties of fish gelatin and commercial milk proteins. J Food Sci 66(1):118–123
Doyle BB, Bendit E, Blout ER (1975) Infrared spectroscopy of collagen and collagen like polypeptides. Biopolymers 14(5):937–957
Fields R (1972) The rapid determination of amino groups with TNBS. Methods Enzymol 25B:464–468
Gilsenan P, Ross-Murphy S (2000) Rheological characterisation of gelatins from mammalian and marine sources. Food Hydrocoll 14(3):191–195
GME (2005). Standard methods for the testing of edible gelatine, Gelatine Monograph. Gelatin Manufacturers of Europe
Gómez-Guillén M, Turnay J, Fernandez-Diaz M, Ulmo N, Lizarbe M, Montero P (2002) Structural and physical properties of gelatin extracted from different marine species: a comparative study. Food Hydrocoll 16(1):25–34
Gómez-Guillén M, Pérez-Mateos M, Gómez-Estaca J, López-Caballero, Giménez B, Montero P (2009) Fish gelatin: a renewable material for developing active biodegradable films. Trends Food Sci Technol 20(1):3–16
Gómez-Guillén MC, Giménez B, López-Caballero ME, Montero MP (2011) Functional and bioactive properties of collagen and gelatin from alternative sources: a review. Food Hydrocoll 25(8):1813–1827
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum proteins by means of the biuret reaction. J Biol Chem 177(2):751–766
Gudmundsson M, Hafsteinsson H (1997) Gelatin from cod skins as affected by chemical treatments. J Food Sci 62(1):37–39
Haug IJ, Draget KI, Smidsrød O (2004) Physical and rheological properties of fish gelatin compared to mammalian gelatin. Food Hydrocoll 18(2):203–213
Jackson M, Choo L, Watson PH, Halliday WC, Mantsch HH (1995) Beware of connective tissue proteins: assignment and implications of collagen absorptions in infrared spectra of human tissues. BBA-Mol Basis Dis 1270(1):1–6
Jambrak AR, Lelas V, Mason TJ, Krešic G, Badanjak M (2009) Physical properties of ultrasound treated soy proteins. J Food Eng 93(4):386–393
Jamilah B, Harvinder K (2002) Properties of gelatins from skins of fish—black tilapia (Oreochromis mossambicus) and red tilapia (Oreochromis nilotica). Food Chem 77(1):81–84
Jamilah B, Tan K, Umi Hartina M, Azizah A (2011) Gelatins from threecultured freshwater fish skins obtained by liming process. Food Hydrocoll 25(5):1256–1260
Jongjareonrak A, Benjakul S, Visessanguan W, Tanaka M (2006a) Effects of plasticizers on the properties of edible films from skin gelatin of bigeye snapper and brownstripe red snapper. Eur Food Res Technol 222(3–4):229–235
Jongjareonrak A, Benjakul S, Visessanguan W, Tanaka M (2006b) Skin gelatin from bigeye snapper and brownstripe red snapper: chemical compositions and effect of microbial transglutaminase on gel properties. Food Hydrocoll 20(8):1216–1222
Karim A, Bhat R (2009) Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins. Food Hydrocoll 23(3):563–576
Kasankala LM, Xue Y, Weilong, Hong SD, He Q (2007) Optimization of gelatine extraction from grass carp (Catenopharyngodon idella) fish skin by response surface methodology. Bioresour Technol 98(17):3338–3343
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lavialle F, Adams RG, Levin IW (1982) Infrared spectroscopic study of the secondary structure of melittin in water, 2-chloroethanol, and phospholipid bilayer dispersions. Biochemistry 21(10):2305–2312
Li H, Pordesimo L, Weiss J (2004) High intensity ultrasound-assisted extraction of oil from soybeans. Food Res Int 37(7):731–738
Liu D, Li L, Regenstein JM, Zhou P (2012) Extraction and characterisation of pepsin-solubilised collagen from fins, scales, skins, bones and swim bladders of bighead carp (Hypophthalmichthys nobilis). Food Chem 133(4):1441–1448
Mason T, Paniwnyk L, Lorimer J (1996) The uses of ultrasound in food technology. Ultrason Sonochem 3(3):S253–S260
Melinda Meador M, Xinping WU, Han A (2011) Fishery products annual. http://www.thefishsite.com/articles/contents/USDAChinaFisheryProd.pdf
Muyonga J, Cole C, Duodu K (2004a) Extraction and physico-chemical characterisation of Nile perch (Lates niloticus) skin and bone gelatin. Food Hydrocoll 18(4):581–592
Muyonga J, Cole C, Duodu K (2004b) Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones of young and adult Nile perch (Lates niloticus). Food Chem 86(3):325–332
Nagarajan M, Benjakul S, Prodpran T, Songtipya P, Kishimura H (2012) Characteristics and functional properties of gelatin from splendid squid (Loligo formosana) skin as affected by extraction temperatures. Food Hydrocoll 29(2):389–397
Narducci O, Jones A, Kougoulos E (2011) Continuous crystallization of adipic acid with ultrasound. Chem Eng Sci 66(6):1069–1076
Nascentes CC, Korn M, Arruda MA (2001) A fast ultrasound-assisted extraction of Ca, Mg, Mn and Zn from vegetables. Microchem J 69(1):37–43
Norland R (1987) Fish gelatin: technical aspects and applications. Photographic gelatin.(Band, SJ ed.): 266–281
Onozato H, Watabe N (1979) Studies on fish scale formation and resorption. Cell Tissue Res 201(3):409–422
Rodrigues S, Pinto GA (2007) Ultrasound extraction of phenolic compounds from coconut (Cocos nucifera) shell powder. J Food Eng 80(3):869–872
Rostagno MA, Palma M, Barroso CG (2003) Ultrasound-assisted extraction of soy isoflavones. J Chromatogr A 1012(2):119–128
Surewicz WK, Mantsch HH (1988) New insight into protein secondary structure from resolution-enhanced infrared spectra. BBA-Protein Struct Mol 952(2):115–130
Surh J, Decker EA, McClements DG (2006) Properties and stability of oil-in-water emulsions stabilized by fish gelatin. Food Hydrocoll 20(5):596–606
Wang Y, Regenstein JM (2009) Effect of EDTA, HCl, and citric acid on Ca salt removal from Asian (silver) carp scales prior to gelatin extraction. J Food Sci 74(6):C426–C431
Wangtueai S, Noomhorm A (2009) Processing optimization and characterization of gelatin from lizardfish (Saurida spp) scales. LWT-Food Sci Technol 42(4):825–834
Wangtueai S, Noomhorm A, Regenstein JM (2010) Effect of Microbial Transglutaminase on Gel Properties and Film Characteristics of Gelatin from Lizardfish (Saurida spp.) Scales. J Food Sci 75(9):C731–C739
Yakimets I, Wellner N, Smith AC, Wilson RH, Farhat I, Mitchell J (2005) Mechanical properties with respect to water content of gelatin films in glassy state. Polymer 46(26):12577–12585
Yudi P, Djagal W, Hafni R (2011) Characteristics of gelatins extracted from fresh and sun dried sea water fish skins in Indonesia. Int Food Res J 18:59–65
Zhang S, Wang Y, Herring JL, Oh JH (2007) Characterization of edible film fabricated with channel catfish (Ictalurus punctatus) gelatin extract using selected pretreatment methods. J Food Sci 72(9):C498–C503
Zhang ZS, Wang LJ, Li D, Jiao SS, Chen XD, Mao ZH (2008) Ultrasound-assisted extraction of oil from flaxseed. Sep Purif Technol 62(1):192–198
Zhang F, Xu S, Wang Z (2011) Pre-treatment optimization and properties of gelatin from freshwater fish scales. Food Bioprod Process 89(3):185–193
Zhu KX, Sun XH, Zhou HM (2009) Optimization of ultrasound-assisted extraction of defatted wheat germ proteins by reverse micelles. J Cereal Sci 50(2):266–271
Acknowledgments
The authors would like to express their sincere thanks to the National High Technology Research and Development Program of China (863 Program) (No, 2011AA100803), the Research Fund for the Doctoral Program of Higher Education(20123604110001), Jiangxi province science and technology support program (20122BBF60060) and Project of Education Department of Jiangxi Province (GJJ12018).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Tu, Zc., Huang, T., Wang, H. et al. Physico-chemical properties of gelatin from bighead carp (Hypophthalmichthys nobilis) scales by ultrasound-assisted extraction. J Food Sci Technol 52, 2166–2174 (2015). https://doi.org/10.1007/s13197-013-1239-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-1239-9