Abstract
Antioxidant properties, physico-chemical characteristics and proximate composition of five wild fruits viz., Garcinia pedunculata, Garcinia xanthochymus, Docynia indica, Rhus semialata and Averrhoa carambola grown in Manipur, India were presented in the current study. The order of the antioxidant activity and reducing power of the fruit samples was found as R. semialata > D. indica > G. xanthochymus > A. carambola > G. pedunculata. Good correlation coefficient (R2 > 0.99) was found among the three methods applied to determine antioxidant activity. Total phenolic content was positively correlated (R2 = 0.960) with the antioxidant activity however, total flavonoid content was not positively correlated with the antioxidant activity. Physico-chemical and proximate composition of these fruits is documented for the first time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Fruits contain high levels of biologically active components that impart health benefits beyond basic nutritional value. Within the biologically active components, natural antioxidants have attracted interest because of their safety and potential therapeutic effect (Rufino et al. 2011). The interest of consumers towards lesser known wild edible fruits has arisen due to their various health beneficial effects and organoleptic properties. These wild fruits have played an important role in supplementing the diet of rural people. Detailed information about the health promoting components of lesser known fruit species could lead to a better understanding of the beneficial effects and an increased consumption of these fruits, including their utilization in functional foods and as ingredients in nutraceuticals, medicines and pharmaceuticals (Yilmaz et al. 2009). Use of wild fruits as a food has decreased due to improvement and hybridization in commercially cultivated fruits. Any scientific evidence for the health benefits of such wild fruits in addition to their nutritional value would be a value addition to the plants producing such fruits. Concerning their medicinal properties, the most commonly studied benefit is their antioxidant effects (Karuppusamy et al. 2011). Assessments of antioxidant properties of natural compounds are very important because of their uses in medicine, food and cosmetics (Halliwell 1996). Epidemiological evidence suggests that antioxidant contained in fruits and vegetables can help to prevent the development of several diseases.
The present communication deals with the antioxidant profile, physico-chemical characteristics and proximate composition of five wild edible fruits viz., Garcinia pedunculata, Garcinia xanthochymus, Docynia indica, Rhus semialata and Averrhoa carambola grown in Manipur, India. These fruits are either consumed raw or made into sweets or preserves. There are several reports on medicinal and others significant values of these fruits. However, basic information like physico-chemical characteristics and proximate composition is limited.
Methods
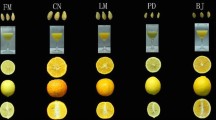
Authentication of the species
Each plant species was properly identified (Fig. 1) and authenticated by submitting a voucher specimen to Botanical survey of India, Eastern circle, Shillong, India. Accession numbers are as follow:
Species | Accession no. |
|---|---|
Garcinia pedunculata Roxb. | 80727(Assam), BSI/ERC/Tech/Herbarium Submission/444. |
Garcinia xanthochymus Hook. f. | 78852 (Assam), BSI/ERC/2012/Plant identification/68. |
Docynia indica (Wall.) Decne. | 78853 (Assam), BSI/ERC/2012/Plant identification/68. |
Rhus semialata Murray | (Assam), BSI/ERC/Tech/Herbarium Submission/444. |
Averrhoa carambola L. | (Assam), BSI/ERC/Tech/Herbarium Submission/444. |
Chemicals
2, 2-Diphenyl-1-picrylhydrazyl (DPPH), 2, 2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt, potassium peroxodisulfate, (±)-6-hydroxy-2, 5, 7, 8-tetramethylchromane-2-carboxylic acid (TROLOX), Aluminium chloride, gallic acid, L-ascorbic acid, quercetin were purchased from Sigma Aldrich, USA. Methanol (spectroscopic grade and GR-grade), petroleum ether (GR-grade) were purchased from E. Merck, Germany. Folin-Ciocalteu’s reagent was purchased from SRL, Mumbai, India.
Preparation of fruit extract for the determination of antioxidant activity, total phenolic content and total flavonoid content
The fruit samples were first washed with running tap water and finally with distilled water. The edible part was chopped into pieces and dried in a circulating air oven at temperature ≤60 °C until a constant dried weight was obtained. The dried sample was ground into powdery form by using Kitchen blender. The pulverized moisture free sample was then macerated in methanol (GR-grade, Merck) for 72 h with intermittent shaking. The clear and coloured solution was decanted and filtered through filter paper (Whatman no.1). The filtrate was then concentrated through vacuum rotary evaporator (Buchi, Switzerland) at temperature below 60 °C. The crude methanolic extract thus obtained was assessed for antioxidant activity, total phenolic and flavonoid content.
Determination of antioxidant activity
DPPH• method
Antioxidant activity by DPPH• method was determined according to the method of Sharma and Bhat (2009) with minor modifications. DPPH• working solution (200 μM) was freshly prepared in methanol (spectroscopic grade) by vortexing for atleast 15–20 min and was used within 3-h of preparation. 1.0 ml of DPPH• working solution (200 μM) was added to 3.0 ml of antioxidant solution dissolved in methanol. The reaction mixture was vortexed for 5–10 s and incubated at room temperature in a dark place for 30 min. After which the absorbance was immediately read at 517 nm against the methanol as blank. Spectrophotometer (Cary-Bio 100, Varian, Australia) was used to record the readings. Stock solution (10 mM) of standard antioxidant compounds was freshly prepared by dissolving in methanol and diluted to their optimum concentration range to give the best concentration response curve (CRC) for the fixed reaction time, 30 min. Percentage DPPH• scavenged was calculated by using the general formula:
where As is the absorbance of the reaction mixture, Ac is the absorbance of the negative control.
Many researchers use linear regression to intrapolate IC50 or EC50. In fact, the data should be subjected to an appropriate non-linear regression model to reflect the true meaning of IC50 or EC50. Plot of % free radical scavenged versus log of concentration of antioxidant was used to construct CRC from which IC50 was evaluated (Fig. 2).
Concentration response curves of % free radical scavenged versus log of concentration of a five wild edible fruits through DPPH method, b five wild edible fruits through ABTS method, c standards through DPPH method, and d standards through ABTS method. GP: Garcinia pedunculata, GX: Garcinia xanthochymus, DI: Docynia indica, RS: Rhus semialata, AC: Averrhoa carambola
ABTS•+ method
Preparation of ABTS radical cation (ABTS•+) solution was prepared according to the method of Rice-Evans et al. (1996) i.e., ABTS and K2S2O8 were dissolved together in distilled water to a final concentration of 7 mM and 2.45 mM respectively. The mixture was incubated in dark at room temperature for 12–16 h before use. ABTS•+ solution is stable from pH 3.0 to 6.5 but optimal at pH 4.5 (Cano et al. 1998). ABTS•+ stock solution thus prepared was diluted with 20 mM sodium acetate buffer, pH 4.5 to an absorbance of 0.7 ± 0.01 at 734 nm at ambient temperature to prepare working solution (w/s) (Ozgen et al. 2006). 3.0 ml of ABTS•+ working solution was mixed with 30 μl of standard/extract solution prepared in methanol and incubated at room temperature for 30 min. Absorbance was read at 734 nm against the reagent blank (3.0 ml + 30 μl MeOH). Similarly, the data were subjected to non-linear regression for the evaluation of IC50.
Reducing power assay
The reducing power was determined according to the method of Oyaizu (1986). Gallic acid (0.05–1.0 mM) was used to construct the standard curve. The reducing power was expressed as gallic acid equivalent (GAE) mg/g extract.
Determination of total phenolic content
Total phenolic content (TPC) was determined according to the method of Singleton and Rossi (1965). Gallic acid (10–50 μg/ml) was used to construct the standard curve. TPC of the extract was expressed as gallic acid equivalent (GAE) g/100 g ext.
Determination of total flavonoid content
Total flavonoid content (TPC) of the methanolic fruit extract was determined according to the method of Meda et al. (2005). Quercetin (2.5–17.5 μg/ml) was used to construct the standard curve. TPC of the extract was expressed as gallic acid equivalent (GAE) g/100 g ext.
Determination of physico-chemical characteristics of the fruit
Physical characteristics of the fruit were assessed from 50 randomly picked freshly harvested mixture of matured unripe and ripe fruits sample (Table 1). Some important physico-chemical characteristics of the fruit juice were also assessed (Table 2). The edible portion of the fruit was first shredded with stainless steel shredder. 100 g of this shredded sample was ground in mortar and pestle and sieved with squeezing through muslin cloth. The crude/pulpy juice thus obtained was centrifuged at 13,200 rpm, 4 °C for 15 min to obtain clear/solid free juice. Extractable juice was expressed in ml per 100 g fresh weight. pH was measured using pH 700 (Eutech Instruments, Singapore). Total soluble solid (TSS) was read by using hand refractometer (Sper scientific, China). Total titratable acidity (TTA) of the fruit was determined by potentiometric titration method (AOAC 2000), expressed in citric acid equivalent (% w/w).
Proximate composition
Determination of moisture content
Moisture content (MC) of the fruit was determined gravimetrically by weighing fresh fruit sample before and after drying in an air circulating oven (NSW-143, Narang Scientific, India). A 20 g of finely shredded fresh sample was weighed (Mettler Toledo, Switzerland) accurately in a clean and dried glass petriplate, W1 and dried in the oven at 70 °C until a constant weight was achieved, W2.
The dried edible portion of the fruit thus obtained was finely ground into powdery form for use in subsequent analysis of proximate composition.
Determination of crude fat content or ether extractives
A known weight of pulverised dried sample was macerated in petroleum ether for 72 h with intermittent shaking. The clear coloured solution was slowly decanted and filtered through Whatman no. 1 filter paper. The residue was redissolved repeatedly in the petroleum ether until decolourisation. The combined extract was concentrated by using rotary vacuum evaporator.
Determination of ash content
1.0 gram of defatted dried sample was put in a clean and preheated crucible, W1 and heated in a gas furnace for about 5–6 h or until a constant weight of greyish white ash, W2 was obtained. Desiccator was used for cooling the crucible while weighing and monitoring the weight.
Determination of fibre content
Fibre content was determined by the non-enzymatic gravimetric method of AOAC (1995) with slightest modifications. A 0.5 g pulverized defatted sample, W1 was placed in an open mouth 250 ml conical flask along with 150 ml of 0.25 N H2SO4 and 0.4 ml of acetone as foam suppressor. The mixture was heated in microwave oven (Samsung-Bio) at 200 °C with some intermittent gap for a total of 20 min. The undigested residue was filtered and rinsed with distilled water to remove any remaining acid. This undigested residue was then subjected to base digestion with 0.3 N NaOH by following the same procedure of acid digestion. The residue thus obtained was scrapped into a clean crucible and dried to a constant weight, W2 in an oven at 100 °C. Then, the crucible containing the sample was heated in a gas flame for about 5 h or until a constant weight of greyish white ash was obtained, W3. Desiccator was used for cooling the crucible while weighing and monitoring the weight.
Determination of crude protein content
Crude protein was determined according to the method of Thiex et al. (2002) with minor modifications. A 0.5 g of pulverized dried sample was digested in 10 ml of H2S04 at 420 °C using copper sulphate and potassium sulphate as catalyst mix. Digested sample was distilled using 40 % NaOH in Kjel-Tech (Pelican, India). Ammonia was absorbed in excess of 4 % boric acid solution and then titrated with standard acid (0.02 N HCL) to estimate total nitrogen content. Crude protein content was evaluated by using the factor 6.25.
Determination of nitrogen and lipid free extract
The nitrogen and lipid free extract (NLFE) which consists of carbohydrate, such as sugars, starches and hemicelluloses can be calculated according to the formula of AOAC (2003).
Determination of energy value
The energy value was estimated by using the conversion factors described in FAO (2003).
Statistical analysis
The experimental results were expressed in mean ± standard deviation (SD) of three independent determinations. The physical dimension of the fruit was expressed in mean ± SD (n = 50). Where applicable, the data were subjected to one way analysis of variance (ANOVA) and the means were separated by Tukey’s post hoc analysis at P < 0.05 using SPSS v.17. Calculation of IC50 was performed using GraphPad Prism® v 5.03. A non-linear regression model, log (inhibitor) vs. normalised response- variable slope was chosen to intrapolate IC50.
Results and discussion
Maceration of pulverized dried sample in methanol was chosen as an extraction method in order to minimize possible artifact formation or sample degradation. Percentage recovery (w/w) of methanolic fruit extract (syrupy consistency) of G. pedunculata, G. xanthochymus, D. indica, R. semialata and A. carambola was found to be 41.00, 21.33, 18.91, 15.10, and 30.00 % respectively.
There are different methods and methodologies for the determination of in vitro antioxidant activity. Unfortunately, no method has been standardised so far and there is no single antioxidant assay for food labelling. It is generally observed that antioxidant activities (IC50s) of standard references vary significantly even by applying a particular method, like DPPH. Hence, the requirement for standard antioxidant assay is very essential in order to compare the results of different laboratories and validation of the conclusions (Sharma and Bhat 2009). In the present study, the modified versions for the determination of antioxidant activity by DPPH• and ABTS•+ method were formulated in order to achieve uniformity and results thereof. The concept of CRC, fixed reaction time and proper interpretation of IC50 were the key elements of the modified versions. Many workers have been interpreted their result from linear regression analysis which is, in fact, an obscurity with respect to the true meaning of IC50 or EC50. For consistency, IC50 is used for inhibition assay while EC50 for stimulation assays, even though there is no fundamental differences between them (Campbell et al. 2012). Use of IC50 or EC50 would be best pronounced for the assay when the reaction conforms to steady state condition with concomitant use of non-regression analysis of the data for the evaluation of IC50 or EC50. Plot of % free radical scavenged versus log of concentration of antioxidant was used to construct CRC from which IC50 was evaluated (Fig. 2). And finally, the value of IC50 should correspond to the final concentration of the test mixture.
IC50 of L-ascorbic acid, TROLOX and gallic acid determined by the modified DPPH method were found to be 11.58, 10.05 and 6.15 μM respectively. IC50 of L-ascorbic acid and TROLOX determined by the modified ABTS method were found to be 11.4 and 10.07 μM (Table 3). In DPPH method, IC50 of L-ascorbic acid corroborated the value of 11.8 μM and 10.2 μM as reported by Sharma and Bhat (2009) and Mishra et al. (2012) respectively. However, IC50 of gallic acid was found to be 6.15 μM as compared to 4.2 μM of Mishra et al. (2012). IC50 of L-ascorbic acid and TROLOX in both the methods were agreeable. However, IC50s of methanolic fruit extracts of ABTS method were slightly higher than DPPH method in each of the respective extracts (Table 3). Possible explanation for such minisculus variation might be due to the solvent system of the assay mixture. DPPH assay was completely performed in organic solvent while in ABTS method, 1 % of the solvent systems was methanol. Hence, the crude methanolic extract might have experienced some matrix interference. During the standardization of ABTS method, a higher ratio of crude methanolic extract solution to ABTS w/s was observed, which is rendering turbidity in the assay mixture. Hence, a critical ratio has been observed. IC50 is inversely proportional to the antioxidant activity and therefore the values were inversed for comparison with reducing power. The order of the antioxidant activity in both free radicals and reducing power assay methods of the methanolic fruit extracts was found as R. semialata > D. indica > G. xanthochymus > A. carambola > G. pedunculata (Table 3). Antioxidant potential of R. semialata was comparable to that of standard antioxidant tested (Table 3). The relationship between the DPPH and ABTS methods was significantly high (R2 = 0.9992) (Fig. 3a). And the relationship of DPPH and reducing power assay methods was also significantly high (R2 = 0.9918) (Fig. 3b). Therefore, all the three methods were highly correlated and validated with each other.
Correlation curves of a IC50 values obtained by DPPH method versus IC50 values obtained by ABTS method, b 1/(GAE mg/g ext.) × 1,000 values obtained by reducing power assay versus IC50 values obtained by DPPH method, c TPC (GAE mg/g ext.) versus 1/IC50 values of DPPH method × 1,000 and d TFC (QE mg/g ext.) versus 1/IC50 values of DPPH method × 1,000
Total phenolic content among the fruits were found in the order of R. semialata > D. indica > A. carambola > G. xanthochymus > G. pedunculata (Table 4). However, total flavonoid content was found in the order of G. xanthochymus > R. semialata > G. pedunculata > D. indica > A. carambola (Table 4). A tendency of co-relationship has been observed between the antioxidant potential and the total phenolic content (R2 = 0.960) (Fig. 3c). However, no similar relationship was observed for the total flavonoid content (Fig. 3d) which is in agreement with other findings of Gálvez et al. (2005), Heinomen et al. (1998) and Matthäus (2002). The possible explanation for such non-correlation ship might be due to the complex structure of the flavonoids or their slow reacting antioxidant potential.
Physical and some of the physico-chemical characteristics of the fruits are enumerated in Tables 1 and 2 respectively. Among the fruits tested, A. carambola, D. indica and G. pedunculata were juicy and the measured juice content of them were respectively 86, 84 and 63 ml per 100 g fresh fruit. Clear juice obtained after centrifugation of the above three fruits were substantial with negligible amount of solid matrices (Table 2). Fruit of G. xanthochymus is less juicy as the pulp was found associated with sticky and gummy substances whereas R. semialata is a dried fruit in nature. Hence, these two fruits were further treated with water decoction procedure in microwave oven so as to enable the assessment of TSS, pH and TTA. Among the three juicy fruits, TSS was found highest in D. indica (ca. 8) followed by ca. 5 in each of the G. pedunculata and A. carambola (Table 2). TSS of each of the decocted fruit extract was also evaluated; however, it is not the true reflectance of its natural juice. The acidity (pH) of the five fruits ranged from 1.35 to 2.84 (Table 2). Among the three juicy fruits, TTA was found highest in G. pedunculata and least in D. indica. However, TTA of water decocted extracts were comparatively higher than the natural fruit juices (Table 2). Such consequence was possibly due to extraction procedure (water decoction treatment in microwave oven) involved in sample preparation.
Table 5 shows the proximate composition of the fruits. MC was found highest in A. carambola (89 %) and least in R. semialata (7.5 %). Crude fat content was found highest in R. semialata (8.9 %) followed by G. xanthochymus (7.57 %) and least in G. pedunculata (0.44 %). Crude protein was found maximum in G. xanthochymus (5.01 %) and minimum in D. indica (1.94 %). N2 free extract or carbohydrate content was found highest in R. semialata and least in G. xanthochymus. Energy value was found highest in R. semialata and least in G. pedunculata. Physico-chemical characteristics and proximate composition of the fruits were documented for the first time and these do not necessarily meant for the superiority of a fruit. The whole experimental analyses were performed from the fruit sample harvested at its right edible stage. Minor variation in the compositional makeup or whole experimental results might arise due to differences in the maturity stages of the fruit, climatic and geographical conditions. Each fruit has its own peculiarity and health beneficial effect, say, G. pedunculata, having the least antioxidant activity among the five fruits under study, has been found to possess (−)-Hydroxycitric acid, which is well recognised for its anti-obesity effect. Likewise, each fruit has its own several reports of significances, which were not mentioned in the present study.
Conclusions
Overall, the data from this study would help in prospecting of such wild underutilised fruits. The present work was primarily aimed to fill up the basic limited experimental results of the selected underutilized wild fruits. Results of the experimental data do not essentially determine the superiority of a fruit. Value addition and commercialization of such wild fruits will uplift the socio-economic conditions of rural people and attract the interest of consumer.
References
AOAC (1995) Official methods of analysis of AOAC (Association of Official Agricultural Chemists). International Method 993.21, Arlington, http://www.eoma.aoac.org/methods/info.asp?ID=8410
AOAC (2000) Official methods of analysis: Method 942.15, 17th edn. AOAC International, Gaithersburg, http://www.eoma.aoac.org/methods/info.asp?ID=15499
AOAC (2003) Official methods of analysis, 17th edn. AOAC International, Gaithersburg, 2 Revision
Campbell RM, Dymshitz J, Eastwood BJ et al (2012) Data standardization for results management. Eli Lilly & Company, Indianapolis, http://www.ncbi.nlm.nih.gov/pubmed/22553865
Cano A, Hernandez-Ruiz J, Garcia-Canovas F, Acosta M, Arnao MB (1998) An end-point method for estimation of the total antioxidant activity in plant material. Phytochem Anal 9:196–202
FAO (2003) Food energy- methods of analysis and conversion factors. Food and Nutrition Paper No. 77. FAO, Rome. ftp://ftp.fao.org/docrep/fao/006/y5022e/y5022e00.pdf
Gálvez M, Martin-Cordero C, Houghton PJ, Ayuso MJ (2005) Antioxidant activity of methanol extracts obtained from Plantago species. J Agric Food Chem 53:1927–1933
Halliwell B (1996) Antioxidants in human health and disease. Annu Rev Nutr 16:33–50
Heinomen IM, Lehtonen PJ, Hopia AI (1998) Antioxidant activity of berry and fruit wines and liquors. J Agric Food Chem 46:25–31
Karuppusamy S, Muthuraja G, Rajasekaran KM (2011) Antioxidant activity of selected lesser known edible fruits from Western Ghats of India. Indian J Nat prod Resour 2(2):174–178, http://nopr.niscair.res.in/handle/123456789/12135
Matthäus B (2002) Antioxidant activity of extracts obtained from residues of different oilseeds. J Agric Food Chem 50:3444–3452
Meda A, Lamien CE, Romito M, Millogo J, Nacoulma OG (2005) Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well at their radical scavenging activity. Food Chem 91:571–577
Mishra K, Ojha H, Chaudhury NK (2012) Estimation of antiradical properties of antioxidants using DPPH˙ assay: a critical review and results. Food Chem 130:1036–1043
Oyaizu M (1986) Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Japanese J Nutr 44:307–315
Ozgen M, Reese RN, Tulio AZ Jr, Scheerens JC, Miller AR (2006) Modified 2, 2-Azino-bis-3-ethylbenzothiazoline-6-sulfonic Acid (ABTS) method to measure antioxidant capacity of selected small fruits and comparison to ferric reducing antioxidant power (FRAP) and 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) Methods. J Agric Food Chem 54:1151–1157
Rice-Evans CA, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 20:933–956
Rufino MSM, Alves RE, Fernandes FAN, Brito ES (2011) Free radical scavenging behaviour of ten exotic tropical fruits extracts. Food Res Int 44:2072–2075
Sharma OP, Bhat TK (2009) DPPH antioxidant assay revisited. Food Chem 113:1202–1205
Singleton VL, Rossi JA Jr (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16(3):144–158, http://ajevonline.org/content/16/3/144.abstract
Thiex NJ, Manson H, Andersson S, Persson JA (2002) Determination of crude protein in animal feed, forage, grain, and oilseeds by using block digestion with a copper catalyst and steam distillation into boric acid: collaborative study. J AOAC Int 85(2):309–317
Yilmaz KU, Ercisli S, Zengin Y, Sengul M, Kafkas EY (2009) Prelimimary characterization of corneliean cherry (Cornus mas L.) genotypes for their physico-chemical properties. Food Chem 114:408–412
Acknowledgments
The first author is grateful to Department of Biotechnology, Govt. of India for granting fellowship and providing necessary facilities to the Institute.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, P.B., Handique, P.J. & Devi, H.S. Antioxidant properties, physico-chemical characteristics and proximate composition of five wild fruits of Manipur, India. J Food Sci Technol 52, 894–902 (2015). https://doi.org/10.1007/s13197-013-1128-2
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-013-1128-2