Abstract
In the view of recent growing interest in utilization of grain fractions as food ingredient, present investigation was carried out to evaluate the roller milling potential of green gram. The effect of conditioning moistures on green gram roller milling were studied. The results showed decrease in flour yield from 85.56 to 58.74 % with increase in conditioning moisture from 10 to 16 %. Higher yield of flour was observed from the first (C1), second (C2) and third (C3) reduction passages; whereas, the first (B1), second (B2) and third (B3) break passages produced less flour. The distribution of protein, dietary fiber, ash and fat in different flour streams and by-products from roller milled fractions of green gram showed wide variation. The protein content increased with increasing numbers of breaks and reductions in the flour streams. The highest protein content of 30.16 % was found in bran duster flour and lowest (11.32 %) in fine seed coat. The protein content of break streams was found lower than reduction streams. The dietary fiber content of coarse seed coat was highest (71.17 %) followed by the fine seed coat (57.22 %). The microstructure studies of milled fractions of green gram showed more deformed and damaged starch granules in reduction flour streams than break flour streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Green gram is one of the important pulse crops cultivated in India since ancient times and it is widely cultivated throughout the Asia. Legumes have an important place in human nutrition, particularly in India. Legume seeds are rich in proteins and complex carbohydrates (dietary fibers) and are important source of minerals and vitamins (Rege 1981). Among the grain legumes, green gram is known for its easy digestibility, low flatulence potential and high protein content (Doughty and Walker 1982). The green gram has three major components, viz., seed coat, cotyledon and embryo, accounting for 12.1, 85.6 and 2.3 % of the whole seed, respectively (Singh et al. 1968).
Roller milling method is traditionally used to fractionate wheat and not used for other grains. Roller milling of wheat generates the fractions which vary in protein content, starch damage, ash content and enzyme activity (Dube et al. 1987; Banu et al. 2010). Roller milling has the potential to fractionate the grain into various streams with unique composition and functionality. Several studies have been conducted in which hull-less barley has been experimentally milled to obtain flour and bran (Wang et al. 1993; Danielson et al. 1996; Bhatty 1997, 1999; Klamczynski and Czuchajowska 1999; Kiryluk et al. 2000). Recently, Kiryluk and coworkers (2000) have shown that by implementing certain adjustments in a wheat roller mill, new and nutritionally valuable barley products may be obtained.
The nutrients are not distributed evenly in the different tissue of the green gram seed. The embryo has higher content of protein and lipids, while starch and crude fibers are concentrated in cotyledons and seed coat respectively (Singh et al. 1968).
The roller milling of green gram fractionates the grain into various fractions and the fractions derived by roller mill have not yet been standardized in terms of quality, composition, or terminology.
The aim of the present study is to fractionate green gram by roller milling to evaluate its milling potential and to study the effect of conditioning moisture on milling yield. The roller milled fractions of green gram were evaluated for the different chemical characteristics and subjected to scanning electronic microscopic study to observe the changes that occur in the milled fractions during roller milling.
Materials and methods
Raw material
Commercial green gram procured from the local market was used for the present study.
Raw material characterization
Analysis of green gram was carried out for physical and chemical characteristics. The green gram was analyzed for moisture content (method 44-15), ash (method 08-01), protein (method 46-10), fat (method 30-10) according to the standard methods (AACC 2000) and dietary fiber (AOAC 1999). Hectoliter weight and the thousand-kernel weight were determined by standard procedures (Pomeranz 1988).
Preparation of green gram
Green gram was cleaned by using the standard apparatus labofix cleaner (Schmidt-Seeger GmbH, Germany) to remove all foreign matters such as dust, dirt and chaff as well as immature and broken seeds. The sample were conditioned to raise the moisture content to 18–20 % followed by drying in oven to the different moisture contents (8,10,12,14 and 16 %). The samples of cleaned green gram were weighed and poured in to the batch rotary drum mixer of 20 kg capacity. The required water quantity to raise the moisture content to 18–20 % was calculated using following equation.
Where:
- Q:
-
is the amount of water (L).
- W:
-
is the weight of seed at initial moisture content (kg).
- M2:
-
is the desired moisture content of seed (%).
- M1:
-
is the initial moisture content of seed (%).
Total quantity of water for the batch was divided into 2–4 fractions and sprinkled on the grains. After each sprinkling, the mixture was rotated for about 5 min and about 1 h in the end. The conditioned grain were removed from the closed drum after 12 h and placed in a rectangular wire mesh for drying in the oven at 65 °C (Singh and Agrwal 2005). The drying time required to reach the desired moisture content was found by drying curve developed for green gram as shown in Fig. 1.
Milling of Green Gram
The cleaned and prepared green gram samples were milled in a lab mill (MLU 202, Buhler, Switzerland). The lab mill consists of three breaks (B1 to B3), three reduction (C1 to C3) passages and one laboratory bran duster.
The break rolls (B1-B3) were adjusted to separate the cotyledon from seed coat and germ with clean separation. The B1 roll gap was adjusted to give big granular clean cotyledon grits with minimum damage to seed coat. The ground stock from the B1 was sifted in the sifter for the size grading. The first scalping was further processed to remove the cotyledon by break passages and sifter. The over-tails from the flour sieves of break passages were sent to the reduction passages. Reduction passages (C1 to C3) were utilized to produce maximum flour by scalping seed coat and germ by making them flakier. Fine seed coat was collected as an over-tail of flour sieve from last reduction passage. The lab milling flow sheet is shown in the Fig. 2. The flour streams were collected from all break (B1 to B3) and reduction (C1 to C3) passages along with coarse and fine seed coat used for the chemical and microstructure studies.
Physicochemical characteristics of streams and milled products
The streams and milled products were analyzed for moisture content (method 44-15), ash (method 08-01), protein (method 46-10), fat (method 30-10), damage starch (method 76-30) according to the standard methods (AACC 2000) and dietary fiber (AOAC 1999). Each analysis was carried out in triplicate and mean value reported.
Microstructure analysis using scanning electron microscopy
The scanning electron microscopy studies of milled fractions of green gram samples were carried out using Leo scanning electron microscope (SEM) model 435VP (UK) (Prabhasankar et al. 2003). Samples were placed on a specimen holder with the help of double-sided scotch tape and sputter-coated with gold (2 min, 2 mbar). Finally each sample was transferred to a microscope where it was observed at 15 kV and a vacuum of 9.75 × 10−5 torr.
Color measurement
The color of the roller milled fractions of green gram was measured in term of lightness (L) and color (+ a: red, −a: green, +b: yellow, −b: blue) using the Hunter Lab Color Measuring System (Color Measuring Labscan XE system, Reston, VA). A white board made from barium sulfate 100 % reflectance was used as a standard. Milled fractions were placed in the sample holder and the reflectance was auto recorded for the wavelength ranging from 360 to 800 nm.
Statistical analysis
The experiments were carried out in three replicates. The data were statistically analyzed and the treatments were tested using Duncan’s multiple range tests (Steel and Tarrie 1980).
Results and discussion
Physico-chemical characteristics of green gram
The green gram selected for the study had the following physico-chemical characteristics: moisture content 10.4 %, thousand kernel weight- 39 g, hectoliter weight- 79 Kg/hectoliter, ash - 3.1 %, protein - 24.15 %, dietary fiber - 16.7 % and fat - 1.67 %.
Milling of green gram
Roller milling of the conditioned green gram samples was carried out by the Lab Mill (MLU-202), which included six grinding stages and their corresponding sifter sections. It consisted of three breaks (B1, B2 & B3) and three reduction (C1, C2 & C3) passages. Six flours streams along with the coarse seed coat and fine seed coat were produced from the mill. The fine seed coat further passed through the laboratory bran duster to recover the flour. Rolls were adjusted to get the good flour yields from green gram. The milling was carried out at 16, 14, 12, 10 & 8 % moisture content to optimization of the conditioning moisture.
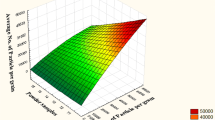
The yields of the roller milled fractions of green gram at different pre conditioned moisture are shown in Fig. 3a. The objectives of break rolls (B1 - B3) are to cut open the green gram and remove the cotyledons from the seed coat with least amount of seed coat contamination. The front break passages releases relatively pure particles of cotyledon and tail break passages cleans up the seed coat and releases smaller pieces of cotyledon along with the more fine pieces of seed coat and germ. The roll gap goes on decreasing from B1 to B3. The flour produced by the break roll is very less, as the job of break roll is not to produce flour. Among the different break passages, it is observed that the B1 passage has produced higher flour followed by the B2 and B3. This could be due to direct impact on the grain at first break passage. The flour yield from the B3 passage gradually decreased with decrease in conditioning moisture from 16 to 8 %. This has resulted because of B3 rolls were more pressed at higher moisture milling where as at low moisture grinding they kept open because of easy separation of seed coat from cotyledon.
a Yields of roller milled fractions of green gram at different pre conditioned moisture and b Yields of straight run flour (SRF) and seed coat at different pre conditioned moisture. B1F break 1 flour, B2F break 2 flour, B3F break 3 flour, C1F reduction 1 flour, C2F reduction 2 flour, C3F reduction 3 flour, BDRF bran duster flour, CSC coarse seed coat, FSC fine seed coat, n = 3
The cotyledons released by the break rolls and graded by sifter are ground at reduction rolls into flour. As shown by the results, more yield of flour has come from the front reduction passages C1 and C2, where as the last reduction passage contributed less quantity of flour. Flour production of reduction passages (C1 - C3) increased with decrease in the moisture content of milled green gram. This is because of higher moisture cotyledon is sticky in characteristic and difficult to sift, whereas optimally mellowed cotyledon is easy for sifting and gives more capacity. The C1 passage contributed highest yield of flour followed by C2 and C3 passages. The C1 flour yield varied from 25 % at 16 % moisture to 36.98 % at 8 % moisture.
The Fig. 3b observes the yields of straight run flour (SRF) and seed coat at different pre conditioned moisture. The coarse seed coat content decreased from 39.07 at 16 % moisture to 10.17 % at 10 % moisture content and increased to 10.29 % at 8 % moisture. The same trend was observed for the total seed coat, which is the combination of coarse and fine seed coat.
Green gram seed contain seed coat of 12.1 %, efficient separation of seed coat from the endosperm was observed at 10 % moisture. The yield of coarse and fine seed coat observed higher at 16, 14 and 12 % moisture with many cotyledon particles in it, where as the lower yield with clean separation was observed at 10 and 8 % moisture.
The milling yield was gradually increased from the 16 % moisture content to 10 % moisture content and then decreased at 8 %. The highest yield of 85.46 % was obtained at 10 % moisture content whereas the lowest of 58.74 % at 16 % moisture. The yield was 85.11 % at 8 % moisture content as shown in Fig. 3. The pre treatment with moisture content of 10 % was found optimum for the green gram roller milling, which has given the higher flour yield with efficient separation of seed coat and cotyledon. The milled fractions produced at 10 % moisture content were studied for the chemical and microstructural analysis.
Chemical characteristics of rolled milled fractions of green gram
The data (Fig. 4) shows that the moisture content of break flours are higher than the reduction flours. In the milling process the milled products are transported by pneumatics, ground into flour by rolls with pressure that results in the moisture loss. Reduction passages ground cotyledon into flour with pressure and transported by more pneumatic lift than break passages, which resulted into the higher moisture loss. Ash content was found less for front break and reduction passages. The lowest ash of 3.01 % was obtained at B1 passages whereas highest of 3.72 % was obtained from the fine bran. The data show the presence of higher protein content in the reduction flours than the break flours. The protein content of 23.68 %, 24.04 % and 24.28 % were observed for B1F, B2F and B3F respectively. The protein content of 24.42 %, 26.56 %, 29.26 % and 30.16 % were observed for C1F, C2F, C3F and BDRF respectively.
Chemical characteristics of roller milled fractions of green gram. Except moisture, all values are on dry weight basis. B1F break 1 flour, B2F break 2 flour, B3F break 3 flour, C1F reduction 1 flour, C2F reduction 2 flour, C3F reduction 3 flour, BDRF bran duster flour, CSC coarse seed coat, FSC fine seed coat, n = 3
The protein content of break passages increased from B1 (23.68 %) to B3 (24.28 %). The same pattern was observed for the reduction passages from C1 (24.42 %) to C3 (29.26 %). The protein content of reduction passages flours was higher than the break passages flours. The highest protein content of 30.16 % was obtained from the bran duster flour. The protein content of 11.32 % and 15.02 % was observed for coarse and fine seed coat respectively. The fiber content of break passages has shown increased pattern from B1 to B3. The same was observed for the reduction passages from C1 (0.45 %) to C3 (0.90 %). The dietary fiber content of coarse bran was found to be highest (71.17 %) followed by the fine bran (57.22 %). The fat content of milled fractions of green gram ranged between 0.65 % content in coarse bran to 3.12 % for fine bran. The data shows presence of higher starch damage values for the reduction passages than the break passages. This could be due to more severity of grinding in the reduction rolls than the break rolls. Similar observations were made by Villanueva et al. (2001). Straight run flour, which is the combination of all flour streams, has shown the damage starch percentage of 4.84 %.
Colour measurement of roller milled fractions of green gram
Table 1 shows the color measurement values for the milled fractions of green gram. The L, which represents the lightness values were higher for the break flours than the reduction flours. This could be due to more roll gap at break rolls does not produce the seed coat powder, where as the reduction rolls are closely set to produce flour which results into the production of seed coat powder. Among the break passages flour the B2F shown the higher L value of 85.91 followed by B1 F (85.46) and B3F (84.45).
Front reduction passages C1F and C2F have shown the L values of 84.21 and 84.52 respectively. Last reduction passage C3F has shown lower L value of 83.67 as the less cotyledon and more seed coat is received by this passage. DBRF observed the L value of 73.93 as this flour contained the more seed coat contamination. The L values for coarse and fine seed coat were 39.64 and 50.91 respectively.
The yellowness value (+b) was higher for the reduction passages flours than the break passages flours. The yellowness value (+b) for the SRF was 20.06. The higher value (+b) could be attributed to carotene content and other pigments present in flours. The greenness value (−a) has not shown significant difference and ranged in between −2.03 and −2.91 for the milled fractions. The color difference (ΔE) showed a significant difference in coarse seed coat, fine seed coat and DBRF.
Scanning electron microscopic studies
The micrographs of B1F, B2F and B3F streams are shown in Fig. 5. The small and large starch granules as well as the protein matrix were observed in the micrograph of break flours. The micrograph shows intact starch granules compactly packed in the protein matrix. The starch exists as an aggregate composed of several individual elliptical granules located in each cell with neighboring discrete protein bodies. Joseph et al. (1993) stated that in the microstructure of black, green and red gram cotyledons elliptical starch granules embedded in a protein were observed. The starch emptied cells was also visible in the micrograph. A proportion of the starch was also seen in single granules. It also observed in micrograph that the aggregates are higher than the single granules. This could be due to adjustment of break roll, which has more clearance. They were set only to scrape the endosperm from the bran.
Figure 6a–d are the micrograph of C1F, C2F, C3F and DBRF. The small and large starch granules as well as the protein matrix were observed in the micrograph. The starch mainly seen as single, unaggregated granules. This looks like the highly compactly packed structure of the grain appear to be broken up into aggregates of protein matrix embedding groups of cellular components mainly starch granules which could be because of the close grinding at reduction rolls. More deformed and damaged starch granules were observed in the micrograph of reduction flours. The deformation and mechanical damage of starches were observed more towards the end reduction passages, which could be due to severity of the grinding during the milling process. The percentage of damaged starch increased, as cotyledon was ground several times in the reduction rolls (C1 to C3 reduction passages). Traces of bran particles were observed in micrographs of DBR flour sample. Figure 7a–d are the micrograph of coarse and fine seed coat with 100X and 1000X. Less quantity of starch granules and protein were observed in the micrograph of coarse seed coat. The micrograph shows more fiber content in coarse seed coat, same was observed during the chemical analysis.
Conclusion
In this study, the results illustrate that roller milling of the green gram generate different products with highly variation in composition. The conditioning moisture has shown the significant influence on the yield of refined flour. The milling yield of refined flour gradually increased from the 16 % conditioning moisture content to 10 % conditioning moisture content. The process was optimized for the roller milling of the green gram with higher yield of refined flour at 10 % conditioning moisture. The chemical characteristics of the milled fractions showed variation in the protein and fiber content. Roller milling of green gram was able to produce the fraction with 30.16 % protein content and dietary fiber content of 71.17 %. The study also observed that roller milling was able to produce >20 % flour with 29 % protein content and 10.17 % coarse seed coat with 71.17 % dietary fiber content. The color measurement values showed higher brightness (L) for flour streams and straight run flour by making it suitable for incorporation into the wheat based products.
Thus the roller milling has proved to be a valuable method for the fractionation of green gram with the generation of protein and fiber rich fractions. Incorporation of protein and fiber rich milled fractions of green gram into the bakery and Indian traditional food products will increase the nutritive value of the food.
References
AACC (2000) American Association of Cereal Chemists. Approved methods. St. Paul, MN, AACC
AOAC (1999) Official Methods of Analysis of AOAC International, method 991.43, 16th edn. Maryland, USA: AOAC International
Banu I, Stoenescu G, Ionescu V, Aprodu I (2010) Physicochemical and rheological analysis of flour mill streams. Cereal Chem 87(2):112–117
Bhatty RS (1997) Milling of regular and waxy starch hull-less barleys for the production of bran and flour. Cereal Chem 74:693–699
Bhatty RS (1999) β-Glucan and flour yield of hull-less barley. Cereal Chem 76:314–315
Danielson AD, Newman RK, Newman CW (1996) Proximate analysis, β-glucan, fiber and viscosity of select barley milling fractions. Cereal Res Commun 24:461–468
Doughty J, Walker A (1982) Legumes in human nutrition. FAO food and nutrition Paper 20, FAO, Rome, p 46
Dube R, Indrani D, Sidhu JS (1987) Flour mill streams. I. Physico-chemical and rheological characteristics. Indian Miller 1:17–34
Joseph E, Crites SG, Swanson BG (1993) Microstructure of black, green and red gram. Food Struct 12:155–162
Kiryluk J, Kawka A, Gasiorowski A, Chalcarz A, Aniola J (2000) Milling of barley to obtain β-glucan enriched products. Nahrung 44:238–241
Klamczynski AP, Czuchajowska Z (1999) Quality of flours from waxy and nonwaxy barley for production of baked products. Cereal Chem 76:530–535
Pomeranz (1988) In: Wheat chemistry and technology vol. 1 (pp 17–21). St. Paul, MN, USA: American of Association of Cereal Chemists
Prabhasankar P, Indrani D, Jyotsna R, Venkateswara Rao G (2003) Scanning electron microscopic and electrophoretic studies of the baking process of south Indian parotta—an unleavened flat bread. Food Chem 82:603–609
Rege DV (1981) Nutritional aspects of legumes: Some research needs. In: Aiyer A, Iyer KR (eds) Proceeding of the workshop on grain legumes. Protein Foods and Nutritional Development Association of India, Bombay, pp 123–132
Singh SK, Agrwal US (2005) Optimization of grain and machine conditions for milling of green gram (Phaseolus aureus). J Food Sci Technol 42:11–16
Singh S, Singh HD, Sikka KC (1968) Distribution of nutrients in the anatomical part of common Indian pulses. Cereal Chem 45:13–18
Steel RGD, Tarrie JH (1980) Principles and procedures of statistics. McGraw-Hill, New York
Villanueva RM, Leong MH, Posner ES, Ponte JG (2001) Split milling of wheat for diverse end-use products. Cereal Foods World 46:363–369
Wang L, Que Q, Newman RK, Newman CW (1993) Enrichment of tocopherols and oil in barley fractions by milling and pearling. Cereal Chem 70:499–501
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sakhare, S.D., Inamdar, A.A., Gaikwad, S.B. et al. Roller milling fractionation of green gram (Vigna radiata): optimization of milling conditions and chemical characterization of millstreams. J Food Sci Technol 51, 3854–3861 (2014). https://doi.org/10.1007/s13197-012-0903-9
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-012-0903-9