Abstract
Six bean (Phaseolus vulgaris L.) cultivars of Himalayan region were analysed for α- amylase inhibitor activity. The α-amylase inhibitor from seeds of screened bean cultivar KR-9, showing maximum inhibitory activity was purified using ammonium sulfate precipitation, gel filtration chromatography (Sephadex G-100) and ion exchange chromatography (DEAE-Sephadex). The inhibitor was purified to homogeneity as judged by native-PAGE with 14.22 fold purification and 71.66% recovery. Purified inhibitor consisted of three subunits of molecular weight 15,488, 18,620 and 26,302 daltons, respectively as determined by SDS-PAGE. It was found to be heat stable up to 30 °C–40 °C and had two pH optima of 5.0 and 6.9. Nature of inhibition was found to be of non-competitive type. The purified inhibitor was found to be effective against α-amylases extracted from larvae of Callosobruchus chinensis, Tribolium castaneum and gut enzyme of Spodoptera littoralis. Larvae of Tribolium castaneum fed on flour mixed with purified inhibitor for 5 days showed 100% larval mortality. Purified α-amylase inhibitor was also found to inhibit human salivary α-amylase, suggesting its potential in prevention and therapy of obesity and use as drug design targets for treatment of diabetes. The gene encoding the inhibitor may be used to develop transgenic plants resistant against insect pests.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants have acquired certain degree of defense mechanisms during evolution, which include secondary chemical compounds toxic to or antimetabolic to insect pests (Franco et al. 2002). Out of these defense compounds, the enzyme inhibitors present in seeds and vegetative organs are found to be important in eliciting resistance to insect attack by inhibiting the gut enzymes of insects (Konarev 1996). α-Amylase inhibitors (α-AI’s) have the ability to impede the activity of α-amylases found mainly in insects and mammals. These inhibitors provide resistance to crop plants against pests by interfering in their digestion/reproduction which causes moderate mortality, prolonged larval developmental time and reduced fecundity. A number of α-amylase inhibitors have been identified and extensively studied in legumes like common bean (Phaseolus vulgaris), mung bean (V. sublobata) (Kokiladevi et al. 2005), rye (Iulek et al. 2000), wheat (Heidari et al. 2005), barley (Waselake et al. 1983), sorghum (Kutty and Pattabiraman 1986) and ragi (Kumar et al. 1998). Insecticidal activity of α-amylase inhibitors are focussed particularly against weevils like cowpea weevil (Callosobruchus maculatus) and adzuki bean weevil (Callosobruchus chinensis) as these are highly dependent on starch for their energy supply.
Utilization of α-amylase inhibitor gene(s) of plant origin as bio-insecticide for developing insect resistant transgenic crop plant has been a major project in crop biotechnological programmes. Transgenic peas, chick peas and rice have been developed using common bean amylase inhibitor through genetic transformation. The identification and screening of plant sources having potent α-amylase inhibitors is needed to develop resistant plant cultivars and this can be made only when the nature of enzyme inhibitor and structure of enzyme-inhibitor complex have been taken into account (Lee et al. 2002).
Keeping in view the above facts and paucity of information available on α-amylase inhibitors in bean cultivars of Himalayan region, the present investigations were undertaken to purify and characterize the α-amylase inhibitor from bean cultivar.
Materials and methods
Seeds of six bean (Phaseolus vulgaris) cutivars (KR-9, KR-24, KR-84, KR-101, KR-133, KRC-5) were procured form Chaudhary Sharvan Kumar Himachal Pradesh Krishi Vishvavidyalaya, Mountain Agriculture Research and Extension Centre Sangla, Kinnaur (HP). The chemicals were procured from Sigma Aldrich (USA), SRL, Pvt Ltd. (India) and Merck (Germany). The chemical and reagents used were of analytical grade.
Preparation of crude extract
Seeds of bean cultivars were ground to make a fine powder. The flour was then extracted using different extraction buffers. Seed flour (100 mg in10 ml) was extracted in 10 mM Tris HCl buffer (pH 7.5) containing 500 mM NaCl, 1% 2- mercaptoethanol, 0.1% Triton-X-100, 2 mM phenyl methyl sulphonyl fluoride (PMSF) at 4 °C for 1 h. The suspensions obtained were then centrifuged at 15,000 rpm for 15 min at 4 °C.
Purification of α -amylase inhibitor from screened bean cultivar
The supernatant was brought to 20–80% saturation with ammonium sulfate at 4 °C and centrifuged at 10,000 rpm for 20 min., the pellet was resuspended in minimum volume of 10 mM Tris HCl buffer (pH 7.5) and dialyzed over night. α-Amylase inhibitor was further purified by gel filtration chromatography of ammonium sulfate precipitated fraction (20–80%) on Sephadex G-100 chromatography column (31 × 2.5 cm) and eluted with 50 mM sodium phosphate buffer (pH 6.9) (Fig. 1). The collected fractions were analyzed for protein content at 280 nm and α-amylase inhibitor activity. Most active fractions were pooled and stored at 4 °C. The active pooled fractions obtained from G-100 gel filtration chromatography were loaded in small lots on ion exchange column, DEAE-Sephadex (A50) (Fig. 1). A flow rate of 12 ml per hour was maintained. The column was first eluted with 50 mM sodium phosphate buffer (pH 6.9) to wash out the unbound protein. The bound proteins were eluted with linear salt gradient (2 bed volumes) of 0.1 M, 0.2 M, 0.3 M and 0.4 M KCl in distilled water. Fractions of 2 ml each were collected and monitored for α-amylase inhibitor activity and the protein content was measured at 280 nm. The active fractions were pooled and concentrated against solid sucrose at 4 °C and used for further studies. The purification experiment was carried out in three replications.
α-Amylase inhibitor activity
The α-amylase inhibitor activity was measured by quantifying the reducing sugar (Maltose equivalent) as described by Bernfeld (1955) .α-Amylase enzyme and α-amylase inhibitor were pre-incubated for 5 min at 30 °C in a metabolic shaking water bath. This was followed by the addition of starch. The reaction was stopped by adding Dinitrosalicylic acid (DNSA) reagent after 5 min. The contents were heated for 10 min in boiling water bath. The same procedure was followed for the control, except for the fact that α-amylase inhibitor was not added and the volume of the reaction mixture was adjusted with sodium phosphate buffer (pH 6.9). Blank did not contain the α-amylase enzyme and the volume was replaced with equal quantities of sodium phosphate buffer (pH 6.9). The absorbance of the colour developed was measured at 530 nm against blank. One unit of α-amylase was defined as the amount of the enzyme that liberated one μmole of maltose under the assay conditions. One unit of α-amylase inhibitor activity was defined as the reduction in amylase activity by one unit.
Estimation of soluble protein
The soluble protein was estimated after each step of purification as described by Lowry et al. (1951).
Characterization of α -amylase inhibitor from screened bean cultivar
Polyacrylamide gel electrophoresis
The purity of α-amylase inhibitor protein obtained was checked by native polyacrylamide gel electrophoresis (native-PAGE) and the method adopted was anionic system of Davis (1964). Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was carried out to determine the subunit composition of the purified inhibitor.
Molecular weight determination
The molecular weight was determined by SDS-PAGE using standard molecular weight markers.
Effect of inhibitor concentration
The effect of varying inhibitor concentration on α-amylase activity was studied. The inhibitor concentration was varied from 6.6 to 73.2 μg in the assay mixture and α- amylase inhibitor activity was monitored at 530 nm.
Effect of temperature on stability of trypsin inhibitor
The inhibitor was incubated for 10 min at 20,30,40,50,60,70,80 and 90 °C. After incubation, the test tubes were immediately cooled in ice bath and inhibitor activity was measured at 530 nm.
Effect of pH
Different buffers viz., acetate buffer (pH 4.0 to 6.0), phosphate buffer (pH 6.0 to 8.0) and Tris buffer (pH 8.0 to 9.0) were used in the reaction mixture and α-amylase inhibitor activity was then monitored.
Effect of pre-incubation temperature on the activity of α-amylase inhibitor
The purified inhibitor was incubated with α-amylase at various temperatures between 25 °C and 50 °C. At each temperature, controls without added inhibitor were taken and α- amylase inhibitor activity was then monitored at 530 nm against the blank.
Effect of reaction time
The effect of reaction time on α-amylase inhibitor activity was studied by stopping the reaction after 5, 15, 30 and 60 min of incubation and inhibitor activity was monitored at 530 nm.
Determination of nature of inhibition
To determine the nature of inhibition, two different fixed inhibitor concentrations were used. Lineweaver and Burk (1934) plot was plotted using different concentrations of substrate in the presence and absence of inhibitor and Km value was determined. Dixons plot was plotted using different concentrations of inhibitor at two fixed concentrations of substrate. The Ki value was determined from the plot as described by Dixon (1953).
Effect of purified α-amylase inhibitor on α-amylase enzyme extracted from larvae of Callosobruchus chinensis
Infested black gram seeds were dissected and the active larvae (100 in number) were homogenized in 2 ml of extraction buffer (50 mM sodium phosphate buffer, pH 6.9) followed by centrifugation at 10,000 rpm for 15 min at 4 °C. The supernatant obtained was stored at 4 °C and used as the source of α-amylase enzyme without any dilution.
Effect of purified α-amylase inhibitor on α-amylase enzyme extracted from larvae of Tribolium castaneum
Larvae of Tribolium castaneum were taken from wheat flour (100 in number) and homogenized in 2 ml of 50 mM sodium phosphate buffer (pH 6.9) followed by centrifugation at 10,000 rpm for 15 min at 4 °C and supernatant was used as the source of enzyme.
Effect of purified α-amylase inhibitor on gut α-amylase enzyme extracted from larvae of Spodoptera littoralis
Midguts from actively growing larvae were dissected out and the extract was prepared by homogenizing them with 50 mM sodium phosphate buffer (pH 6.9) in chilled test tube using a glass rod. The homogenate thus obtained was filtered through filter paper and the filtrate was then used as the enzyme extract.
Effect of α-amylase inhibitor on larvae of Tribolium castaneum
Purified α-amylase inhibitor activity was tested against Tribolium castaneum. The wheat flour (2 g) was mixed with 1 ml (333 μg) of purified α-amylase inhibitor (Treatment). Feeding assay was conducted by feeding five larvae of Tribolium castaneum on treated flour. Same number of larvae was placed on flour mixed with 1 ml of distilled water (control). The per cent mortality and weight of flour eaten was recorded.
Effect of purified α-amylase inhibitor on human salivary amylase
Fresh human saliva was taken as a source of α-amylase enzyme and inhibition assay was preformed as described earlier.
Statistical analysis
All the biochemical estimations were done in three replications with duplicates for each replicate. For plotting graphs only mean values were used. The purification experiment and electrophoresis were repeated three times. In feeding bioassay the experiment was conducted in three sets and C.D. was calculated for treatment, time interval and the interaction between the two.
Results and discussion
The α-amylase inhibitor was purified to 14.22 fold with 71.66% recovery from screened KR-9 bean cultivar by ammonium sulphate precipitation and subsequent chromatographic separation on Sephadex G-100 and DEAE-Sephadex (Table 1). Ho and Whitaker (1993) purified inhibitor to 18.5 fold by ethanol fractionation and DEAE-cellulose chromatography from white kidney bean. Kokiladevi et al. (2005) reported 63.7% recovery with 7.48 fold purification of α-amylase inhibitor from Vigna sublobata following ammonium sulphate precipitation, Sephadex G-50 and reversed phase-high profile liquid chromatography. Hivrale et al. (2011) purified an alpha amylase inhibitor from Achyranthes aspera seeds to 9.99 folds.
Native PAGE confirmed the homogeneity of the purified α-amylase inhibitor with relative mobility of 0.66. Similarly, it was also purified to homogeneity from Phaseolus vulgaris (Mirkov et al. 1995), Lablab purpureus (Janarthanan et al. 1999), as judged by native PAGE. Subunit composition of the purified α-amylase inhibitor was detected using SDS-PAGE, which revealed the inhibitor to be composed of three subunits with molecular weight of 15,488, 18,620 and 26,302 daltons. Heat labile alpha amylase inhibitor from white kidney beans was reported to be composed of three subunits α, β, and γ with molecular weights of 7800, 14000 and 22000, respectively by SDS-PAGE (Yamaguchi 1993). A similar heat labile heterotrimer was reported from white kidney bean by Wato et al. (2000). Sawada et al. (2001) reported the inhibitor from P.vulgaris to be a glycoprotein with molecular weight of 45,000 having subunit molecular weights of 14,000 and 30,000 daltons. However, Suzuki and Ishimoto (1999) reported four subunits in purified α-amylase inhibitor from P. vulgaris with molecular weight ranging from 14,000–20,000 daltons. Hivrale et al. (2011) also detected two alpha amylase inhibitor activity bands with different molecular weights, on starch polyacrylamide gel. On contrary, SDS-PAGE revealed single band in case of Phaseolus vulgaris (Yang et al. 2008) and rye (Iulek et al. 2000).
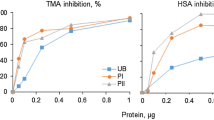
No trypsin inhibitor activity was found in the purified inhibitor during present studies. When tested for the presence of carbohydrates by Molisch and Anthrone’s tests, negative results were obtained. However the inhibitor from Phaseolus bean cultivars was reported to be a glycoprotein by Sawada et al. (2001) and Yang et al. (2008). In present studies, the inhibition was found to increase upto 85% with the increase in concentration of purified α-amylase inhibitor in the assay, however, at higher concentrations the degree of inhibition was constant. Strumeyer and Fisher (1983) reported inhibition to increase linearly up to 70–75% with the increase in concentration of purified wheat α-amylase inhibitor. Kutty and Pattabiraman (1986) found inhibition to be linear up to 80% with increasing levels of sorghum inhibitor concentration and this inhibitory pattern deviated from linearity at higher concentrations.
The purified inhibitor from KR-9 cultivar was found to be stable upto 30 °C–40 °C for 10 min and lost its activity at higher temperatures (Fig. 2). Similarly heat labile α-amylase inhibitors were reported from P. vulgaris seeds by Grant et al. (1995) and Kotaru et al. (1989). However, Sasikiran et al. (2004) found heat stable α-amylase inhibitor from lesser yam bean (D. esculenta). Hivrale et al. (2011) reported purified α-amylase inhibitor (6 KDa) to be heat stable. The purified α-amylase inhibitor was found to have two pH optima of 5.0 and 6.9 from KR-9 cultivar of bean (Fig. 2). The optimum pH for common bean inhibitor was found to be 5 (Grossi-de-Sa et al. 1997) and for maize it was 6.8 (Labra et al. 1995). Frels and Rupnow (1985) found the extent of inhibition to be less below pH 4.5 and above 6.0 and no inhibition was detected at pH 7.5.
In the present studies the maximum activity for the purified α-amylase inhibitor from KR-9 cultivar of bean was observed at 30 °C–35 °C. Similar results were observed in case of bean seeds by Frels and Rupnow (1985) and Power and Whitaker (1977) and for rye α-amylase inhibitor by Granum (1978). The time period required for incubation with amylase enzyme to achieve maximum inhibition was 5 min in the screened been cultivar (KR-9). Strumeyer and Fisher (1983) reported 10 min for wheat inhibitor, Granum (1978) observed 15–30 min for rye α-amylase inhibitor and it was 25 min for α-amylase inhibitor from yam bean (Sharma and Pattabiraman 1982). The Lineweaver Burk plot (Fig. 3) and Dixon’s plot (Fig. 3) revealed the inhibition pattern of purified inhibitor from KR-9 cultivar to be of non-competitive type. These results are in agreement with those of Marshall and Lauda (1975) and Frels and Rupnow (1985) who reported non-competitive mechanism of inhibition for P. vulgaris inhibitor. However, the nature of inhibition by α-amylase inhibitor from yam bean was found to be of uncompetitive type (Sharma and Pattabiraman 1982). The purified inhibitor was reported to be effective against larval extracts of Callosobruchus chinensis,Tribolium castaneum and gut α-amylase of Spodoptera littoralis. When 26.6 μg of the purified α-amylase inhibitor was included in the assay mixture, amylase units inhibited (α-AUI/ml) were found to be 25.74 ± 0.02, 16.22 ± 0.103 and 13.51 ± 0.072, respectively. Insecticidal activity of α-amylase inhibitor from common bean seeds (Ishimoto et al. 1999) was found against C. maculatus and C. chinensis. Heidari et al. (2005) observed broad inhibition specificity of α-amylase inhibitor purified from wheat against α-amylase of rice weevil (Sytophilus oryzae), red flour beetle (T. castaneum) and other bruchid insects. Sasikiran et al. (2004) found the α-amylase inhibitor purified from lesser yam to show inhibitory activity against rice weevil, sweet potato and coffee bean weevil. Khan (2011) also reported the inhibitory activity of proteinaceous inhibitors from seeds of chickpea, kidney bean, maize, wheat and millet against alpha-amylase from red flour bettle, Tribolium castaneum. During present studies, the Tribolium castaneum larvae showed significant decline in feeding after 3 days and 100% larval mortaility was observed after 5 days, when the larvae were fed on flour mixed with purified α-amylase inhibitor. In control, the weights (g) of flour eaten by the larvae on third and fifth days were 1.843 ± 0.08 and 1.565 ± 0.01, respectively. The larvae fed on flour mixed with 333 μg of inhibitor showed significant decline in feeding and the weight of flour eaten were 1.911 ± 0.05 and 1.746 ± 0.008, respectively after 3 and 5 days of feeding. The C.D. values for treatment (T), interval (I) and interaction (T x I) were 0.02, 0.025 and 0.034. This may be due to direct inhibition of digestive enzymes of the larvae. In the control after 45 days four larvae out of five emerged as adult. Similarly, 60% mortality of Callosobruchus chinensis larvae and 50% larval mortality of C. maculatus was observed when bioassay were done from Dipteryx alata (Bonavides et al. 2007) and Carica papaya seeds (Farias et al. 2006), respectively. Hivrale et al. (2011) also reported the survival of the larvae of Callosobruchus maculatus fed on diet containing seed powder of A. aspera to be severely affected and the highest mortality rate occurred on the fifth day of feeding. The transgenic pea seeds (Morton et al. 2000) containing α-amylase inhibitor from P.vulgaris seeds was found to be effective against pea weevil (Bruchus pisorum). De Sousa-Majer et al. (2007) reported 98% of larval morality of Bruchus pisorum at an early instar from transgenic pea seeds which contained α-amylase inhibitor gene from the common bean
The α-amylase inhibitor purified from P. vulgaris (KR-9) was found to be effective on human salivary α-amylase. The α-AUI/ml of the purified inhibitor was found to be 16.70 ± 0.037. Similarly, purified inhibitor from P.vulgaris (Yoshikawa et al. 1999), wheat (Heidari et al. 2005), A. aspera (Hivrale et al. 2011) and rye (Iulek et al. 2000) was found to be effective against human salivary amylase. However, protienaceous alpha amylase inhibitors from chick pea, kidney bean, maize, wheat and millet seeds did not inhibit human saliva α-amylase activity (Khan 2011). Similarly, no inhibitor activity of purified α-amylase inhibitor from Job’s tears (Ary et al. 1989) was found against human salivary enzyme. Ethanol and Hexane extracts of Phyllanthus amarus were reported by Tamil et al. (2010) to exhibit considerable alpha-amylase inhibitory activities.
Conclusion
Present studies were undertaken to uncover new α-amylase inhibitor, which may be used in genetic modification of crops i.e. gene encoding the inhibitor of KR-9 bean cultivar may be used to develop transgenic plants to confer resistance against insect pests. Inhibitory activities of α-amylase inhibitor against human salivary amylase suggested its potential in prevention and therapy of obesity and it can be used as drug design targets for treatment of diabetes. So, there is a need for the identification of effective α-amylase inhibitors with desirable characteristics from new sources.
References
Ary MB, Richardson M, Shewry PR (1989) Purification and characterization of an insect α-amylase inhibitor/endochitinase from seeds of job’s tears. (Coix lechryma jobi). Biochim Biophys Acta 999:260–266
Bernfeld P (1955) Amylases, α and β. In: Colowick SP, Kalpan NO (eds) Methods in enzymology. Academic, New York, pp 149–158
Bonavides KB, Pelegrini PB, Laumann RA, Grossi-de-Sa MF, Bloch JC, Melo JA, Quirino BF, Noronha EF, Franco OL (2007) Molecular identification of four different α-amylase inhibitors from baru (Dipteryx alata) seeds with activity toward insect enzymes. J Biochem Mol Biol 40:494–500
Davis BJ (1964) Disc electrophoresis-II: methods and application to human serum proteins. Ann New York Acad Sci 121:404–427
De Sousa-Majer MJ, Hardie DC, Turner NC, Higgins TJV (2007) Bean α-amylase inhibitors in transgenic peas inhibit development of pea weevil larvae. J Econ Entomol 189:1416–1422
Dixon M (1953) The determination of enzyme inhibitor constant. Biochem J 55:170–171
Farias LR, Costa FT, Souza LA, Pelegrini PB, Grossi-de-Sa SM, Bloch JC, Laumann RA, Noronha EF, Franco OL (2006) Isolation of a novel Carica papaya α-amylase inhibitor with deleterious activity towards Callosobruchus maculatus. Pestic Biochem Physiol 87:255–260
Franco OL, Rigden DJ, Melo FR, Grossi-de-Sa MF (2002) Plant α-amylase inhibitors and their interaction with insect α-amylases. Eur J Biochem 269:397–412
Frels JM, Rupnow JH (1985) Characterization of two α-amylase inhibitors from black bean (Phaseolus vulgaris). J Food Sci 50:72–78
Grant G, Edwards JE, Pusztai A (1995) α-Amylase inhibitor levels in seeds generally available in Europe. J Sci Food Agric 67:235–238
Granum PE (1978) Purification and characterization of α-amylase inhibitor from rye flour. J Food Biochem 2:103–107
Grossi-de-Sa MF, Mirkov TE, Ishimoto M, Colucci G, Bateman KS, Chrispeels MJ (1997) Molecular characterization of a bean α-amylase inhibitor that inhibits the α-amylase of the mexican bean weevil Zabrotes subfasciatus. Planta 203:295–303
Heidari R, Zareae S, Heidarizadeh M (2005) Extraction, purification and inhibitory effect of α-amylase inhibitor from wheat (Triticum aestivum var. Zarrin). Pakistan J Nutr 4:101–105
Hivrale VK, Chougule NP, Giri AP, Chhabdaa PJ, Kacholea MS (2011) Biochemical characterisation of α-amylase inhibitors from Achyranthes aspera and their interactions with digestive amylases of coleopteran and lepidopteran insects. J Sci Food Agric 91:1773–1780
Ho MF, Whitaker JF (1993) Purification and partial characterization of white kidney bean α-amylase inhibitor from two experimental cultivars. J Food Biochem 17:15–33
Ishimoto M, Yamada T, Kaga A (1999) Insecticidal activity of an α-amylase inhibitor like protein resembling a putative precursor of alpha amylase inhibitor in the common bean, (Phaseolus vulgaris L.). Biochim Biophys Acta 1432:104–112
Iulek J, Franco OL, Silva M, Slivinski CT, Bloch JC, Rigden DJ, Grossi-desa MF (2000) Purification, biochemical characterization and partial primary structure of a new α-amylase inhibitor from Secale cereale (rye). Int J Biochem Cell Biol 32:1195–1204
Janarthanan S, Venugopal KJ, Ignacimuthu S (1999) Purification and characterization of α-amylase inhibior from seeds of wild variety of Lablab purpureus that show resistance to the bruchid Callosobruchus maculatus. Indian J Expl Biol 37:778–781
Khan N (2011) In vitro effects of protienaceous alpha amylase inhibitors on red flour Bettle, Tribolium castaneum. Sci Res Rep 1:101–104
Kokiladevi E, Manickam A, Thayumanavan B (2005) Characterization of alpha-amylase inhibitor in Vigna sublobata. Bot Bull Acad Sinica 46:189–196
Konarev AV (1996) Interaction of insect digestive enzymes with plant protein inhibitors and host parasite co-evolution. Euphytica 92:84–89
Kotaru M, Yeh HY, Yoshikawa H, Ikeuchi T, Iwami K, Ibuki F (1989) Activity changes in cranberry bean (Phaseolus vulgaris) α-amylase inhibitor by chemical modification and enzymatic digestion. J Nutr Sci Vitaminol 35:71–80
Kumar A, Galaev IYu, Mattiasson B (1998) Isolation and separation of α-amylase inhibitors I-1 and I-2 from seeds of ragi (Indian finger millet, Eleusine coracana) by metal chelate affinity precipitation. Bioseparation 7:129–136
Kutty AVM, Pattabiraman TN (1986) Isolation and characterization of an amylase inhibitor from sorghum seeds specific for human enzymes. J Agric Food Chem 34:552–557
Labra AB, Lopez AC, Gallardo NM, Rodriguez SV (1995) Further characterization of the 12 kDa protease/alpha amylase inhibitor present in maize seeds. J Food Biochem 19:27–41
Lee SC, Gepts PL, Whitaker JR (2002) Protein structures of common bean (Phaseolus vulgaris) α-amylase inhibitors. J Agric Food Chem 50:6618–6627
Lineweaver H, Burk D (1934) The determination of enzyme dissociation constants. J Am Chem Soc 56:658–666
Lowry OH, Roseburgh NJ, Farr AK, Randall RJ (1951) Protein measurements with Folin’s phenol reagent. J Biol Chem 193:265–275
Marshall JJ, Lauda CM (1975) Purification and properties of phaseolamin an inhibitor of α-amylase from the kidney bean (Phaseolus vulgaris). J Biol Chem 250:8030–8037
Mirkov TE, Evans SV, Washletrom J, Gomez L, Young NM, Chrispeels MJ (1995) Location of the active site of the bean α-amylase inhibitor and involvement of a Trp, Arg, Tyr triad. Glycobiology 5:45–50
Morton RL, Schroeder HE, Bateman KS, Chrispeels MJ, Armstrong E, Higgins TJV (2000) Bean α-amylase inhibitor-1 in transgenic peas (Pisum sativum) provides complete protection from pea weevil (Bruchus pisorum) under field conditions. Proc Natl Acad Sci USA 97:3820–3825
Power JR, Whitaker JR (1977) Purification and some physical and chemical properties of red kidney bean (Phaseolus vulgaris) α-amylase inhibitor. J Food Biochem 1:217–238
Sasikiran K, Rekha MR, Padmaja G (2004) Purification and partial characterization of proteinase and α-amylase inhibitors from lesser yam (Dioscorea esculenta). Int J Food Prop 7:185–199
Sawada S, Takeda Y, Kanamori M, Tashiro M (2001) Purification and characterization of alpha-amylase inhibitors from the seeds of three cultivars of the genus Phaseolus. J Japanese Food Sci Tech 48:182–188
Sharma KK, Pattabiraman TN (1982) Natural plant enzyme inhibitors: purification and properties of an amylase inhibitor from yam bean (Dioscorea alata). J Sci Food Agric 33:255–262
Strumeyer DH, Fisher BR (1983) Automated assay of alpha amylase inhibitor proteins by continuous flow analysis. Anal Biochem 130:506–513
Suzuki K, Ishimoto M (1999) Characterization of the third α-amylase inhibitor (α-AI-3) in common bean (Phaseolus vulgaris L.). Breed Sci 49:275–280
Tamil IG, Dineshkumar B, Nandhakumar M, Senthilkumar M, Mitra A (2010) In vitro study on α-amylase inhibitory activity of an Indian medicinal plant, Phyllanthus amarus. Indian J Pharmacol 42:280–282
Waselake RJ, MacGregor AW, Hill RD, Duckworth HW (1983) Purification and characteristics of an endogenous α-amylase inhibitor from barley kernels. Plant Physiol 73:1008–1012
Wato S, Kamei K, Arakawa T, Philo JS, Wen J, Hara S, Yamaguchi H (2000) A chimera like alpha amylase inhibitor suggesting evolution of Phaseolus vulgaris α-amylase inhibitor. J Biochem 128:139–144
Yamaguchi H (1993) Isolation and characterization of the subunits of heat labile alpha amylase inhibitors from Phaseolus vulgaris, white kidney bean. J Biosci Biotechnol Biochem 57:297–302
Yang MY, Zhang XQ, Ma Y, Shen J, Song JR, Zhu HL (2008) Purification and partial characterization of a glycoprotein alpha-amylase from white kidney bean. J Food Biochem 32:72–84
Yoshikawa H, Kotaru M, Tanaka C, Ikeuchi T, Kwabata M (1999) Characterization of kintoki bean (Phaseolus vulgaris) α-amylase inhibitor: inhibitory activities against human salivary and porcine pancreatic α-amylases and activity changes by proteolytic digestion. J Nutr Sci Vitaminol 45:797–802
Acknowledgment
Financial assistance rendered by ASPEE, Agriculture Research and Development Foundation, Malad (W) Mumbai in the form of scholarship and contingency grant is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, M., Sharma, P. & Nath, A.K. Purification of a novel α-amylase inhibitor from local Himalayan bean (Phaseolus vulgaris) seeds with activity towards bruchid pests and human salivary amylase. J Food Sci Technol 51, 1286–1293 (2014). https://doi.org/10.1007/s13197-012-0631-1
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-012-0631-1