Abstract
The quality of refined groundnut oil, as affected by frying Poori, was assessed with respect to two types of frying operations viz., continuous frying and intermittent frying. Continuous frying was carried out consistently for 8 h, whereas intermittent frying was performed for 2 h everyday for 4 days for a total of 8 frying hours. The purpose of the study was to compare the level of deterioration that occurred during the two operations. Among the parameters studied, peroxide value (11.3 ± 0.26 meqO2/kg), anisidine value (172.4 ± 2.71), diene value (1.57 ± 0.095), oxidized fatty acid (2.6 ± 0.17%) and viscosity (103.8 ± 2.5 mPa s−1), were found to be higher after 8 h due to intermittent frying. The corresponding values 4.9 ± 0.15, 133.3 ± 0.49, 0.811 ± 0.04, 0.38 ± 0.023 and 81.8 ± 2.02 were observed in continuous frying. Parameters such as iodine value, unsaturated fatty acids, saponification value and smoke point decreased significantly (P < 0.5) due to intermittent frying. Results showed that intermittent frying caused more quality degradation to GNO than continuous frying.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Deep-fat fried foods have gained worldwide popularity because of the flavor, aroma and crisp appearance that develop during frying. Oil is exposed to oxygen and moisture at a high temperature, i.e., 180 °C, for a long time during the frying process. As a result, various chemical processes such as oxidation, hydrolysis, polymerization and fission can take place (Augustin et al. 1987).
It is imperative to assess the quality of oil to avoid the use of deteriorated oil, which may have health consequences if consumed as part of the fried product. However, cost of production should also be evaluated as it may increase due to the early disposal of frying oil. The fast food industry has adopted various methods of maintaining the quality and increasing the useful life of frying oils. They include the use of active and passive filters, antioxidants and proper maintenance of the fryer equipment (Paul and Mittal 1997). In many instances where deep-fat frying is employed, the oil utilized is not heated continuously (Perkins and Akkeren 1965). Deep-fat frying involves immersing a food item in a large quantity of heated oil or fat, which is normally replenished and reused several times before being disposed (Aladedunye and Przybylski 2009). Heating periods are generally shorter in domestic use and the oil used for frying is saved for further use (Seppanen and Csallany 2006). Continuous frying is carried out where large-scale production of fried products is required (Winkler et al. 2007). In India, a large quantity of fried products are consumed in the form of breakfast, snacks and other items, such as the traditional item Poori, a wheat based product. Poori is not only prepared in household-kitchen, but is gaining momentum in restaurants, industrial and institutional canteens as well.
Deep-fat frying is a complicated thermal chemical process producing fried foods with desirable color, flavor and texture (Fritsch 1981). Oils with high linolenic acid have been reported to be poor frying oils since they rapidly deteriorate under frying conditions (Bracco et al. 1981; Gutierrez et al. 1988; Warner and Mounts 1993; Khan et al. 2011). In addition, Warner and Mounts (1993) found that foods fried in these oils develop rancid and fishy flavors, which greatly reduce their shelf-life. Oleic acid is more stable towards oxidation both at ambient storage temperatures and at the high temperatures that prevail during cooking and frying of foods. Hence, oils with high amounts of oleic acid are slower to develop oxidative rancidity during shelf life or undergo oxidative decomposition during frying than those oils that contain high amounts of polyunsaturated fatty acids (PUFA) (Abdulkarim et al. 2007). Groundnut oil (GNO) is rich in oleic acid and has more stability over other PUFA rich oils viz. sunflower, safflower etc. It has also been considered to be superior to soybean oil during frying, developing fewer flavour defects with long term use (Young 1996). Considerable importance has been ascribed to the role of the oleate/linoleate ratio (O/L) and iodine value (IV) in governing product shelf life. High O/L ratio and low IV have been associated with greatly enhanced shelf life and decreased rancidity of the product (Andersen and Gorbet 2002). IV of crude GNO is between 86 and 107, being close to olive oil IV (75–94) and lower than soybean oil IV (120–143) (Padley et al. 1994). Moreover, GNO has a high smoke point of 229.4 °C, which enhances its suitability for deep frying. Thus GNO is considered premium oil for frying, consequently, frying and cooking constitute of its greatest use throughout the world (Sanders 2002). In India, the oil is used in several states viz., Maharashtra, Karnataka, Tamil Nadu, Andhra Pradesh, Madhya Pradesh and Bihar (Susheelamma et al. 2002).
Keeping in view the above factors, continuous and intermittent frying operations were conducted by frying Poori using GNO as a frying medium. The objective of the study was to compare the effect of continuous frying, simulating large scale frying operation practiced commercially, with that of intermittent frying, which simulates batch frying operation, on GNO quality.
Materials and methods
Materials
A fresh batch of refined GNO, manufactured by Kaleesuwari Refinery Pvt. Ltd., Chennai, India, was purchased from the local market. The dough for the preparation of Poori was made using whole wheat flour. All the frying operations were carried out in a stainless steel pan (Model 686528, Meilleur du chef, Capacity ~5 L, diameter 28 cm). A digital thermometer was kept suspended into the oil while not touching the base of the pan. All the chemicals and solvents used were of analytical grade.
Frying operations
Both the frying operations were started individually with oil quantity of 3 kg. In each frying operation, 5.76 kg of dough was prepared with 3.45 kg of whole wheat flour, 2.18 kg of potable water and 0.13 kg of table salt. The dough was then evenly distributed into 288 pieces of balls weighing 20 g each and then equal numbers of Poories were made by flattening them and frying in GNO, subsequently. The oil was heated for 17 ± 2 min from room temperature (27 °C) to attain the frying temperature of 180 °C and the same was maintained at 180 ± 1 °C during frying. Poories were fried in groups and number of Poories in each group varied with the decrease in oil level. However, it was ensured that an equal numbers of Poories are fried after every nth hour of continuous and intermittent frying, respectively.
Continuous frying
A total number of 288 Poories were fried continuously for 8 h during continuous frying. After every 2 h, 50 g of oil was withdrawn for testing, which was not replenished. At the end of 8 h frying, 1.76 kg oil was recovered as left over. A total of 4 samples were collected during this course.
Intermittent frying
The same number of Poories was fried in intermittent frying which was performed for 4 days, 2 h each day, for a total of 8 h of frying. 72 Poories were fried each day and 50 g of oil was withdrawn for testing, which was not replenished. Hence, A total of 4 samples were collected during this course. After frying, oil was cooled to room temperature for 45 min, transferred to a glass jar with properly tightened lid and kept in dark until every subsequent frying. At the end of 8 h frying, 1.52 kg oil was recovered as left over.
Oil analysis
The oil quality parameters viz., free fatty acids (FFA), peroxide value (PV), p-ansidine value (AV), diene and triene values, unaltered triacylglyceride (TAG) and partial glycerides viz. diacylglyceride (DAG) and monoacylglyceride (MAG) were determined for fresh GNO as well as for samples collected during continuous and intermittent frying. Among these parameters, FFA and PV were analyzed immediately after cooling the samples whereas AV was analyzed on the same day. However, IV, unsaturated fatty acids, oxidized fatty acid, saponification value (SV), unsaponifiable matter (USM), viscosity and smoke point were also determined for fresh GNO as well as samples collected at the end of the of the frying operations i.e., after 8 h frying.
FFA, PV, IV, unsaturated fatty acids, SV and AV were determined as per the AOCS Official Methods Ca 5a-40, Cd 8–53, Cd 1–25, Ce 1–62, Cd 3–25 and Cd 18–90, respectively (AOCS 1998). Fatty acid methyl esters (FAME) of the oil samples were prepared by AOCS official method Ce 1–62. FAMEs were analyzed on a Fisons 8000 series gas chromatograph (Fisons Co., Italy), equipped with a hydrogen flame ionization detector (FID) and a fused silica capillary column (100 m × 0.25 mm i.d.), coated with 0.20 μm SP2560 (Supelco Inc., Bellefonte, PA) as the stationary phase. The oven temperature was programmed from 140 to 240 °C at 4 °C/min with an initial hold at 140 °C for 5 min. The injector and FID were at 220 °C and 240 °C, respectively. A reference standard FAME mix (Supelco Inc.) was analyzed under the same operating conditions to determine the peak identity. The FAMEs were expressed as relative area percentage. AV is defined by convention as 100 times the absorbance measured in a 1 cm cell of a solution resulting from the reaction of 1 g oil with 100 ml mixture of solvent and reagent. It was determined using double beam spectrophotometer (model UV-160A; Shimadzu Corporation, Kyoto, Japan). Anisidine (p-methoxyaniline) reagent grade was purchased and recyrstallized from ethanol before use. Isooctane used was of HPLC grade. Absorption readings were made at 350 nm in 1 cm cell.. The AOCS Official Method Ti 1a-64 (AOCS 1998) was followed to determine diene and triene values by measuring λmax at 230 nm and 268 nm using the above mentioned spectrophotometer. Oxidized fatty acid was determined by insolubilization method of IUPAC II.D.12. (IUPAC 1979). Oil samples were saponified and the unsaponifiable matter was extracted. The soap solution was then decomposed by 1 N hydrochloric acid followed by the separation of oxidized acids with hexane for 12 h. The aqueous acid layer was run off and the solvent layer was filtered. Separating funnel and filter funnel were washed with hexane. Oxidized acids being insoluble in petroleum ether, were dissolved in hot ethanol by thoroughly washing the separating funnel and filter paper followed by the evaporation of ethanol and weighing the oxidized acids.
A viscometer (model RI: 3: M; Rheology International, Shannon, Ireland), equipped with spindle No. 4 was employed to measure the viscosity of oil samples at 29 °C. All the measurements were carried out with the spindle rotating speed at 50 rpm.
For measuring smoke point, oil samples were placed in a 200 ml beaker. Oil was filled just below the brim of the beaker so that the smoke could be visible before a dark background. A thermometer was kept suspended in the center of the beaker so that it did not touch the bottom. GNO was heated at the rate of 5–6 °C/min and the temperature was noted when a thin continuous stream of bluish smoke appeared.
Unaltered TAG and partial glycerides were determined by solid–liquid adsorption chromatography (SLAC) as per the AOCS Official Method Cd 11c-93. (AOCS 1998). Silica gel was used as adsorbent. Unaltered TAG was eluted with 10% diethyl ether in petroleum ether (v/v), DAG with 25% diethyl ether in petroleum ether (v/v) and MAG with 100% diethyl ether. Oil samples were eluted at a flow rate of 2 mL/min using 250 mL of solvent for each fraction as noted above. The effluents were separately collected for each fraction and evaporated to dryness until constant weights of fractions were obtained.
Statistical analysis
All the aforementioned physico-chemical analyses were conducted in triplicates for two different independent trials of both continuous and intermittent frying and mean scores are reported. Standard deviations (SD) were calculated using MS Excel. Mean separation was accomplished by Duncan’s Multiple Range Test (DMRT) using STATISTICA software. Significant difference between the results of two frying conditions viz. continuous frying and intermittent frying was calculated at 5% level.
Results and discussion
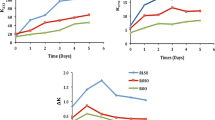
Water, steam and oxygen initiate the chemical reactions in the frying oil and food. Water being a weak nucleophile attacks the ester linkage of TAG and produces DAG, MAG and FFA. FFA can be formed by hydrolysis as well as by the cleavage and oxidation of double bonds (Paul and Mittal 1997). However; it is used as an indicator of hydrolytic rancidity of vegetable oils (Manral et al. 2008). GNO with an initial value of 0.076 ± 0.002% (as oleic acid) was used as the frying medium in this study (Fig. 1a). It is noteworthy that the increase in FFA in both continuous and intermittent frying proceeded in the same pattern and did not differ significantly (P < 0.05) from each other. Chung et al. (2004) showed that FFA contents in frying oil increase with the number of fryings. However, in the present study it can be noted that values after the 8 h frying in both the operations decreased, possibly, because of the loss of low molecular weight fatty acids through volatilization (Sulieman et al. 2006) which in turn leads to the difficulty to assess the level of deterioration of oil. Moreover, titration method is unsuitable for FFA determination when FFA is caused either by hydrolysis or oxidation (Fritsch 1981; Manral et al. 2008; Sulieman et al. 2006). It is accepted that hydrolysis of oil/fat results in formation of FFA, DAG, MAG and glycerol (Gutierrez et al. 1988). Therefore, to know the extent of hydrolysis which may have occurred during both continuous and intermittent frying, determination of unaltered TAG and partial glycerides were carried out (Table 1). During both the frying operations, the levels of DAG and MAG increased and TAG decreased from their initial values of 3.8, 1.2 and 95.1, respectively. However, at 4 h, intermittent frying had greater values than continuous frying (P < 0.05) and the same trend was found to be continued upto 8 h of frying. After 8 h, intermittent frying oil had 16.8% DAG and 11.6% MAG whereas the respective values for continuous frying were 13.8% and 7.1%. The result showed that intermittent frying oil was susceptible to hydrolysis more than continuous frying oil.
In intermittent frying PV was always higher (P < 0.05) than that of continuous frying at different time intervals (Fig. 1b) despite the inconsistency in values for both the operations. This may be caused by the instability of peroxides (Fritsch 1981). Peroxide value increased from 1.3 ± 0.16 meqO2/kg (fresh oil) to 11.3 ± 0.26 meqO2/kg at the end of the intermittent frying and 4.9 ± 0.15 meqO2/kg at the end of the continuous frying. This remarkable difference (P < 0.05) in PV in intermittent frying can be attributed to the fact that when oil cools down from the normal temperature of frying, the solubility of oxygen in the oil increases. This accelerates oxidation reactions and hence the productions of hydro peroxides take place. This phenomenon is repeated with each cycle of heating and cooling. Hydroperoxides thus formed are, however, highly unstable at higher temperatures and readily undergo decomposition, producing most of the decomposition (secondary) products (Paul and Mittal 1997). This was supported by our results obtained from the determination of AV, a measurement of aldehyde content (secondary oxidation products) principally 2, 4-dienals and 2-alkenals (Tompkins and Perkins 1999). The steady increase in AV during both the frying operations was observed (Fig. 1c). GNO with initial AV of 8.99 ± 0.117 increased as high as 172.4 ± 2.71 and 133.3 ± 0.49 in intermittent and continuous frying, respectively. Hence, oxidative deterioration in GNO was more pronounced during intermittent frying.
The formation of diene and triene during both the operations are presented in Fig. 1d and e. Oxidation can cause double-bond position shifts in PUFA. In this process, conjugated diene and triene structures are formed, which can be indicated by UV absorption (Warner 1996). When linoleic acid is oxidized to form hydroperoxides, a shift in one of the double bonds occurs, producing a conjugated diene that can be measured by UV absorbance at 230 nm. The increment of absorptivity at 230 nm, which is an indicator for the formation of conjugated dienes was significant (P < 0.05). Diene values substantially increased with increasing frying time, notably after 4 h onwards during intermittent frying. The high values during intermittent frying could be attributed to its higher PV, which led to a greater degree of primary oxidation. Moreover, the variation of absorptivity at 268 nm attributed to the formation of conjugated trienes as well as unsaturated ketones and aldehydes (Sulieman et al. 2006).
The parameters which were studied after 8 h of both continuous and intermittent frying (Table 2) also showed greater deterioration occurred in intermittent frying. Iodine value, which is a measure of unsatsuration, decreased from 99.06 ± 0.35 to 87.83 ± 0.671 in continuous frying and 81.85 ± 0.064 in intermittent frying. The decrease in IV with time of frying could be attributed to the changes in fatty acids taking place with duration of frying (Tynek et al. 2001). Reblova et al. (1999) also reported a decreasing trend in iodine value of the oil during deep-fat frying. The greater decrease in unsaturation of intermittent frying oil corroborates with the determination of relative percentage of oleic acid (monounsaturated fatty acid), and PUFAs viz. linoleic and linolenic acids. Although the unsaturated fatty acids decreased evidently as the fryings proceeded, a pronounced effect was observed during intermittent frying. Oleic acid from its initial value 39.7 ± 0.024 decreased to 32.5 ± 0.084 after continuous frying and 30.0 ± 0.051after intermittent frying. A similar trend was found for linoleic acid as well. However, linolenic acid from its initial value 1.12 ± 0.011 was completely eradicated after the both the frying operations.
As the previous data showed intermittent frying was mainly affected by oxidation, the results of oxidized fatty acids confirmed those findings. There was an increase in% oxidized fatty acids after both continuous and intermittent frying, but a significantly greater increase was observed in intermittent frying (P < 0.05). The mechanism of thermal oxidation involves the initiation, propagation and termination of the reaction. In the initiation step alkyl radical is formed which can further react with alkyl radicals, alkoxy radicals and peroxy radicals to form dimers and polymers. Polymers, in turn, accelerate the oxidation process and degrade the oil further, thereby increasing the viscosity (Choe and Min 2007). From its initial value of 55.6 ± 2.75 mPa s−1, viscosity increased to 81.8 ± 2.03 mPa s−1 after continuous frying whereas that of intermittent frying was 103.8 ± 2.51 mPa s−1. It is well discussed in the literature that formation of polymers is aggravated with increased frying temperature and total number of fryings. (Choe and Min 2007; Cuesta et al. 1993). However, in the present study the significant increase (P < 0.05) of viscosity in the intermittent frying oil is noteworthy and can be attributed to the fact that solubility of oxygen increases as the oil cools down from frying temperature (as discussed before). Thus intermittent frying oil was exposed to greater oxidation and subsequent polymerization despite maintaining same frying temperature and equal number of fryings as that of continuous frying. The polymers are high molecular weight compounds that gel the oil phase and increase the viscosity (Yaghmur et al. 2001). SV is an indicator of average molecular weight as it is inversely related to molecular weight of oil. (Knothe 2002). In the present study SV with the initial value 190.31 ± 0.89 was found to decrease more in intermittent frying (174.46 ± 1.44) than in continuous frying (180.66 ± 1.15), which confirmed the presence of high molecular weight substances. Furthermore, the reduction in values of USM and smoke point was greater in intermittent frying, which substantiated our findings that oil deterioration took place more in intermittent frying (where there was substantial time gap between usage period and holding period) than continuous frying (without any holding period).
Conclusion
The results showed that intermittent frying oil was highly prone to oxidation, which in turn caused more damage to the GNO and thus affected its quality to a greater extent than did continuous frying. However, hydrolysis was also found to contribute in deterioration, but to a much lesser extent. Therefore the common practice of heating and reheating of cooking oil and frying causes more damage to oil in comparison to the practice of frying food continuously in large scale. In other words, oil, during frying, heated for a shorter period, cooled and kept for further use undergoes more damage than the oil heated continuously for greater period of time. GNO, as deep fat frying medium, is preferable due to its thermal stability and the present study adds data of the pattern of its thermal degradation to the existing knowledge. Moreover, the results also depict that oxidation and subsequent polymerization of frying oil is not only effected by frying temperature and total number of frying, the conditions of batch frying (simulated in intermittent frying) also has a considerable impact.
References
Abdulkarim SM, Long K, Lai OM, Muhammad SKS, Ghazali HM (2007) Frying quality and stability of high-oleic Moringa oliefera seed oil in comparison with other vegetable oils. Food Chem 105:1382–1389
Aladedunye FA, Przybylski R (2009) Degradation and nutritional quality changes of oil during frying. J Am Oil Chem Soc 86:149–156
Andersen PC, Gorbet DW (2002) Influence of year and planting date on fatty acid chemistry of high oleic acid and normal peanut genotypes. J Agric Food Chem 50:1298–1305
AOCS (1998) Official methods and recommended practices of the American Oil Chemists’ Society. AOCS, Champaign
Augustin MA, Asap T, Heng LK (1987) Relationships between measurement of fat deterioration during heating and frying in RBD olein. J Am Oil Chem Soc 64:1670–1675
Bracco U, Dieffenbacher A, Kolarovic L (1981) Frying performance of palm oil liquid fractions. J Am Oil Chem Soc 58:6–12
Choe E, Min DB (2007) Chemistry of deep-fat frying oils. J Food Sci 72:R77–R86
Chung J, Lee J, Choe E (2004) Oxidative stability of soybean and sesame oil mixture during frying of flour dough. J Food Sci 69:574–578
Cuesta C, Sanchez-Muniz FJ, Garrido-Polonio C, Lopez-Varela S, Arroyo R (1993) Thermooxidative and hydrolytic changes in sunflower oil used in frying with a fast turnover of fresh oil. J Am Oil Chem Soc 70:1069–1073
Fritsch CW (1981) Measurement of frying fat deterioration: a brief review. J Am Oil Chem Soc 58:272–274
Gutierrez R, Quijano G, Dobarganes MC (1988) Analytical procedures for the evaluation of used frying fats. In: Varela G, Bender AE, Morton ID (eds) Frying of food: principles, changes, new approaches. Ellis Horwood, Chichester, pp 141–154
IUPAC (1979) Standard methods for the analysis of oils, fats and derivatives. Pergamon, Oxford
Khan MI, Asha MR, Bhat KK, Khatoon S (2011) Studies on chemical and sensory parameters of coconut oil and its olein blends with sesame oil and palmolein during wheat flour-based product frying. J Food Sci Technol 48:175–182
Knothe G (2002) Structure indices in FA chemistry. How relevant is the iodine value? J Am Oil Chem Soc 79:847–854
Manral M, Pandey MC, Jayathilakan K, Radhakrishna K, Bawa AS (2008) Effect of fish (Catla catla) frying on the quality characteristics of sunflower oil. Food Chem 106:634–639
Padley FB, Gunstone FD, Harwood JL (1994) Occurrence and characteristics of oils and fats. In: Gunstone FD, Harwood JL, Padley FB (eds) The lipid handbook. Chapman and Hall, London, pp 47–223
Paul S, Mittal GS (1997) Regulating the use of degraded oil/fat in deep-fat/oil food frying. Crit Rev Food Sci Nutr 37:635–662
Perkins EG, Akkeren LAV (1965) Heated fats. IV. Chemical changes in fats subjected to deep fat frying processes-cottonseed oil. J Am Oil Chem Soc 42:782–786
Reblova Z, Kudrnova J, Trojakova L, Pokorny J (1999) Effect of rosemary extracts on the stabilization of frying oil during deep fat frying. J Food Lipids 6:13–23
Sanders TH (2002) Groundnut (Peanut) oil. In: Gunstone FD (ed) Vegetable oils in food technology. CRC, New York, pp 231–241
Seppanen CM, Csallany AS (2006) The effect of intermittent and continuous heating of soybean oil at frying temperature on the formation of 4-hydroxy-2-trans-nonenal and other α-, β-unsaturated hydroxyaldehydes. J Am Oil Chem Soc 83:121–127
Sulieman ARM, Makhzangy A, Ramadan MF (2006) Antiradical performance and physicochemical characteristics of vegetable oils upon frying of French fries: a preliminary comparative study. J Food Lipids 13:259–276
Susheelamma NS, Asha MR, Ravi R, Vasanth Kumar AK (2002) Comparative studies on physical properties of vegetable oils and their blends after frying. J Food Lipids 9:259–276
Tompkins C, Perkins EG (1999) The evaluation of frying oils with the p-anisidine value. J Am Oil Chem Soc 76:945–947
Tynek M, Hazuka Z, Pawlowicz R, Dudek M (2001) Changes in the frying medium during deep frying of food rich in proteins and carbohydrates. J Food Lipids 8:251–261
Warner K (1996) Evaluation of lipid quality and stability. In: McDonald RE, Min DB (eds) Food lipids and health. Marcel Dekker, New York, pp 345–369
Warner K, Mounts TL (1993) Frying stability of soybean and canola oils with modified fatty acid compositions. J Am Oil Chem Soc 70:983–988
Winkler JK, Warner K, Glynn MT (2007) Effect of deep-fat frying on phytosterol content in oils with differing fatty acid composition. J Am Oil Chem Soc 84:1023–1030
Yaghmur A, Aserin A, Mizrahi Y, Nerd A, Garti N (2001) Evaluation of argan oil for deep-fat frying. Lebensm-Wiss u-Technol 34:124–130
Young T (1996) Peanut oil. In: Hui YH (ed) Bailey’s industrial oil and fat products. Wiley, New York, pp 377–392
Acknowledgements
Authors are grateful to the Director, CFTRI for his keen interest in the study. Thanks are due to Dr. KSMS Raghavarao, Dr. BR Lokesh, Mr. KK Bhat, Dr. K Venkatesh Murthy and Dr. R Ravi. A special thank to Dr. Chetana R. for her support during frying study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Das, A.K., Babylatha, R., Pavithra, A.S. et al. Thermal degradation of groundnut oil during continuous and intermittent frying. J Food Sci Technol 50, 1186–1192 (2013). https://doi.org/10.1007/s13197-011-0452-7
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-011-0452-7