Abstract
Low field Nuclear Magnetic Resonance was used in comparison to yield and physicochemical measurements to assess the effects of salt and protein injection on the properties of saithe (Pollachius virens) fillets during chilled and frozen storage. Saithe fillets injected with various combinations of salt, homogenized fish proteins, gelatine and fish protein hydrolyzate, were compared to the properties of untreated fillets. Addition of salt or fish protein hydrolyzate resulted in increased yield after cooking and water holding capacity compared to other treatments. Transversal relaxation data fitting resulted in three water populations with relaxation times of 27–45 ms, 60–99 ms and 187–341 ms. Relaxation times and respective populations showed significant correlation to various physicochemical properties, that muscle water behaviour was changed by salt and protein injection and indicated protein denaturation during frozen storage. Fish protein hydrolyzate injected fillets were most stable through storage, while gelatine injected fillets were most denatured during frozen storage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 2006, more than 110 million metric tons or 77% of the world’s fish production was used for human consumption (FAO 2008). Fish fillets are the dominating products on the world fish market. However, since the yield during filleting operations is only 30–50%, up to 70% of the fish may end up as by-products or be discarded (Kristbergsson and Arason 2006). Significant additional nutritional, economical and environmental value can therefore be obtained by increasing the yield of filleting operations by improving processing prior to filleting and by using the by-products in an optimal way, for example in the production of functional proteins.
The use of functional proteins as additives in food products has increased over the last years. These can increase water and fat binding properties of the products and improve texture and stability. The functionality and sensory attributes of the proteins is however dependent on their type, origin and handling (Cunningham et al. 1988; Kristinsson and Rasco 2000; Shaviklo et al. 2011; Hefnawy and Ramadan 2011). Soy proteins have been used in the food industry to improve water- and fat- binding in frozen products (Cunningham et al. 1988). Kristinsson and Rasco (2000) showed that less drip was observed in frozen salmon patties with added fish protein hydrolyzates than with egg albumin or soy protein concentrate. Fish protein products, which have undergone various isolation methods, are also commercially available as concentrates or dried products, ready to use for the same purpose. Thorarinsdottir et al. (2004) showed that fish proteins injected into cod fillets could improve the water holding capacity of the fillets to a greater extent than injection with soy proteins. Moreover, improving yield and water holding properties of fish fillets by injection of proteins, made from by-products produced during the fillet production, can lead to higher quality and value of the final product.
Salting by brining or brine injection with low salt concentrations (1–6%) is a known process for improving the yield and water holding capacity of fish fillets (Barat et al. 2002; Akse et al. 1993). The salt uptake is however affected by several factors, such as species, muscle type, fish size and weight, chemical composition of the muscle and the brine, salting method, rigor status etc. (Ismail and Wootton 1992; Jittinandana et al. 2002; Wang et al. 2000). Salting leads to binding of chloride ions (Cl−) to the charged amino acid residues of the thick muscle filaments, leading to the onset of electrostatic repulsive forces causing an increase in the filament spacing, as well as by the rupturing of intra- and intermolecular bonds. This gives the opportunity for water to flow between the filaments leading to increased water-protein bonds, and thus increased water holding capacity (Offer and Trinick 1983; Schmidt et al. 2008).
Low field Nuclear Magnetic Resonance (LF-NMR) proton relaxation measurements are a widely applicable method for studying muscle behaviour during processing or storage. Transversal relaxation time measurement have shown to give strong correlations to various physicochemical properties of fish and meat muscle, such as moisture content (Andersen and Rinnan 2002), water holding capacities and drip loss (Jepsen et al. 1999; Erikson et al. 2004) and has been used to indicate pH-induced structural changes occurring in the muscle post mortem (Bertram et al. 2000) as well as the effects of frozen storage on protein denaturation in fish (Steen and Lambelet 1997). The technique can give valuable information about the distribution of water throughout the muscle and how it is changed by various handling or processing (Jepsen et al. 1999; Erikson et al. 2004; Bertram and Andersen 2007; Bertram et al. 2009; Aursand et al. 2009) and is therefore an excellent technique for studies on the effects of protein injection to fish muscle.
The objectives of this study was to investigate the effect of injection of various combinations of homogenized fish protein, gelatine, fish protein hydrolyzates and salt brine on the composition, water holding capacity and yield through processing and chilled and frozen storage by means of low field NMR, compared to measurements of physicochemical properties and yield. The overall aim of the project was to study if added salt and/or proteins, produced from fish by-products, can maintain or increase the yield, quality and stability of saithe fillets during chilled or frozen storage.
Materials and methods
Experimental design
Fresh, skinless saithe (Pollachius virens) fillets (n = 182) with an average weight of 452 ± 332 g, from fish caught by trawl on May 3rd 2009 at Hvalbakshalli, east of Iceland were used in the study. The fillets were injected with six combinations of brine and protein solutions 4 days post catch (Table 1). The fish protein used was made from fresh and frozen-thawed homogenized fish protein mince (HFP(a) and HFP(b) respectively), a commercial fish protein hydrolyzate (FPH) and dried collagen peptides (gelatine). All injection solutions were prepared using tap water at 0–1 °C. The homogenized fish proteins (HFP) were produced from fresh and frozen mince from saithe cut-offs. The mince was washed in a 1:4 fish-to-water ratio, thereafter sieved (1000 μm) to dispose of insoluble and undesirable material. The mince was homogenized at approximately 3000 psi in a special homogenizer and directly injected to the fillets as described below. Two types of homogenized fish protein solutions were prepared, i.e. HFP(a) produced from fresh saithe mince made from cut-offs and frames (by-products) after filleting, while HFP(b) was produced by frozen saithe mince made from flaps and backbones from skinless fillets. No salt was added to the HFP solutions and the protein concentration was set to 3% (w/w). A commercial fish protein hydrolyzate (FPHyd) concentrate, produced by the hydrolysis of by-products from cod, was diluted with cold water, in a 1:3 fish-to-water ratio, as recommended by the producer, just prior to injection. The concentrate was firmly stirred prior to dilution to prevent precipitation. To prevent foaming 0.03% of anti-foaming agent (AFEK-FDV2K-25) was added to the solution. The dried fish collagen peptides (gelatine) was dissolved and diluted in cold tap water to form a 2% (w/w) gelatine concentration. The salt brine used in the study was prepared from food grade pure dried vacuum salt (>99.9% NaCl) and tap water to form a 3.6% NaCl (w/w) brine.
An automatic brine injection system (Dorit INJECT-O-MAT, PSM-42F-30I, Auburn NSW, Australia) with 42 needles in two rows was used for injections, using 1 bar pressure. The temperature of the injection solutions and the processing room were 5 °C and 16 °C respectively. The fillets were injected twice, first with a 3.6% salt solution, followed by injection of the various protein solutions, with the exception of the fillets injected solely with brine (Salt) or the fish protein hydrolyzate (FPHyd) injected fillets. These fillets were only injected once. This was due to a high salt content in the commercial hydrolyzate concentrate compared to the other protein solutions, making further salt injection into that group unnecessary. This procedure of injection of salt and proteins separately was chosen due to concerns of possible salt induced changes in the structure of the proteins prior to injection. An additional group was left without injection to form a control group. An overview of the brine and protein injections used in the study can be seen in Table 1. The fillets were either stored under chilled (+2 °C) conditions or plate frozen in iron pans before stored in frozen storage (−24 °C) for 1 week and 1 month respectively before being thawed and analyzed. The fillets were thawed by placing them on a grid at +2 °C for approximately 36 h. The chilled samples were stored in expanded polystyrene (EPS) boxes with a cooling mat on top, until analyzed 4 days after processing.
Yield measurements
The fillets were weighed prior to and after each processing step, i.e. the raw material, after injection, after storage (chilled, frozen for 1 week, frozen for 1 month) and after cooking at each sampling point. The injection and storage weight yield was determined by comparing the weight of the fillets after brine and protein solution injection and after storage respectively to the weight of the raw material according to the equation:
where mraw material and mi represents the weight of the fillet before processing (raw material) and after each processing step (after injection or after storage) respectively. After injection the fillets were placed on a grid for 15 min to allow excess liquid to drain off before the fillets were weighed again. For evaluation of the storage yield the frozen samples were thawed at +2 °C for approximately 36 h before weighing. The storage yield was determined by comparing the weight of the fillets after storage to the weight of the raw material. Drip loss during storage was calculated according to the equation:
where mbefore storage and mafter storage represented the weight of the fillets before and after storage respectively.
The cooking yield was evaluated by steam cooking three pieces from each sample group at 95–100 °C for 8 min in a Convostar oven (Convotherm, Elektrogeräte GmbH, Eglfing, Germany). The pieces were taken from the middle part of the fillet. The samples were cooled down to room temperature prior to weighing. The cooking yield was calculated as the ratio between the weight of the pieces after and before cooking.
The total yield of the fillets was calculated by multiplying the storage yield and the cooking yield:
Physicochemical measurements
The moisture, salt and protein content were measured both in the protein solutions and the middle part of the fillets at all sampling times (chilled, frozen for 1 week and frozen for 1 month). All samples were minced in a mixer (Braun Electronic, type 4262, Kronberg, Germany) prior to physicochemical and NMR analyses.
The moisture content was measured by drying 5 g of minced muscle mixed with sand in a ceramic bowl for 4 h at 103 ± 2 °C. The moisture content was based on the weight differences before and after the drying of three replicates for each sample (ISO 6496 1999). The salt content was measured with the Volhard Titrino method (AOAC 2000) and the total protein content was obtained from the total nitrogen content (TN*6.25) and analyzed with the Kjeldahl method (ISO-5983 2005). Water holding capacity (WHC) of the fillets was determined with the centrifugal method described by Eide et al. (1982). Approximately 2 g of the samples were weighed precisely into a vial and centrifuged (Sorvall RC-5B, Dupoint Company, USA) at 210 g (1300 rpm) and temperatures in the range of 2–5 °C for 5 min. The WHC(%) is calculated as the ratio of water in the sample after centrifugation to water in the sample before centrifugation. The pH of the fish protein solutions was measured by inserting a combined electrode (SE 104—Mettler Toledo, Knick, Berlin, Germany), connected to a portable pH meter (Portames 913, Knick, Berlin, Germany) into the protein solution. Results of chemical measurements are presented as an average of three measurements with standard deviation.
Low field NMR measurements
A low field Bruker mq 20 benchtop NMR analyzer (Bruker Optics GmbH, Rheinstetten, Germany) with a frequency of 20 MHz was used for measurements of proton transversal (T2) relaxation times with a Carr-Purcell-Meiboom-Gill (CPMG) pulse sequence (Carr and Purcell 1954; Meiboom and Gill 1958). The minced samples were placed in 10 mm sample tubes. Four replicates were made from each sample group and all measurements were performed at ambient temperature. The echo time τ was set to 250 μs and the number of collected echoes was 8100. The Receiver Gain (RG) was set to 70 dB, the Recycle Delay (RD) was 10 s, the Number of Scans (NS) was 16 and no dummy shots were used.
Data analysis
Statistical analysis and plotting of figures was performed in Microsoft Excel 2007 (Microsoft Corporation, US). A two tail t-test, assuming unequal variances, as used to distinguish between significant NMR-variables within the groups at each sampling time (chilled (C), frozen for 1 week (F1) and frozen for 1 month (F2)).
NMR data was collected with the Bruker Minispec software and successively maximum-normalized to allow comparison of samples with different size and water content. The data was normalized by setting the maximum echo to a value of 100 and scaling the other echoes accordingly. Transversal relaxation data was fitted to a multi-exponential curve by using the Low-field NMR toolbox for Matlab (The Mathworks Inc. Natric, MA), as described by Pedersen et al. (2002) according to the equation:
where N is the number of exponentials fitted, t stands for time, T2i is the relaxation time, a2i its corresponding water population and ξ(t) is the model error. The relative amount of water in each water pool is then found by the equation:
Residual analysis of the exponential fittings was used to decide the number of exponential used for the fittings. The weighted amount of water in each population compared to the total amount of observed water was calculated.
Multivariate analysis on weighted principal components (PCA) was performed on all data using Unscrambler® (Version 9.8, CAMO ASA, Trondheim, Norway) to identify similarities and differences between samples. A weighted PCA of the fitted NMR parameters and physicochemical quality parameters of the muscle was made. All variables in the PCA were weighed with the inverse of the standard deviation to correct for different scales of the variables and the data was centred prior to analysis. Individual Partial Least Square Regression (PLS1) models, with Martens Uncertainty Test (Martens and Martens 2001) were then made to identify the significant effect of the NMR parameters on each physicochemical quality property. The obtained NMR parameters were set as the X-matrix while each individual physicochemical quality parameter was set as the Y-matrix. All models were fully cross validated. All significant levels were set to p < 0.05.
Results and discussion
Physicochemical properties of the protein solutions
The physicochemical properties of the salt and protein solutions used in the study can be seen in Table 2. The homogenized fish protein (HFP) solutions and the gelatine solution contained higher moisture content than the salt brine and the fish protein hydrolyzate (FPHyd). However, significantly higher protein content was observed in the commercial FPHyd solution than in the other groups. None of the HFP or gelatine solutions contained any additional salt, while the FPHyd contained the same salt content as the salt brine (3.6%). The solutions had pH values from 5.5 in the gelatine solution to 7.7 in the FPHyd solution. Martínez-Alvarez and Gómez-Guillén (2005) observed solubility of actin and myosin heavy chain in cod fillets brined in an 18% (w/w) NaCl brine with an initial brine pH of 6.5, but this was not observed in cod muscle salted in brine with an initial pH of 8.5. Kristinsson and Rasco (2000) also showed that acidity affected the temperature needed to denature 50% of the myofibrillar proteins. This was found to be 29–35 °C at pH 7 but only 11–27 °C at pH 5.5. The difference in pH in the injection solutions could therefore affect the solubility and denaturation of myofibrillar proteins in this study.
Yield results
Results from all yield measurements can be seen in Table 3. The fillets gained approximately 5% weight when injected with salt, but about 15% when injected by the homogenized fish protein solutions (HFP(a) and HFP(b)). Addition of gelatine, alone or in combination with HFP(a) had no additional effects on the weight gain compared to fillets only injected with salt or HFP(a) respectively. A similar weight gain was obtained in the fish protein hydrolyzate (FPHyd) injected fillets as in the salt injected fillets.
Significantly increased drip during storage was observed in all injected groups compared to the untreated control group at all storage conditions. Drip is caused by partial denaturation of proteins during storage, which in turn leads to lower WHC (Shenouda 1980; Mackie 1993). The binding of water in fish muscle is especially susceptible to frozen storage, where substantive cross-linking and deformation of proteins may occur. Formaldehyde-mediated denaturation of proteins during frozen storage is known to induce a decrease in protein solubility and lead to loss of protein functional properties, including water holding capacity and gel-forming ability (Steen and Lambelet 1997). Water is also lost during frozen storage due to cell rupturing caused by ice crystal formation. However, significantly higher total storage yield was observed in the injected groups, except for the gelatine injected group, compared to the control group after chilled storage. This was especially evident in the homogenized fish protein injected fillets (HFP(a), HFP(b) and HFP(a)+Gel) having approximately 5% higher weight than the raw material. No significant difference was however observed in the total storage yield between these HFP injected groups. Fillets from all treatments experienced increased drip during frozen storage, but the difference in drip was rarely significant between 1 week and 1 month of storage. Only the HFP(a) injected groups (HFP(a) and HFP(a)+Gel) showed slightly less drip after 1 month of frozen storage than after 1 week of frozen storage. No significant difference was found in the total storage yield in the injected fillets compared to the control group after 1 week of frozen storage, except in the HFP(a) injected group, which had a lower total storage yield than the control group. However, after 1 month of frozen storage a slightly higher total storage yield was observed in the HFP injected groups compared to the control or salt injected group. The difference was only significant in the HFP(b) injected group.
A significantly higher cooking yield was observed in the salt injected and the FPHyd injected fillets compared to the control group after chilling storage, while the HFP injected groups showed significantly lower cooking yield compared to the control group. After frozen storage for 1 month the highest cooking yield was obtained for the fillets injected with salt, HFP(a)+Gel or FPHyd. Protein injected fillets showed less change in cooking yield during frozen storage compared to the salted injected or untreated fillets.
In chilled fillets and fillets frozen for 1 week, only the salt injected and FPHyd injected fillets showed significantly higher cooking yield than the control. It is possible that the fact that these groups were only injected once, while the others were injected twice, affected the yield of the fillets. After frozen storage for 1 month no significant difference was found between the different treatments. This is in agreement with Thorarinsdottir et al. (2004), who showed no effective increase in yield from protein addition after cooking in cod fillets.
Physicochemical properties
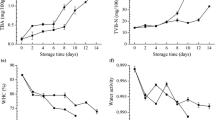
The moisture and protein content and the water holding capacity (WHC) of the saithe fillets after chilled and frozen (1 week and 1 month) storage are shown in Fig. 1. The raw material had a moisture content of 81.5 ± 1.2%, protein content of 18.5 ± 0.4% and a salt content of 0.2 ± 0.1% prior to processing. The salt content increased to 0.5 ± 0.1% in all injected groups, except the FPHyd injected group which had a salt content of 0.4 ± 0.1% after injection. No significant changes were observed in the salt content during storage. The addition of salt and proteins resulted in a higher moisture content (83.3–85.4%) than in the control group (81.4 ± 0.4%) in correlation with the moisture content of the injected solutions (R 2 = 0.816, n = 6, p < 0.05). However moisture was lost during frozen storage in all groups, except the FPHyd injected group. The relative protein content was lower in the salt and protein injected fillets compared to the control fillets, since the injected solutions contained mostly water. A relative increase in protein content was on the other hand observed during frozen storage, coupled to the moisture loss experienced.
Salt injection had a positive impact on the water holding capacity (WHC) compared to the control fillets both for the chilled fillets and those frozen stored for 1 week. This is in agreement with several studies which have shown that salt addition before freezing can increase the WHC and thus decrease thaw drip (Mahon and Schneider 1964). There was however a slightly higher drip observed in the salt injected fillets in this study compared to the control fillets, indicating that the injection process and settings (salt concentration, injection pressure, number of needles etc.) may affect the drip as well. Injection of the fish protein solutions showed no improvements in water holding capacity (WHC) compared to the control fillets neither after chilled nor frozen storage. A significant decrease in the WHC was observed during frozen storage compared to the chilled fillets in all groups. However this decrease was lowest in the FPHyd injected samples, which had significantly higher WHC after 1 month of frozen storage compared to other treatments, except the control and HFP(a) injected fillets. The changes in WHC during frozen storage were negatively correlated (R 2 = 0.5372, n = 7, p < 0.05) with the moisture content added by injection, indicating that the injected moisture is more susceptible to be lost as drip during frozen storage than moisture occurring naturally in the muscle.
Low field NMR results
Transversal relaxation data obtained by LF-NMR generally corresponds to the behaviour of water and fat in the muscle and can be divided into several proton populations. Most studies report 2–3 water populations in fish and meat muscle, depending on their proton relaxation properties (Jepsen et al. 1999; Erikson et al. 2004; Bertram and Andersen 2007; Bertram et al. 2009; Aursand et al. 2009). Bertram and Andersen (2007) and Bertram et al. (2009) identified three water populations in pork at 1–3 ms (T2B), suggested to correspond to water closely associated with macromolecules and proteins, at 40–80 ms (T21), suggested to correspond to myofibrillar water or water located within organized protein structures and at 200–400 ms (T22) corresponding to extra-myofibrillar water. Jepsen et al. (1999) found on the other hand three populations of 37, 79 and 448 ms in frozen cod and 39, 84 and 353 ms for frozen salmon, while Jensen et al. (2002) found four water populations of 37, 56, 126 and 361 ms in minced cod muscle using the PARAFAC Slicing method.
Tri-exponential fitting of the transversal relaxation data gave the most precise fitting in this study (Table 4). The fitting resulted in relaxation times ranging from 27 to 45 ms (T2a), from 60 to 99 ms (T2b) and from 187 to 341 ms (T2c) (Table 4), giving similar results as the study of Jepsen et al. (1999). This observation of three water pools indicated that a simple model of myofibrillar and extra-myofibrillar water was not applicable in the present study, possibly due to the mincing of the samples or because of the injection of proteins, giving rise to the formation of additional water pools in the fillets.
No significant difference was seen in the relaxation times between the chilled salt injected fillets and the control fillets, although the added salt affected the water distribution. The amount of water in the middle relaxing water pool (A2b) increased as a result of muscle swelling and water uptake caused by the electro-repulsive forces within the muscle structure caused by the added salt (Erikson et al. 2004), while the amount of water in the shortest relaxing water pool (A2a) decreased. Injection of salt in combination with HFP(a) and/or gelatine resulted in the longest relaxation times, indicating that the injection of these proteins affected the characteristics of these water pools the most compared to the control fillets. Aursand and others (2009) showed an increasing trend in the faster relaxing component T21 and its water population coupled to the salt-induced swelling of myofibers in frozen-thawed salmon, but this can only partly explain the observed relaxation behaviour of the varying treatments in this study, since no significant difference was found in the salt content of the fillets after injection. However, according to Nagashima and Suzuki (1981) most of the water in a gelled network of macromolecules is loosely bound, which could explain the increase in the more restricted relaxation times and associated amount of water in each water pool in the gelatine injected fillets. No significant difference was seen between the relaxation times or water populations of the FPHyd injected fillets and the control group, in agreement with the similar moisture content observed in these groups.
A general increasing trend was observed in the shortest relaxing water population (A2a) during frozen storage, with a clear negative linear correlation to the water population of the second fastest relaxing water population (A2b) (R 2 = 0.906, n = 84, P < 0.0001). This is agreement with Jensen and Jørgensen (2003) who found four water populations in frozen cod mince (37, 56, 126 and 361 ms respectively), where the amount of water associated with the two fastest relaxing components were directly correlated to the changes obtained in the denaturation profiles during storage, as assessed by Differential scanning calorimetry (DSC). The study suggested that the increased amount of water in the shortest relaxing water pool at the expense of the second fastest relaxing water pool was due to myosin and sarcoplasmatic protein denaturation. This indicates that the highest degree of denaturation of myosin and sarcoplasmatic proteins after 1 week of frozen storage was observed in the HFP(b) injected fillets, while the fillets injected with gelatine, solely or in combination with HFP(a), were the most denatured after 1 month frozen storage.
A weighted principal component analysis (PCA) with all NMR and physicochemical properties measured was made, indicating connections between the various parameters and samples in which these qualities could be found (Fig. 2). The first three components described 82% of the overall variation between the samples, the first one describing the variation found in the WHC between the samples and total yield after cooking, while PC2 primarily described the effect of increasing moisture and protein content in the fillets. Finally the third principal component PC3 (14%) described the effect of added salt on drip, cooking yield etc.
Weighted Principal Component Analysis (PCA) bi-plot of scores and loadings for the first three principal components of saithe fillets injected with various protein brines after chilled storage(C), frozen storage for 1 week (F1) and frozen storage for 1 month (F2). PC1, PC2 and PC3 explained 38% 30% and 14% of the variation respectively. Group names have been shortened for clarification of the figure: Cont stands for the control fillets, S for salt injected fillets, H(a) and H(b) for the hydrolyzed fish protein injected fillets, G for gelatine injected fillets and F for fillets injected with the commercial fish protein hydrolyzate solution
To establish which NMR parameters gave significant correlations to the physicochemical properties, individual PLS1 models were made, with the NMR parameters as the X-matrix and each physicochemical property as the Y-variable. Table 5 shows that muscle drip during storage was significantly (p < 0.05%) correlated to the amount of water in the two fastest relaxing components (A2a and A2b) as well as the middle relaxation time T2b. The amount of A2a increased on the expense of A2b in samples with increased drip. This is in agreement with the earlier statement that samples with a high amount of water in the fastest relaxing component, and a low amount of water in the second fastest relaxing component indicated high protein denaturation (Jensen and Jørgensen 2003), which in turn leads to reduced water holding capacity and increased drip. All relaxation times showed a significant correlation to the WHC, while only the middle relaxation time, T2b, was correlated to the storage drip, indicating that this pool was most sensitive to moisture loss from the muscle due to drip. The total storage yield showed on the other hand significant correlations to amount of water in the middle and slowest relaxing water populations A2b and A2c and since no significant correlation was found between the cooking yield and the NMR parameters, the same water populations, along with the shortest relaxation time T2a, were correlated with the total yield after cooking.
All NMR parameters, except the amount of water in the slowest relaxing water pool (A2c) showed significant correlation to the moisture content and WHC of the fillets respectively. The protein content showed significant correlation to the relaxation times and amount of water of the two faster relaxing components. No correlations were on the other hand found between the salt content and the NMR parameters due to the small variations in salt content between the treatments.
Conclusions
Injection of the commercial FPHyd resulted in the most stable yield during chilled and frozen storage, indicating that protein injection can be used to stabilize the properties of saithe fillets. Addition of gelatine, alone or in combination with homogenized fish proteins (HFP(a)) had no additional effects on the weight gain compared to fillets only injected with salt or HFP(a) respectively. Tri-exponential fitting of transversal relaxation data gave significant correlations between the relaxation times and the amount of their respective water populations to all physicochemical properties, except the salt content and cooking yield obtained. Changes within the two shorter relaxation times also indicated increased protein denaturation due to the frozen storage and correlated with the changes in drip and water holding capacity. The NMR results indicated that the FPHyd fillets fillets most resembled the control fillets, while the water distribution and muscle structure was most affected in the HFP(b) injected fillets frozen for 1 week and the HFP(a)+Gel injected frozen stored for 1 month. The study showed that protein addition can be used to stabilize and improve quality of saithe fillets, but the isolation processes, choice of concentrations etc. need to be optimized further to reach the desired functional properties of the muscle.
References
Akse L, Gundersen B, Lauritzen K, Ofstad R, Solberg T (1993) Saltfisk: saltmodning, utprovning av analysemetoder, misfarget saltfisk. Fiskeriforskning, Tromsö, In Norwegian
Andersen CM, Rinnan Å (2002) Distribution of water in fresh Cod. LWT 35:687–696
AOAC 976.18 (2000) Association of official analytical chemists. Official methods of analysis, 17th edn. AOAC, Arlington
Aursand IG, Veliyulin E, Böcker U, Ofstad R, Rustad T, Erikson U (2009) Water and salt distribution in Atlantic salmon (Salmo salar) studied by low-field 1H NMR, 1H and 23Na MRI and light microscopy: effects of raw material quality and brine salting. J Agric Food Chem 57:46–54
Barat JM, Rodríguez-Barona S, Andrés A, Fito P (2002) Influence of increasing brine concentration in the cod salting process. J Food Sci 65(7):1922–1925
Bertram HC, Andersen HJ (2007) NMR and the water-holding issue of pork. J Anim Breed Genet 124:35–42
Bertram HC, Andersen HJ, Karlsson AH (2000) Comparative study of low-field NMR relaxation measurements and two traditional methods in the determination of water holding capacity of pork. Meat Sci 57:125–132
Bertram HC, Karlsson AH, Rasmussen M, Pedersen OD, Dønstrup S, Andersen HJ (2001) Origin of multiexponential T-2 relaxation in muscle myowater. J Agric Food Chem 49:3092–3100
Bertram HC, Meyer RL, Andersen HJ (2009) A look at NMR relaxometry applications in meat science–Recent advances in coupling NMR relaxometry with spectroscopic, thermodynamic, microscopic and sensory measurements. In: Gudjonsdottir M, Belton P, Webb G (eds) Magnetic resonance in food science–changes in a changing world. RSC Publishing, Cambridge, pp 241–250
Carr HY, Purcell EM (1954) Effects of diffusion on free precession in nuclear magnetic resonance experiments. Am J Physiol 94:630–638
Cunningham NL, Bonkowski AT, Tuley WB (1988) Soy protein use in meat, seafood. J Am Oil Chem Soc 65:1871–1873
Eide O, Børresen T, Ström T (1982) Minced fish production from capelin (Mallotus villosus). A new method for gutting, skinning and removal of fat from small fatty fish species. J Food Sci 47:347–354
Erikson U, Veliyulin E, Singstad TE, Aursand M (2004) Salting and desalting of fresh and frozen-thawed cod (Gadus morhua) fillets: a comparative study using 23Na MRI, low-field 1H NMR, and physicochemical analytical methods. J Food Sci 69:107–114
FAO (2008) The state of world fisheries and aquaculture. FAO Fisheries Department, Food and Agricultural Organization of the United Nations, Rome
Hefnawy HTM, Ramadan MF (2011) Physicochemical characteristics of soy protein isolate and fenugreek gum dispersed systems. J Food Sci Technol. doi:10.1007/s13197-010-0203-1
Ismail N, Wootton M (1992) Fish salting and drying: a review. Asean Food J 7(4):175–183
ISO-6496 (1999) Determination of moisture and other volatile matter content. The International Organization for Standardization, Genf
ISO-5983 (2005) Animal feeding stuffs—Determination of nitrogen content and calculation of crude protein content. The International Organization for Standardization, Genf
Jensen KN, Jørgensen BM (2003) Effect of storage conditions on differential scanning calorimetry profiles from thawed cod muscle. LWT 36:807–812
Jensen KN, Guldager HS, Jørgensen BM (2002) Three-way modelling of NMR relaxation profiles from thawed cod muscle. J Aquat Food Prod Technol 11(3/4):201–214
Jepsen SM, Pedersen HT, Engelsen SB (1999) Application of chemometrics to low-field 1H NMR relaxation data of intact fish flesh. J Sci Food Agric 79:1793–1802
Jittinandana S, Kenney PB, Slider SD, Kiser RA (2002) Effect of brine concentration and brining time on quality of smoked rainbow trout fillet. J Food Sci 67(6):2095–2099
Kristbergsson K, Arason S (2006) Utilization of by-products in the fish industry. In: Oreopoulou V, Russ W (eds) Utilization of by-products and treatment of waste in the food industry. Springer, US, pp 233–258
Kristinsson HG, Rasco BA (2000) Fish protein hydrolyzates: production, biochemical and functional properties. Critical reviews. Food Sci Nutr 40(1):43–81
Mackie IM (1993) The effects of freezing fish proteins. Food Rev Int 9(4):575–610
Mahon JH, Schneider CG (1964) Minimizing freezing damage and thawing drip in fish fillets. Food Technol 18:1941
Martens H, Martens M (2001) Multivariate analysis of quality-An introduction. Wiley, West Sussex
Martínez-Alvarez O, Gómez-Guillén MC (2005) The effect of brine composition and pH on the yield and nature of water-soluble proteins extractable from brined muscle of food (Gadus morhua). Food Chem 92:71–77
Meiboom S, Gill D (1958) Modified spin-echo method for measuring nuclear times. Rev Sci Instrum 29:688–691
Nagashima N, Suzuki E (1981) Pulsed NMR and state of water in foods. In: Rockland LB, Stewart GF (eds) Water activity: influences on food quality. Academic, New York, pp 247–264
Offer G, Trinick J (1983) On the mechanism of water holding in meat: the swelling and shrinking of myofibrills. Meat Sci 8:245–281
Pedersen HT, Bro R, Engelsen SB (2002) Towards rapid and unique curve resolution of low-field NMR relaxation data: trilinear SLICING versus two-dimensional curve fitting. J Magn Res 157:141–155
Schmidt FC, Cariciofi BAM, Laurindo JB (2008) Salting operational diagrams for chicken breast cuts: hydration-dehydration. J Food Eng 88(1):36–44
Shaviklo GR, Thorkelsson G, Arason S, Sveinsdottir K (2011) Characteristics of freeze-dried fish protein isolated from saithe (Pollachius virens). J Food Sci Technol. doi:10.1007/s13197-011-0285-4
Shenouda SYK (1980) Theories of protein denaturation duing frozen storage of fish flesh. Adv Food Res 26:275–311
Steen C, Lambelet P (1997) Texture changes in frozen cod mince measured by low-field nuclear magnetic resonance spectroscopy. J Sci Food Agric 75:268–272
Thorarinsdottir KA, Gudmundsdottir G, Arason S, Thorkelsson G, Kristbergsson K (2004) Effects of added salt, phosphates, and proteins on the chemical and physicochemical characteristics of frozen cod (Gadus morhua) fillets. J Food Sci 69(4):E144–E152
Wang D, Tang J, Correia LR (2000) Salt diffusivities and salt diffusion in farmed Atlantic salmon muscle as infuenced by rigor mortis. J Food Eng 43:115–123
Acknowledgements
The authors would like to thank Rannís Technology Development fund for support to the projects “Fast measuring techniques in food processing” (Project number 071320008) and Homogenisation-increased value of fish mince (Project number 071321007), as well as the AVS R&D Fund of Ministry of Fisheries in Iceland for funding of the latter project (Project number R 011–08). The authors would also like to thank Nordisk Innovation Center (NICe) for funding of the project “Maximum resourse utilisation—Value added by fish by-products” (Project number 04252) and FISK Seafood for provision of facilities and raw material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gudjónsdóttir, M., Karlsdóttir, M.G., Arason, S. et al. Injection of fish protein solutions of fresh saithe (Pollachius virens) fillets studied by low field Nuclear Magnetic Resonance and physicochemical measurements. J Food Sci Technol 50, 228–238 (2013). https://doi.org/10.1007/s13197-011-0348-6
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-011-0348-6