Abstract
In breast cancer, axillary lymph node involvement directly impacts the patient survival and prognosis. Sentinel lymph node biopsy (SLNB) is a procedure of choice for axillary staging in early breast cancer. Currently, management options for axilla management are axillary lymph node dissection and sentinel node biopsy in node positive and in node negative respectively. Accuracy of current clinical methods for evaluating axilla is low. Hence, to select patients for appropriate procedure, ultrasound (USG) combined with fine-needle aspiration cytology (USG-FNAC) using vascular pedicle–based nodal mapping method is emerging as a good tool to address above issues. We evaluated the feasibility of ultrasound and needle aspiration cytology in a tertiary care center. All early breast cancer patients with clinically node-negative axilla and having palpable nodes with less than or equal to 5 cm tumor size in breast were screened by ultrasound of axilla to categorize the nodes as suspicious or non-suspicious based on radiological features and vascular pedicle–based nodal mapping method of axilla. Patients having suspicious nodes underwent ultrasound of axilla and needle aspiration; if found positive, patient underwent axillary node dissection. Sentinel node biopsy (SLNB) performed in all patients found negative on needle aspiration and in all patients having non-suspicious nodes on ultrasound axilla. Final histopathology was taken as gold standard. The sensitivity, specificity, accuracy, positive predictive value, and negative predictive value were calculated for ultrasound (USG) and ultrasound-guided needle aspiration (USG-FNAC). A total of 100 patients were included in which 58 had non-suspicious and 42 had suspicious nodes on ultrasound of axilla. Among suspicious group, 24 were positive on ultrasound-guided needle aspiration cytology and 18 were negative. In non-suspicious nodes, sentinel node biopsy was performed. Sensitivity, specificity, positive predictive value, and negative predictive value for ultrasound were 61.5%, 75.6%, 69.5%, and 68.5% respectively. For ultrasound-guided needle aspiration (USG-FNAC), sensitivity, specificity, and positive and negative predictive value are 83%, 100%, 100%, and 72.6% respectively. The accuracy of ultrasound (USG) and ultrasound-guided needle aspiration (USG-FNAC) was 69% and 88.1%. The result of our study indicates the feasibility of USG and USG-FNAC in a high-volume center with good accuracy of around 70–80%. Approximately one-fourth (24%) of the total patients were taken up for axillary lymph node dissection (ALND) without performing SLNB.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Breast cancer is the most common malignant tumor among women, representing 31% of all cancers [1, 2]. In India, it is constantly growing. In 2012, there have been 144,937 cases of breast cancer with over 70,218 deaths in India [3]. The presence of axillary involvement in breast cancer determines patient’s survival and the staging of the disease, and it plays an important role in local control [1,2,3]. The addition of mammography to ultrasonography seems not to provide significant benefits in predicting ALN status in breast cancer patients [4]. Until recently, axillary lymph node dissection (ALND) was considered the reference method for detecting lymph node involvement [5, 6]; originally, axillary staging was achieved through an axillary lymph node dissection (ALND) [7]. However, axillary dissection is reported to have a positive result in 30% of palpable tumors and in 10% of non-palpable tumors in patients with clinically negative axillary involvement. The remaining 70 to 90% undergo axillary dissection unnecessarily. The rate of axillary lymph node (ALN) metastases is very low in patients diagnosed at an early stage [8, 9]. In 2011, the American College of Surgeons Oncology Group (ACOSOG) Z-0011trial revolutionized the surgical approach to axillary management for early-stage breast cancer patients [10]. Currently the use of sentinel lymph node biopsy (SLNB) has increased in frequency as an alternative procedure for patients with early breast cancer. Purushotham et al. and Cox et al. mentioned that axillary lymph node dissection (ALND) has been the reference standard for diagnosis, but sentinel lymphadenectomy has replaced ALND as the primary staging procedure in many centers because sentinel lymphadenectomy is associated with less morbidity [11, 12]. The SLNB procedure is time-consuming and requiring either blue dye or Technetium-99 (Tc-99) radioisotope or both. Both these agents can be used individually with accuracy of around 90–95%, but if used in combination, the accuracy touches up to 98–99%. But both these agents are not easily available at all tertiary healthcare institutes of our country. Preoperative ultrasound of axilla in a routine fashion use can help reduce the false positivity of clinical examination and help in avoiding unnecessary axillary dissection and also in better selection of patients for sentinel node biopsy [13]. Also SLNB requires (1) preoperative lymphoscintigraphy; (2) intraoperative availability of the nuclear medicine physician; and (3) intraoperative SN pathologic examination that may be postponed to definitive pathologic evaluation, with return of the patient to the operative room for delayed ALND when sentinel node turns out to be positive, based on the individual surgical attitude. Temple et al., in a study on 233 women, revealed that this procedure carries a 4–14% rate of complications, including lymphedema, paresthesia, chronic pain, and immobility [14]. This emphasizes the need to identify less invasive and diagnostic procedures for the regional staging of patients with early-stage breast cancer. An alternative pathway is represented by axillary imaging techniques, such as PET/TC, MRI, and ultrasonography. However, a substantial variability in the accuracy of PET/TC and MRI was reported, with a sensitivity and specificity for identifying metastatic lymph nodes of 56% and 96% for PET/TC, and 66% and 93% for MRI [15]. Prior studies have shown a clear indication for the use of the procedure in patients with large tumors, but the indications for axillary ultrasound and ultrasound-guided FNA of patients with smaller tumors are less well-defined [16, 17]. Ultrasonography (USG) can detect suspicious lymph nodes in clinically negative armpits and select patients for needle biopsy, reducing the number of unnecessary sentinel node biopsies. Cools-Lartigue et al., in their study, showed that with an axillary metastasis prevalence of 38.7%, the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of axillary US alone in detecting axillary node metastases were 55, 88, 74, 75, and 75%, respectively [18]. Axillary US with FNAB may avoid unnecessary SLNB in a significant number of patients [19]. It may also decrease the rate of false negatives by detecting lymph nodes with extensive metastatic involvement and whose drainage pathways are blocked, that is, they would not be marked by dye and /or radiopharmaceutical. Alvarez et al. has cited that the preoperative axillary ultrasound (US) is a frequently performed procedure in patients diagnosed with primary breast cancer [20].
Ultrasound-guided fine-needle aspiration (FNA) is a quick non-morbid method of staging disease in the axilla. Fan Zhang et al. conducted a study aimed to explore the clinical usefulness of ultrasound-guided fine needle aspiration cytology (USG-FNAC) for the evaluation of axillary lymph nodes in patients with early-stage breast cancer [21]. A positive ultrasound-guided FNA result obviates sentinel lymphadenectomy, allowing the patient to proceed directly to ALND or neoadjuvant chemotherapy. US-guided FNA of non-palpable indeterminate and suspicious axillary lymph node is simple, minimally invasive, and reliable technique for the initial determination of axillary lymph node status in breast cancer patients [22]. Because ultrasound-guided FNA is not as sensitive as sentinel lymphadenectomy, the false-negative rate of ultrasound-guided FNA is too high to replace sentinel lymphadenectomy entirely, and patients with negative findings at ultrasound-guided FNA will still need to undergo sentinel lymphadenectomy for evaluation of the axilla [16]. Preoperative axillary ultrasound (US) is a frequently performed procedure in patients diagnosed with primary breast cancer. Ultrasound-guided fine needle aspiration (USG-FNA) allows to find a subgroup eligible for one-stage axillary surgery [23]. This technique has demonstrated good sensitivity and specificity, particularly in conjunction with fine-needle aspiration biopsy (FNAB)/core biopsy, in the preoperative diagnosis of nodal metastasis in breast cancer. The specificity of US-FNA in clinically or radiologically suspicious nodes was100%, and the negative predictive value was 33% [24]. It has been used to identify patients who can forego SLNB and proceed directly to ALND. In terms of preoperative evaluation for ALN metastasis, physical examination has low sensitivity between 34 and 76%. Ultrasonography of ALN has superior diagnostic accuracy in many studies when combined with US-guided fine-needle aspiration biopsy (US-FNA) of sonographically suspicious lymph nodes. Studies show a reduction in 15% of the risk of false-negative sentinel node when combining the use of ultrasound to biopsy fine needle aspiration (US-FNA) [25, 26]. The sensitivity and specificity of US examination in the evaluation of ALN metastasis have been reported to be 36–92 and 69–100%. Addition of US-FNA to axillary US may increase the specificity to 93–100%. Kusum Kapila et al. showed an association was seen between metastatic carcinoma on FNAC and axillary US features of a maximum length of ≥ 1.5 cm, the absence of hilar fat, and a CT of > 3 mm [27]. However, the majority of previous studies on US-FNA have consisted of small patients with high incidence of metastatic lymph nodes, and thus, a study targeting a larger sample of unspecified individuals is needed [28].
The purpose of this study is to evaluate the accuracy of axillary ultrasound and FNAC among Indian patients with early breast cancer in a busy high-volume center. Logistics issues and resource constraints are two constant threats to avail health-related facilities by the public of developing nation like India. This study can guide us to utilize ultrasound and ultrasound-guided FNAC as a routine evaluation tool in the preoperative assessment of axillary lymph nodes in early breast cancer and facilitate decision-making optimal management of axilla. A study demonstrated that US-FNAC is a feasible and effective triage during axillary staging for newly diagnosed breast cancer patients [29].
Material and Methods
It was a prospective cohort study conducted from October 2017 to December 2018 in a tertiary care health center in India and the work has been reported in line with the STROCSS criteria. With the following:
Primary Outcome
-
1.
To evaluate the accuracy of focused axillary ultrasound for assessment of axillary lymph nodes in early breast cancer patients.
-
2.
To evaluate the accuracy of ultrasound-guided fine-needle aspiration cytology (FNAC) of axillary lymph nodes in early breast cancer patients.
Secondary Outcomes
-
1.
Accuracy of clinical examination
-
2.
Number of patients avoided sentinel node biopsy.
The Inclusion Criteria
-
1.
Patients with histopathological diagnosis of cancer breast.
-
2.
Patients with early breast cancer (stages I and II)
-
3.
Clinical N0 or N1 nodal status (cN0/N1)
The Exclusion Criteria
-
1.
Locally advanced breast cancer.
-
2.
Distant metastasis at presentation.
-
3.
Poor performance status or patient unfit for surgery.
-
4.
cN2 and N3 nodal disease
-
5.
DCIS
-
6.
Male breast cancer
Sample Size
100 patients
Ultrasound Technique and Node Characterization
Axillary ultrasound performed by a single experienced radiologist using high-frequency linear transducer using either Siemens Acuson S2000 HELX (Erlangen, Germany, 14L5 transducer, 5–10 MHz) or Sonosite Micromax (Bothell, WA, USA; L38e, 5–10 MHz) ultrasound machines. Primary axillary lymphatic drainage from the breast is predominantly to the pectoral group of nodes, although any axillary node group can contain the “sentinel” nodes that receive lymph directly from the breast [30]. The nodes were assessed on the basis of their imaging appearances and evaluated in terms of:
-
(a)
presence or absence of hilum;
-
(b)
whether or not there is asymmetrical cortical thickening > 3 mm with eccentric hilum;
-
(c)
cortical thickening > 3 mm with central hilum; cortical thickness will be measured at its thickest part [1, 2].
The lymph nodes with asymmetrical cortical thickening of more than 3 mm with eccentric hilum and the nodes with absent fatty hilum were considered suspicious, whereas cortical thickening > 3 mm with central hilum was considered an indeterminate finding. In both these settings, the most suspicious lymph node will be subjected to fine-needle aspiration cytology [1]. The three groups of level I nodes were divided into the lateral group (deep), the subscapular group (posterolateral), and the pectoral group (anteromedial). A systematic approach to evaluating level I nodes begun with the third segment of the axillary artery and the accompanying axillary vein, which run through the deep portion of level I, serving as the first important landmark. Lymph nodes of the lateral group were seen near the axillary vein. These nodes predominantly drain the upper extremity. The second useful landmark used at a cross-sectional and ultrasound imaging of the axilla was the subscapular artery, which was the largest branch of the axillary artery and the only branch that was seen arising from the inferior surface of the axillary artery in level I of the axilla After the trunk of this vessel, with its characteristic hook shape identified, the main terminal branches, the thoracodorsal artery, and the circumflex scapular arteries were identified. Short segments of the circumflex scapular artery and its branches were seen as they dive into the muscles that form the posterior wall of the axilla: the latissimus dorsi, the subscapularis, and the teres major (30). The thoracodorsal artery is found along the margin of these posterolateral muscles as it continues through the axillary fat along the chest wall. Lymph nodes of the subscapular group were located along the course of this vessel and the axillary portion of its terminal branches. These nodes were also frequently seen in isolation in the axillary fat. This group of nodes predominantly drains the scapular region and the posterior chest wall. The third important landmark, the lateral thoracic artery, was found along the anteromedial margin of level I and was seen running parallel and posterior to the lateral margin of the pectoralis minor muscle, with branches into the muscle. The lateral thoracic artery was one of the two primary arteries that supply the breast, and which arises from the terminal portion of the second segment of the axillary artery. Lymph nodes of the pectoral group were found along its course, as well as centrally in the axillary fat. Primary axillary lymphatic drainage from the breast is predominantly to the pectoral group of nodes, although any axillary node group can contain the “sentinel” nodes that receive lymph directly from the breast (30). Most level I nodes were found in the axillary fat without any adjacent structures. Frequently, however, they are seen next to a vessel or close to a particular muscle, and occasionally, the hilar vessels to a particular node were traced back to their artery of origin. We devised a method to label the lymph nodes at level 1 of axilla with respect to the proximity to neurovascular structures, i.e., axillary vein, latissimus dorsi pedicle.
All these neurovasculature and muscular structures around axilla as shown in Fig. 1 were used as landmarks in order to locate the node, and any suspicious node along these structure were labeled as “a/b/c/d.” By doing so, it made our study a bit convenient for delineating the node location during ultrasound (USG) and during fine needle aspiration cytology (FNAC) helped in labeling the lymph node. Suppose we performed the fine needle aspiration cytology (FNAC) of node located along the latissimus dorsi pedicle, labeled it as “c.” If it turned out to be positive on cytology, we did the axillary node dissection (ALND), and during axillary dissection, we harvest the same nodes located along the latissimus dorsi pedicle and sent it separately for histopathological examination labeling it as “c” too and then compare the ultrasound and ultrasound-guided needle aspiration cytology (USG-FNAC) with the final histopathological report of the same node.
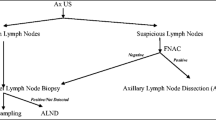
If the axillary lymph node found to be negative or indeterminate, patient underwent SLNB. Technique used to do the sentinel lymph node biopsy: If the axillary lymph node found to be negative or indeterminate on ultrasound-guided needle aspiration cytology (USG-FNAC) and also if there are no suspicious lymph nodes in the axilla on ultrasound (USG), all these patients were taken for sentinel lymph node biopsy (SLNB). In our study, we used a single agent dye (methylene blue) sentinel lymph node biopsy (SLNB) technique. On the day of surgery, 2–3 ml of methylene blue dye was injected subdermal at periareolar site. Milking was done towards axilla, and we waited for 5–7 min after the injection of dye. After this, small 3–4-cm incision is given in axilla near pectoralis major fold. A gentle dissection is done, and all blue nodes (up to 3) were harvested, defattened on the operation theater table, and sent for histopathological examination. In case no blue node was found, we followed the blue lymphatic streak and removed the receiving node which received the lymphatics. The nodes obtained from sentinel node biopsy (SLNB) was sent for frozen section, and if positive, axillary node dissection (ALND) was performed, and if negative, no axillary dissection was performed. Final histopathology report was considered the gold standard, and both the ultrasound-guided fine needle aspiration cytology (US-guided FNAC) and sentinel lymph node biopsy ( SLNB) were compared with it. The node obtained from SLNB sent for frozen section and if positive, axillary lymph node dissection (ALND) was performed and if negative, no axillary dissection was performed. Final histopathology report was considered the gold standard and both the US-guided FNAC and SLNB were compared with it.
-
1.
sensitivity = true positive(true positive + false negative)
-
2.
specificity = true negative(true negative + false positive)
-
3.
accuracy = (true positive + true negative)(true positive + true negative + false positive + false negative)
-
4.
positive predictive value (PPV) = True positive(true positive + false positive)
-
5.
negative predictive value (NPV) = True negative(false negative + true negative)
Status of sentinel nodes on frozen section and final histopathology report and detail on number of sentinel node harvested are described in details on Table 2 and Table 3 respectively.
Finally the sensitivity, specificity, diagnostic accuracy, negative predictive value (NPV) and positive predictive value (PPV), and false-negative rate of USG, US-guided FNAC were calculated which helped in clinical decision-making as well as the therapeutic implications of relying on axillary US-FNAC findings.
For localization of the nodal structure during ultrasonography, we have devised a method for easy targeting of suspicious nodes during FNAC and isolating the same node during surgery of axilla. We have used the vascular structure as an anatomical landmark using ultrasonography and marked the location of nodes in axilla denoted by alphabetical letters as shown in Fig. 1.
Results
-
(1)
Age: Median age of the cohort was 51 years (23–85 years).
-
(2)
Clinical presentation: Out of 100 patients, majority of patients presented with the lump as a major presenting complaint, i.e., 87%.
-
(3)
Size of lump in breast: 75% of patients had a lump size between 2 and 5 cm. In 80% of patients, clinically, there were no axillary nodes.
-
(4)
Clinical stage: 59% of patients were cT2N0, 19% of patients were cT1N0 and in 17% of patients were cT2N1. However, cT1N1 stage was found in 4% of patients.
-
(5)
Biopsy: 98% were infiltrating ductal cancer.
-
(6)
Hormonal profile: 67% were luminal type, 15% were Her 2 neu–enriched and only 11% were basal-type.
-
(7)
Mammogram: 50% were of BIRADS V, 20% were BIRADS IV and VI each.
-
(8)
On screening ultrasonography: out of the total 100 patients, 58 were benign and the rest 42 were suspicious.
-
(9)
Radiological characteristics: both increased cortical thickness and loss of fatty hilum suspicious features were present in 43% patients shown in Fig. 2. There is an isolated increased cortical thickness in 43% of the patients.
-
(10)
Location of suspicious nodes in axilla: As per vascular pedicle nodal mapping of axilla using ultrasonography, 54% patients had suspicious nodes located along the latissimus dorsi pedicle, while 38% were located along pectoralis minor as shown in Fig. 2.
-
(11)
Radiological features of lymph nodes predicting possibility of metastasis in nodes: Both these factors (increased cortical thickness and loss of fatty hilum) were good predictors of malignancy in our study. But presence of loss of fatty hilum among suspicious nodes was good predictor of malignancy in nodes as compared with cortical thickness as shown in Fig. 3.
On USG-FNAC of 42 patients who were found to be suspicious on screening ultrasound axilla, 24 (57%) were positive and 18 (42.8%) were negative. Twenty-four (57%), who were positive on FNAC, were also positive on the final histopathology (HPR). False-negative rate of FNAC in this group is around 17%.
-
(12)
Sentinel lymph node biopsy (SLNB) was performed in total of 76 (58 patients did not have suspicious nodes in axilla and 18 had suspicious radiological features, but found negative on FNAC report) patients out of 100. Sixty (78.9%) patients were negative on SLNB frozen, but on final HPR 7(11.5%), patients were found to be positive.
Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy of ultrasonography (USG) and ultrasound-guided fine-needle aspiration cytology (USG-FNAC) are shown in Table 1 and Fig. 4.
In our study, simply using ultrasound-guided fine-needle aspiration cytology (USG-FNAC), 24% of patients (nearly 1/4) were spared, who otherwise were supposed to subject for SLNB for axillary assessment.
Conclusion
-
By using ultrasound and guided needle aspiration cytology for assessment of axillary nodes in breast cancer patients, these modalities offer quite an easy, cheap, readily available, and accepted accurate method especially in busy high-volume centers in India.
-
This study was not at all an attempt to compare sentinel node biopsy (standard of care for axillary staging in all early breast cancers) with ultrasound and needle aspiration cytology.
-
The main intention of this study was to just circumvent the technical issues, logistics, and resource limitation associated with sentinel node biopsy procedure.
-
Our study showed quite good and acceptable result in isolating and retrieving the targeted node by just following the vascular pedicle–based nodal mapping method for axilla to locate the suspicious node without using any tagging or marking of node from where FNAC was performed. This finding can be used as a practicing tool in a busy high-volume facing logistics issues and resource constraints.
-
To date, no modality that can boast an accuracy exceeding 90–95% has been validated prospectively. Consequently, many patients with node-negative disease are subject to SLNB. Although this is currently unavoidable at the level of institutional policy, the reality is that thousands of patients are subjected to an unnecessary surgical procedure, with the burden of cost bearing heavily on healthcare systems. Thus, the ability to accurately predict which patients are likely to have involved axillary nodes would be of great benefit.
-
In practice, where on-site cyto-pathologist is not available, learning to properly smear and stain slides is a desirable skill for the radiologist performing US-FNAC.
References
Banerjee M, George J, Song EY, Roy A, Hryniuk W (2004) Tree-based model for breast cancer prognostication. J Clin Oncol Off J Am Soc Clin Oncol 22(13):2567–2575
Cianfrocca M (2004) Prognostic and predictive factors in early-stage breast cancer. Oncologist 9(6):606–616
Krag D, Weaver D, Ashikaga T, Moffat F, Klimberg VS, Shriver C, Feldman S, Kusminsky R, Gadd M, Kuhn J, Harlow S, Beitsch P, Whitworth P Jr, Foster R Jr, Dowlatshahi K (1998) The sentinel node in breast cancer — a multicenter validation study. N Engl J Med 339(14):941–946
Alkuwari E, Auger M (2008) Accuracy of fine-needle aspiration cytology of axillary lymph nodes in breast cancer patients: a study of 115 cases with cytologic-histologic correlation. Cancer. 114(2):89–93
Reynolds C, Mick R, Donohue JH, Grant CS, Farley DR, Callans LS, Orel SG, Keeney GL, Lawton TJ, Czerniecki BJ (1999) Sentinel lymph node biopsy with metastasis: can axillary dissection be avoided in some patients with breast cancer? J Clin Oncol Off J Am Soc Clin Oncol 17(6):1720–1726
Lovrics PJ, Chen V, Coates G, Cornacchi SD, Goldsmith CH, Law C, Levine MN, Sanders K, Tandan VR (2004) A prospective evaluation of positron emission tomography scanning, sentinel lymph node biopsy, and standard axillary dissection for axillary staging in patients with early stage breast cancer. Ann Surg Oncol 11(9):846–853
Somasundar P, Gass J, Steinhoff M, Koeliker S, Dizon D, Cady B, Taneja C (2006) Role of ultrasound-guided axillary fine-needle aspiration in the management of invasive breast cancer. Am J Surg 192(4):458–461
Swenson KK, Nissen MJ, Ceronsky C, Swenson L, Lee MW, Tuttle TM (2002) Comparison of side effects between sentinel lymph node and axillary lymph node dissection for breast cancer. Ann Surg Oncol 9(8):745–753
Mincey BA, Bammer T, Atkinson EJ, Perez EA (2001) Role of axillary node dissection in patients with T1a and T1b breast cancer: Mayo Clinic experience. Arch Surg Chic Ill 1960 136(7):779–782
Bruneton JN, Caramella E, Héry M, Aubanel D, Manzino JJ, Picard JL (1986) Axillary lymph node metastases in breast cancer: preoperative detection with US. Radiology. 158(2):325–326
Feng Y, Huang R, He Y, Lu A, Fan Z, Fan T, Qi M, Wang X, Cao W, Wang X, Xie Y, Wang T, Li J, Ouyang T (2015) Efficacy of physical examination, ultrasound, and ultrasound combined with fine-needle aspiration for axilla staging of primary breast cancer. Breast Cancer Res Treat 149(3):761–765
Jain A, Haisfield-Wolfe ME, Lange J, Ahuja N, Khouri N, Tsangaris T, Zhang Z, Balch C, Jacobs LK (2008) The role of ultrasound-guided fine-needle aspiration of axillary nodes in the staging of breast cancer. Ann Surg Oncol 15(2):462–471
Ahn HS, Kim SM, Jang M, La Yun B, Kim S-W, Kang E et al (2013) Comparison of sonography with sonographically guided fine-needle aspiration biopsy and core-needle biopsy for initial axillary staging of breast cancer. J Ultrasound Med 32(12):2177–2184
Choy N, Lipson J, Porter C, Ozawa M, Kieryn A, Pal S, Kao J, Trinh L,Wheeler A, Ikeda D, Jensen K, Allison K,Wapnir I (2015) Initial results with preoperative tattooing of biopsied axillary lymph nodes and correlation to sentinel lymph nodes in breast cancer pa- tients. Ann Surg Oncol 22(2):377–382
Gipponi M, Fregatti P, Garlaschi A, Murelli F, Margarino C, Depaoli F, Baccini P, Gallo M, Friedman D (2016) Axillary ultra- sound and fine-needle aspiration cytology in the preoperative stag- ing of axillary node metastasis in breast cancer patients. Breast 30: 146–150
Mainiero MB, Cinelli CM, Koelliker SL, Graves TA, Chung MA (2010) Axillary ultrasound and fine-needle aspiration in the preoperative evaluation of the breast cancer patient: an algorithm based on tumor size and lymph node appearance. Am J Roentgenol 195(5):1261–1267
Bedi DG, Krishnamurthy R, Krishnamurthy S, Edeiken BS, Le-Petross H, Fornage BD et al (2008) Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. Am J Roentgenol 191(3):646–652
Cools-Lartigue J, Sinclair A, Trabulsi N, Meguerditchian A, Mesurolle B, Fuhrer R, Meterissian S (2013) Preoperative axillary ultrasound and fine-needle aspiration biopsy in the diagnosis of axillary metastases in patients with breast cancer: predictors of accuracy and future implications. Ann Surg Oncol 20(3):819–827
Holwitt DM, Swatske ME, Gillanders WE, Monsees BS, Gao F, Aft RL, Eberlein TJ, Margenthaler JA (2008) Scientific presentation award: the combination of axillary ultrasound and ultrasound-guided biopsy is an accurate predictor of axillary stage in clinically node-negative breast cancer patients. Am J Surg 196(4):477–482
Kim WH, Kim HJ, Jung JH, Park HY, Lee J, Kim WW, Park JY, Cheon H, Lee SM, Cho SH, Shin KM, Kim GC (2017) Ultrasound-guided fine-needle aspiration of non-palpable and suspicious axillary lymph nodes with subsequent removal after tattooing: false-negative results and concordance with sentinel lymph nodes. Ultrasound Med Biol 43(11):2576–2581
Houssami N, Ciatto S, Turner RM, Cody HS, Macaskill P (2011) Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg 254(2):243–251
Zhang F, Zhang J, Meng Q, Zhang X (2018) Ultrasound combined with fine needle aspiration cytology for the assessment of axillary lymph nodes in patients with early stage breast cancer. Medicine (Baltimore). [cited 2018 Dec 11];97(7). Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5839868/
Kuenen-Boumeester V, Menke-Pluymers M, de Kanter AY, Obdeijn I-MA, Urich D, Van Der Kwast TH (2003) Ultrasound-guided fine needle aspiration cytology of axillary lymph nodes in breast cancer patients. A preoperative staging procedure. Eur J Cancer 39(2):170–174
Hu X, Zhou X, Yang H, Wei W, Jiang Y, Liu J (2018 Jun) Axillary ultrasound and fine needle aspiration biopsy in the preoperative diagnosis of axillary metastases in early-stage breast cancer. Oncol Lett 15(6):8477–8483
Park S, Koo JS, Kim GM, Sohn J, Kim SI, Cho YU et al (2018) Feasibility of charcoal tattooing of cytology-proven metastatic axillary lymph node at diagnosis and sentinel lymph node biopsy after Neoadjuvant chemotherapy in breast cancer patients. Cancer Res Treat Off J Korean Cancer Assoc 50(3):801–812
Liu Q, Xing P, Dong H, Zhao T, Jin F (2018) Preoperative assessment of axillary lymph node status in breast cancer patients by ultrasonography combined with mammography: a STROBE compliant article. Medicine (Baltimore) 97(30):e11441
Verbanck J, Vandewiele I, De Winter H, Tytgat J, Van Aelst F, Tanghe W (1997) Value of axillary ultrasonography and sonographically guided puncture of axillary nodes: a prospective study in 144 consecutive patients. J Clin Ultrasound 25(2):53–56
Park SH, Kim MJ, Park B-W, Moon HJ, Kwak JY, Kim E-K (2011) Impact of preoperative ultrasonography and fine-needle aspiration of axillary lymph nodes on surgical management of primary breast cancer. Ann Surg Oncol 18(3):738–744
Agha RA, Borrelli MR, Vella-Baldacchino M, Thavayogan R, Orgill DP, for the STROCSS Group (2017) The STROCSS statement: strengthening the reporting of cohort studies in surgery. Int J Surg 46:198–202
Shrivastava V, Singh S, Singh S, Maurya AK, Maurya AK, Singh M, Singh M, Sam S, Sam S, Gupta SK, Gupta SK (2016) The accuracy of USG and USG guided FNAC axilla in predicting nodal metastasis in a clinically lymph node negative cancer breast patient. Int J Res Med Sci 5(1):196–200
Availability of Data and Material
Not applicable.
Code Availability
Not applicable.
Author information
Authors and Affiliations
Contributions
Not applicable.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
IECPG-292/07.09.2017, RT 2/16.10.2017
Consent to Participate
Taken.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Singh, R., Deo, S.V.S., Dhamija, E. et al. To Evaluate the Accuracy of Axillary Staging Using Ultrasound and Ultrasound-Guided Fine-Needle Aspiration Cytology (USG-FNAC) in Early Breast Cancer Patients—a Prospective Study. Indian J Surg Oncol 11, 726–734 (2020). https://doi.org/10.1007/s13193-020-01222-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-020-01222-3