Abstract
The purpose of this study was to compare early oncologic outcomes of oncoplastic breast surgery and conventional breast conservation surgery in patients of locally advanced breast cancer. A single-center, prospective, non-randomized study enrolled select cases of locally advanced breast cancer (TNM T3/T4, N0/1/2) who after neoadjuvant chemotherapy, were considered for breast conservation surgery with oncoplasty techniques. The specimen volume resected, the mean margins and mean closest margin obtained were noted. The re-surgery rates, complication rates, and incidence of locoregional recurrence were also noted. Variables were compared with a retrospective cohort of similar patients who had undergone conventional breast conservation surgery. Fifty-seven patients underwent OBS (group 1) and were compared with 43 cases that had undergone conventional BCS (group 2). Majority of the patients in group 1 (73 %) had cT3 with N0 or N+ and a minority (17 %) were with limited skin involvement (cT4 and N0/N+). Relatively larger sized, post-NACT tumors could undergo OBS(4.4 vs 2.3 cm). Relatively greater proportion of tumors in central and lower quadrants were addressed by oncoplasty than traditional BCS (17/57, 29 % vs 4/43, 9 %, p = 0.04). The mean specimen volume excised in group 1 was more than that in group 2. (187.54 vs 125.19; p = 0.01). The mean of the margins were obtained more in group 1 (1.04 vs 0.69 cm); p < 0.01) as also the mean closest margin (0.86 vs 0.49 cm; p < 0.01). The incidence of close or involved margins was lesser in the OBS group (8 vs 24 %). Overall incidence of complications was similar in both groups (8/57, 14 % vs 4/43, 9 %; p = 0.34 NS). The median follow-up period of group 1 is 18 months (range 06–30 months) while group 2 is 34 months (14–44 months. There was no recurrence in group 1, but there were 5 cases (11 %) in group 2. Oncoplasty breast surgery offers more opportunity for breast conservation and oncologic safety than conventional breast conserving surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breast conservation therapy (BCT) has become the standard of care for early breast cancer as it has been shown it has an equivalent survival benefit compared to conventional mastectomy [1]. However, the optimal surgical management of patients with locally advanced breast cancer(LABC) remains undefined. The possibility of breast conservation in cases of LABC has been demonstrated in single-institution experiences with small numbers and long follow-up. There are also single institutional experiences with large number of LABC patients, but with short follow-up results [2–4]. The only prospective randomized trial, LAMANOMA, failed due to insufficient accrual of the patients [5]. Oncoplastic breast surgery (OBS) offers optimal oncological treatment as well as improved overall aesthetic outcomes. Studies have demonstrated that in early breast cancers and large operable breast cancers, as compared to conventional breast conservation surgery (wide local excision or lumpectomy with a gross margin of 1 cm plus axillary lymph node dissection), OBS achieves wider excision of tumor, superior mean volume of specimen, and potentially reduces margin involvement. It is more effective from aesthetic-functional point of view and does not compromise local control and survival [6–8]. Locally advanced breast cancer (LABC) accounts for a large proportion of breast cancers in India; 40–60 % in some series. In these patients, breast conservation rates are poor [9, 10]. OBS offers an attractive alternative in these cases to enable breast conservation. The purpose of this study was to demonstrate if OBS techniques offer any advantage over conventional BCS in terms of surgical outcomes in cases of LABC who have been pre-treated with NACT. The parameters studied were specimen volume resected, the margin status, complication rates, and local recurrence seen in the limited follow-up seen.
Patients and Methods
This was a single-center, prospective observation study carried out in an oncology center of a teaching hospital, over a 30-month period, since Jan. 2012 to Aug 2014. The study was approved by the Institutional Committee of Research Ethics. Written informed consent was obtained from all patients. The study enrolled patients of LABC (AJCC TNM 2010) which classically includes Stage III A: cT3N1, T3N2, T1N2, T2, N2, and Stage III B: cT4N0, T4N1, T4N2. Though not part of LABC as per AJCC, Stage IIB: cT3N0 too has been included in our study.
Workup and Staging
All patients underwent a detailed physical examination. Physical examination of the breast along with digital sonomammography was utilized to accurately size and mark the tumor. The pre-treatment tumor site and size were marked on the breast with either subcutaneous methylene blue or indelible henna. All patients underwent CECT of the chest, ultrasonogram of the abdomen, and a bone scan. All patients underwent a trucut biopsy to get an accurate histopathological diagnosis and receptor status.
Neoadjuvant Chemotherapy
Patient received doxorubicin-based neoadjuvant chemotherapy in which they received 4 cycles of Inj Doxorubicin 60 mg/m2 and Inj Cyclophosphamide 600 mg/m2 on Day 1 at a 21-day interval.
Pre-surgery Evaluation
Patient underwent a physical as well as mammographic assessment of tumor response. Patients who demonstrated a decrease in tumor size to an extent >25 % of the original size were termed responders and were considered for OBS. The following patients are not considered for breast conservation:
-
(i)
Extensive peau d orange
-
(ii)
Extensive skin involvement( infiltration or ulceration)
-
(iii)
Chest wall involvement
-
(iv)
Multicentric disease
Limited skin involvement/ peau d orange as well as a multifocality limited to one quadrant were not considered a contraindication provided they could be excised en bloc at the time of surgery.
Surgery
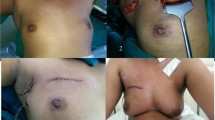
Oncoplastic surgical techniques used in the patients included the use of volume displacement (periareolar, superior and inferior pedicle techniques, quadrantectomy with glandular remodeling, and dermo-glandular flaps) or volume replacement (mini LD myofascial or myocutneous flap) (Figs. 1 and 2) The choice of technique to be used took into consideration following factors:
-
(i)
Tumor site (quadrant) and size
-
(ii)
Tumor: Breast ratio
-
(iii)
Position of tumor in relation to nipple areolar complex
-
(v)
Degree of ptosis of ipsilateral breast as well as contralateral breast
-
(vi)
Extent of skin excision anticipated.
Tumor excision was performed with the aim of including the tumor with at least 1 cm of healthy tissue far from the macroscopic margins. Frozen section from edges of tumor cavity was not done routinely. It was resorted to only if there was suspicion that residual tumor may have been left. The tumor bed was marked by placing clips in the four dimensions. Patients were not offered symmetrization surgery for contralateral breast at same sitting. The same was offered after completion of adjuvant therapy.
Pathological Assessment
Surgical specimens were identified according to their topography and spatial position. The volume of each specimen was calculated by multiplying measurements of the length, width, and height and correlated with values obtained by volumetric method. The specimen are inked in six dimensions, fixed, and sectioned. The maximal diameter of residual tumor was noted. The anatomo-pathologic response assessment was done. Presence of residual disease, macroscopic (residual nidus over small areas), and microscopic (residual scatter cells over original volume) disease foci were evaluated. Margins were assessed by radial (perpendicular) margin assessment technique. Six margins were taken: superior, inferior, medial, lateral, posterior, and anterior). Margin was considered a negative margin if tumor cells were >2 mm from the cut edge. It was considered close margin if tumor cell nest were present <2 mm from the cut edge and positive margin if tumor cell nests were seen at the cut edge. The mean of the six margins was calculated to get the mean margin for specimen as a whole. The closest margin obtained in each specimen was noted separately.
Adjuvant Therapy
Patient received 12 cycles of Inj Paclitaxel at weekly intervals. Inj Transtuzumab was given if patients were Her2neu positive. Patient also received standard adjuvant radiation therapy to the chest wall and with boost to the tumor bed.
Post-Operative Assessment
Patients were followed up at three monthly intervals for the first 2 years then at six monthly intervals. Clinical assessment was done at each visit and mammogram at six monthly intervals.
Control Group
The control group was historical and included patients who had undergone conventional BCS previously. This included patients who had received the aforesaid neoadjuvant chemotherapy and then undergone lumpectomy with a gross 1-cm margin or a formal quadrantectomy along with axillary lymph node dissection. These patients had received the same adjuvant therapy as outlined previously. Only those patients for whom complete medical records were available have been included in this study.
Outcome Measure
Primary outcome measures included volume of specimen resected, residual tumor size, mean margins, and mean closest margin. The mean margin of a tumor was defined as having the mean of the six dimensions measured in each specimen. The secondary outcome measures included incidence of revision surgery done, incidence of complications, and incidence of local recurrence.
Statistical Analysis
Outcome analysis has been carried out by SPSS Ver 20.0. Statistical analysis for significance between variables was performed by unpaired Students t test, Fisher’s exact test, and chi square test.
Results
During the study period, so far, neoadjuvant chemotherapy was initiated in 154 cases of LABC. Out of these, 141 were considered for surgery after 4 cycles of NACT. Sixty-two cases (44 %) were considered optimal to be offered BCS by oncoplasty. However, five patients refused BCS and opted for MRM. Fifty-seven patients have undergone OBS and constitute group 1. Forty-three cases that have undergone conventional BCS constitutes group 2. The patients in group 1 are younger than those in group 3 (mean age 46.9 yrs. (±13.1) vs 54.3 yrs. (±5.3) p = 0.03; Mann-Whitney U test). Majority of patients in group 1 (73 %) were cT3 with N0 or N+ and a minority ( 27 %) were those with limited skin involvement ( cT4 and N0/N+). It was seen that the pre-NACT tumor size was similar in both groups. However, we could subject relatively larger sized, post-NACT tumors to breast conservation surgery by oncoplasty rather than by conventional approach (Table 1). Relatively, a greater proportion of tumors in the central and lower quadrants were addressed by oncoplasty than traditional BCS ( 17/57 29 % vs 4/43 9 %, p = 0.04) (Table 1). The clinicopathologic profile in terms of pathological T and N stage, grade, and hormone receptor status was similar between the two groups (Table 2). It was seen that mean specimen volume excised in the oncoplasty group was more than that in the conventional BCS group. (187.54 vs 125.19; p = 0.01). The mean of the margins obtained too was more in the former group (1.04 vs 0.69 cm); p < 0.01) as also was the mean closest margin (0.86 vs 0.49 cm; p < 0.01) (Table 3). It was seen that close or involved margins occurred lesser in OBS group as compared to conventional BCS (8 vs 24 %) (Table 3). One patient had to undergo revision surgery in Group 1 as compared to three in group 2. Further study of histopathological response pattern seen in patients receiving NACT in group 1 revealed that only 26/57 (47 %) cases had universal concentric regression while 44 % has actually patchy regression in the form of micro-, macro-, or mixed-pattern fragmentation. Macrofragmentation with macroscopic tumors was seen in 15 cases (26 %). Microfragmentation was seen in 9 cases (16 %). Macro- and microfragmentation with in situ carcinoma was seen in 1 case ( 2 %). Pathologic CR was seen in 3 cases ( 4 %), while one and two cases had progressive and stable disease, respectively.
Overall, the incidence of complications was similar in both groups ( 8/57, 14 % vs 4/43, 9 %; p = 0.34 NS, chi-square test). The OBS group ( group 1 ) had four cases of wound infection and one case each of partial necrosis nipple areolar complex; skin flap necrosis, hematoma, and seroma. The group 2 patients had two cases of wound infection and one each of hematoma and seroma. The median follow-up period of group 1 is 18 months (range 6–30 months) which is much shorter than the conventional BCS group which is 34 months (14–44 months; p = 0.04, Mann-Whitney U test). While there was no recurrence seen in the former group as yet, there have been five cases (11 %) of recurrences seen in group 2 during the follow-up.
Discussion
Locally advanced breast cancer is a heterogeneous group which comprises larger than 5-cm tumors (T3) or skin/chest wall involvement (T4). In these cases, a large-volume tissue resection or a large amount of skin loss results in poor aesthetic results and this proves an impediment to breast conservation therapy in LABC. However, studies have shown that breast conservation surgery (BCS) for operable breast cancer after neoadjuvant chemotherapy is feasible and safe and associated with acceptable local recurrence rate [11–13]. It has been well-documented that the integration of oncoplastic surgical techniques has enabled more extensive resections of large tumors. This can be achieved through alternative incisions that enable the dissection of larger breast volume while maintaining good cosmetic results.
We have studied a heterogenous group of cases of LABC who were subjected to NACT and followed by breast conservation surgery by oncoplasty breast surgery approach. We found that we could achieve breast conservation with acceptable aesthetic results in larger, post-NACT tumors by utilizing OBS than we could previously achieve by traditional wide local excision.
A major advantage of OBS over traditional BCS is the larger volume of breast parenchyma which can be resected thus allowing assessment of pathological response following the complete excision of the original tumor area. In this study, it is seen that OBS resulted in larger specimen volume size as well as wider mean margins being obtained. This is similar to the experience of other authors too [6–8]. In our patients, total excision was made possible by tattooing the tumor skin projection preoperatively as suggested by Mathieu et al. [14]. The skin tattooing permitted localization of the tumor area, and oncoplastic surgical techniques, in turn, certainly permit the excision of the entire preoperatively ink-marked tumor area regardless of tumor response. It may be argued that these wider parenchymal excisions are done to achieve symmetry with the contralateral breast and not necessarily to obtain wider surgical margins; hence, comparing the mean margin status obtained in OBS and that obtained in conventional BCS may be fallacious. However, it may be pointed out that the mean closest margin, a measure not mentioned in previous studies but studied in this study, is also significantly more in OBS than in conventional BCS. This suggests that OBS indeed yields wider margins than conventional BCS.
The histopathologic response pattern to the NACT seen in our study suggests that while 47 % tumors had concentric pattern of regression, another 44 % had actually a “patchy” regression. In the latter group there would be viable tumor cell nests outside the zone of post-NACT clinical tumor size. Although this study did not focus on the prognostic relationship among these findings, it is in consonance with other studies that have shown that the variety of pathologic responses seen indicates that the entire pre-chemotherapy tumor area should be removed [10, 15]. For some authors, the diversity of pathologic findings indicates the need for mastectomy [16, 17]. Other authors argue that the low recurrence rates reported in selected cases of T3 and T4 show that these patients could rather undergo conserving surgery [3, 18]. This study indicates that oncoplastic surgical techniques are a useful approach to achieve this aim of getting adequate margins as well as achieving a cosmetically acceptable breast conservation. Indeed, as seen in this study, the incidence of close or involved margins is significantly less in patients undergoing OBS than those who underwent conventional BCS.
Although some authors [6, 19] have reported more number of complications in OBS leading to a delay in the start of adjuvant therapy, in this study, the complication rates were similar in both groups, and there was no delay noted in the start of adjuvant therapy. In this study, no locoregional recurrence has been seen in OBS while 11 % cases in conventional BCS have demonstrated local relapse. However, it is to be noted that the follow-up for patients undergoing OBS has been relatively short as compared to those who underwent conventional BCS. Whether the wider margins enabled by OBS results in lower incidence of locoregional recurrence remains to be seen on a longer follow-up. Other studies with longer follow-up have indicated a recurrence rates of 6–10 % following OBS which is comparable to that of conventional BCS [20, 21]. The study may be criticized on the aspect that it is not a prospective, randomized study; rather, it relies on comparing OBS with a retrospective cohort. It is unlikely that a prospective randomized trial can be conducted to compare OBS with conventional BCS in post-NACT, LABC cases. This is so because the heterogeneity of presentation in LABC prevents randomization of cases into these two arms. A tumor not amenable to breast conservation by conventional methods may be amenable to the same by oncoplastic technique. Moreover, as in our center where breast conservation is now being routinely done by OBS techniques, it would be morally unethical to offer OBS to one set of patients and not to the other. Hence, evidence supporting advantages of OBS over conventional BCS is likely to be only garnered in similar studies like this one which are conducted over a longer period in a larger cohort. Prima facie, OBS represents a major improvement in surgical de-escalation in breast surgery enabling surgeons to improve the frequency of conservative procedures in LABC without compromising oncologic safety.
It is logical to assume that oncoplasty breast surgery may extend the indication of breast conservation in LABC. Indeed, in our study, it is demonstrated that by OBS, we can offer breast conservation to larger, post-NACT tumor sizes than when traditional lumpectomy is employed. It is also seen that OBS can be offered successfully to tumors in lower quadrants as well as central quadrant. These are sites where the cosmetic results are poor if traditional lumpectomy technique is utilized.
Conclusion
The use oncoplastic surgical techniques in selected locally advanced breast cancer patients who have undergone NACT represent a good option to offer breast conservation treatment. It allows removing the entire area supposedly affected by disease with favorable cosmetic outcomes. It appears to offer better oncologic safety than conventional BCS approach.
References
Fisher B, Anderson S, Bryant J, et al. (2002) Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 347:1233–1241
Parmar V, Hawaldar R, Badwe RA (2010) Safety of partial breast reconstruction in extended indications for conservative surgery in breast cancer. Indian J Surg Oncol 1:256–262
Chen AM, Meric-Bernstam F, Hunt KK, et al. (2004) Breast conservation after neoadjuvant chemotherapy: the MD Anderson cancer center experience. J Clin Oncol 22:2303–2312
Parmar V, Krishnamurthy A, Hawaldar R, et al. (2006) Breast conservation treatment in women with locally advanced breast cancer—experience from a single centre. Int J Surg 4:106–114
Sinacki M, Jassem J, van Tienhoven G (2005) Conservative local treatment versus mastectomy after induction chemotherapy in locally advanced breast cancer: a randomised phase III study (EORTC 10974/22002, LAMANOMA)—why did this study fail? Eur J Cancer 41:2787–2788
Clough KB, Lewis JS, Couturaud B, et al. (2003) Oncoplastic techniques allow extensive resections for breast conserving therapy of breast carcinomas. Ann Surg 237:26–34
Kaur N, Petit JY, Rietjens M, et al. (2005) Comparative study of surgical margins in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol 12:539–545
Giacalone PL, Roger P, Dubon O, et al. (2006) Comparative study of the accuracy of breast resection in oncoplastic surgery and quadrantectomy in breast cancer. Ann Surg Oncol 14:605–614
Rustogi A, Budrukkar A, Dinshaw K, Jalali R (2005) Management of locally advanced breast cancer: evolution and current practice. J Cancer Res Ther 1:21–27
Akhtar M, Akulwar V, Gandhi D, Chandak K (2011) Is locally advanced breast cancer a neglected disease? Indian J Cancer 48:403–405
Cance WG, Carey LA, Calvo BF, et al. (2002) Long-term outcome of neoadjuvant therapy for locally advanced breast carcinoma: effective clinical down staging allows breast preservation and predicts outstanding local control and survival. Ann Surg 236:295–302
Wolmark N, Wang J, Mamounas E, et al. (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 30:96–102
Hortobagyi GN, Singletary SE, Strom EA (eds) (2010) Locally advanced breast cancer. Diseases of the breast, 4 edn. Lippincott Williams and Wilkins, Philadelphia, pp. 750–751
Mathieu MC, Bonhomme-Faivre L, Rouzier R, Seiller M, Barreau-Pouhaer L, Travagli JP (2007) Tattooing breast cancers treated with neoadjuvant chemotherapy. Ann Surg Oncol 14:2233–2238
Bonadonna G, Valagussa P, Brambilla C, et al. (1998) Primary chemotherapy in operable breast cancer: eight year experience at the Milan cancer institute. J Clin Oncol 16:93–100
Singletary SE, McNeese MD, Hortobagyi GN (1992) Feasibility of breast-conservation surgery after induction chemotherapy for locally advanced breast carcinoma. Cancer 69:2849–2852
Abraham DC, Jones RC, Jones SE, et al. (1996) Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer 78:91–100
Gentilini O, Intra M, Gandini S, et al. (2006) Ipsilateral breast tumor reappearance in patients treated with conservative surgery after primary chemotherapy. The role of surgical margins on outcome. J Surg Oncol 94:375–379
Mazouni C, Naveau A, Kane A, et al. (2013) The role of oncoplastic breast surgery in the management of breast cancer treated with primary chemotherapy. Breast 13:1189–1193
Zucca Matthes AG, Uemura G, Kerr L, et al. (2012) Feasibility of oncoplastic techniques in the surgical management of locally advanced breast cancer. Int J Surg 10:500–505
Bogusevicius A, Cepuliene D, Sepetauskiene E (2014) The integrated evaluation of the results of oncoplastic surgery for locally advanced breast cancer. Breast J 20:53–60
Acknowledgment
Ms. Harleen Chabbra, Translational Psychiatry Lab, NIMHANS, Bangalore for statistics support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Institutional Committee of Research Ethics. Written informed consent was obtained from all patients.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Chauhan, A., Sharma, M.M. & Kumar, K. Evaluation of Surgical Outcomes of Oncoplasty Breast Surgery in Locally Advanced Breast Cancer and Comparison with Conventional Breast Conservation Surgery. Indian J Surg Oncol 7, 413–419 (2016). https://doi.org/10.1007/s13193-016-0549-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-016-0549-6