Abstract
Thymic tumors represent 0.2–1.5 % of all malignancies with an incidence of 0.15 per 100,000 population. Thymic tumors are most common tumors of the anterior mediastinum accounting for 20 % of all mediastinal tumors and 50 % of all anterior mediastinal tumors. Over 90 % of all thymic tumors occur in anterior mediastinum, remainder occurring in neck or other mediastinal areas especially aortopulmonary window and retro cardiac area which are common sites for ectopic thymic tissues and possible explanation for failure in some cases of simple thymectomy to improve Myasthenia Gravis(MG). The aim of this review is to discuss histologic classification, diagnostic features, evaluation, management and prognosis of thymic tumors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The word thymus is derived from Greek word thumos meaning heart or soul possibly because of its location or else comes from the herb thyme to which it resembles [1]. The thymus is a specialized pinkish grey colored soft lobulated organ located anatomically in anterior superior mediastinum in front of heart and behind sternum. It is a central lymphoid organ functioning in T lymphocyte maturation which are critical cells of adaptive immune system [2, 3]. It develops from third pharyngeal pouch along with parathyroid gland, grows during infancy, reaches maximum weight by puberty, following which it involutes eventually leading to a scattered lymphocytes present within adipose tissue.
Thymic tumors represent 0.2–1.5 % of all malignancies with an incidence of 0.15 per 100,000 population [4]. Thymic tumors are most common tumors of the anterior mediastinum accounting for 20 % of all mediastinal tumors and 50 % of all anterior mediastinal tumors [5]. Over 90 % of all thymic tumors occur in anterior mediastinum, remainder occurring in neck or other mediastinal areas especially aortopulmonary window and retro cardiac area which are common sites for ectopic thymic tissues and possible explanation for failure in some cases of simple thymectomy to improve Myasthenia Gravis(MG).
The aim of this review is to discuss histologic classification, diagnostic features, evaluation, management and prognosis of thymic tumors.
Pathology
Thymus is composed of thymocytes, lymphocytes and epithelial stroma. Six types of epithelial cells have been identified in mature thymus, four exist in cortical region and two in medullary region. Type six forms Hassalls corpuscles that are characteristic of thymus [6].
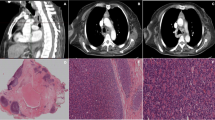
Although lymphomas, neuroendocrine tumors(NET) and germ cell tumors all may arise within the thymus, only thymomas, thymic carcinomas and thymolipomas arise from true thymic elements. The normal contour of thymus is biconcave or flat but the diseased thymus displays a more convex margin.
Thymomas grossly are lobulated, firm, tan pink to gray tumors that may contain cystic spaces, calcification or hemorrhage. They may be encapsulated, adherent to surrounding structures or frankly invasive. Microscopically thymomas arise from thymic epithelial cells although thymocytes or lymphocytes may predominate histologically. True thymomas contain cytologically bland cells and should be distinguished from thymic carcinomas.
Like thymoma, thymic carcinoma is an epithelial tumor but cytologically exhibit malignant features. Extensive local invasion and distant metastases are common. Low grade tumors(LGT) include squamous cell carcinoma, mucoepidermoid carcinoma, basaloid carcinoma. High grade tumors(HGT) include lymphoepithelial like carcinoma, small cell carcinoma, undifferentiated, sarcomtoid and clear cell carcinoma [7]. LGT have a more favourable clinical course (Median Survival(MS) 24.4 months to >6.6 years) when compared with HGT(MS 11.3–15 months) [8].
NETs are often macroscopically large, encapsulated masses. A characteristic feature of these tumors is the presence of nests of tumor cells that are detached from the surrounding stroma and contain areas of necrosis. They usually show cytologic and architectural features of neuroendocrine differentiation and hence can be positive for CD56,Neuron Specific Enolase(NSE),Chromogranin, Somatostatin and Synaptophysin [9].
Currently term non-invasive and invasive are preferred over benign or malignant designation.
Histological Classification
To unify the pathology of thymic neoplasms in view of histologic variability and intratumoral heterogeneity, the WHO in 1999 proposed a histological classification system which was revised in 2004 (Tables 1 and 2). It is based on histological assessment of the morphology of the neoplastic epithelial cells and the non-neoplastic lymphocytic component [10, 11].
The WHO classification has prognostic significance. WHO Type A-B2 tumors are more likely to present with locoregional disease than WHO Type B3-C [12]. The histology can help predict resectability. WHO Type A tumors are mostly non-invasive hence completely resectabe as compared with WHO Type B tumors.
Clinical Features
Sex distribution in thymomas is roughly equal though SEER data suggests a male predominance. Peak age of onset in thymomas with MG is 4th to 5th decade and without MG peaks in 7th or 8th decade [13, 14]. Nearly one half patients are asymptomatic and incidentally discovered on routine examination or investigation. In symptomatic patients,50 % are related to paraneoplastic syndromes(PNS) predominantly MG,40 % present with pain and pressure symptoms and rest have generalized symptoms such as weight loss or tiredness [15].
Thymic carcinoma which comprises 1 % of thymic malignancies have a male predominance with mean age at diagnosis being 47–60 years [16]. In comparison to thymomas being indolent, thymic carcinomas have high malignant potential and can be aggressive with poor prognosis. Initial presentation is usually with cough, chest pain, phrenic nerve palsy, SVC syndrome or as an incidental finding [17]. Local invasion of contiguous mediastinal structures is present in upto 80 % at the time of diagnosis. Metastases or lymph node spread is present in 40 % at presentation. The usual sites for metastases are bones, lungs, pleura and liver.
NETs account for <5 % of all mediastinal neoplasms with mean age of 54 years(range 15–100 years) and male predilection [18]. One third cases are incidentally diagnosed and rest have symptoms secondary to PNS. Metastases are reported to occur in 20–30 % of cases affecting liver, lung, pleura and bones. Patients can also present with pressure symptoms.
Paraneoplastic Syndromes (PNS)
Thymomas are associated with several PNS. Approximately 30 % of thymomas develop MG while as 10–12 % patients with MG have thymomas [19]. MG is characterized by development of autoimmune antibodies to acetylcholine receptors on the postsynaptic neuromuscular junction. Symptoms at presentation include blurred vision, dysphagia and muscle weakness. Pure red cell aplasia(PRCA) is the second most common PNS.30–50 % patients with PRCA have associated thymomas. Examination of bone marrow reveals an absence of erythroid precursors and in 30 % decrease in platelets and leucocyte. Hypogammaglobulinemia is reported in 3–6 % cases and manifests itself as combined deficiency of both T and B lymphocyte leading to increased risk of infections. Other paraneoplastic conditions associated include rheumatoid arthritis, thyroiditis, SLE, ulcerative colitis, acute pericarditis, myocarditis, cushings syndrome, sensory motor neuropathy and hemolytic anemias [20]. PNS are uncommon in thymic carcinoma having no association with MG. However in well differentiated thymic carcinoma (WDTC), PNSs have been described. About one third patients in NETs are associated with Cushings Syndrome, MEN-1. Unlike carcinoids of GIT, NETs are rarely associated with carcinoid syndrome. Lambert Eaton Syndrome, peripheral neuropathy and proximal myopathy are also associated with NETs.
Staging of Thymic Tumors
The Masaoka Staging System is the most commonly used to assess stage (Table 3). It was originally designed in 1981 and several modifications to this have been offered, but proved to be of no significant advantage over original [21, 22]. It assesses the degree of invasion of capsule and surrounding structures. A WHO Staging System using the TNM Classification has been published in 1994.
Investigations
Blood Tests
It includes complete hemogram, tumor markers like Beta HCG, AFP to rule out GCTs;T3,T4,TSH as clinically indicated to rule out mediastinal goitre; gammaglobulins; ACTH, ADH in NETs. To rule out MG, serum antiacetylcholine receptor antibody levels measured to avoid respiratory failure during surgery.
Imaging
It includes Chest X Ray-PA & lateral view which may show mediastinal mass & in 15 % may show calcification. Contrast enhanced CT chest is done to delinate local extent, nodal involvement & metastases. MRI is more sensitive in defining tumor contour, capsule invasion, intratumoral signals & vascular involvement.
Nuclear Medicine Imaging
The role of PET/CT in diagnosis, treatment planning, recurrences has been highlighted by various studies & has gained acceptance [23]. The role of somatostatin receptor scintigraphy has been studied in NETs. Gallium 67 is taken by lymphomas & can help in differential diagnosis.
Biopsy
NCCN guidelines recommend CT guided FNAC or trucut biopsy for tissue diagnosis in locally advanced or unresectable tumours avoiding transpleural approach.
Management
Thymic tumours require a multidisciplinary team approach involving thoracic surgeons, radiation and medical oncologists, diagnostic imaging specialists, pulmonologists to determine optimal plan of treatment.
The NCCN guidelines [24] from treatment point of view has divided thymic tumors into three categories-localised/resectable, locally advanced/unresectable and metastatic groups. Determination of resectability must be done by experienced thoracic surgeons. Surgical resection (total thymectomy and complete excision of tumour) is appropriate in resectable tumours. Patients with R0 resection in thymomas with no capsule invasion (CI) are candidates to be kept under surveillance. If RO resection with CI or thyroid carcinoma, postoperative radiotherapy (PORT) is considered. If R1 resection in thymomas, PORT is given and if R1 resection in thymic carcinomas, PORT and chemotherapy should be considered. In R2 resection, PORT and chemotherapy is considered.
Role of Surgery
Complete surgical resection is the mainstay of therapy & is the most important predictor of long term survival. Although median sternotomy is the most commonly used approach, bilateral anterolateral thoracotomies with transverse sternotomy or clam shell procedure is preffered with advanced or laterally displaced large tumors. VATS & Robotic Assisted Surgery have been used with acceptable outcomes but long term results are not available [25].
Goal of surgery is complete excision of the lesion with total thymectomy and complete resection of contiguous and noncontiguous disease. Complete resection(CR) may require the resection of adjacent structures including pericardium, phrenic nerve.pleura.lung and even major vascular structures. Extended thymectomy involves excision of all tissues anterior to pericardium from diaphragm to neck and laterally from one phrenic nerve to the other. During thymectomy, the pleural surfaces should be examined for metastases. CR is reported to be 100 % in stage I tumors. The success rate falls with higher stages (Table 1). CR is the most important prognostic factor since patients with stage III disease can have a similar survival rate if complete resection is feasible. The histology also can help predict resectability as WHO type B being invasive have less chance of CR compared with WHO type A [10].
Role of debulking or subtotal resection is controversial with conflicting datas in favour and against [26, 27]. Partial resection has been argued to provide better survival than a simple biopsy in unresectable cases [28].
Role of Radiotherapy
Radiotherapy is indicated in incompletely resected, locally advanced, unresectable, recurrent thymic tumors. In stage 1 tumors with CR, adding RT has not shown any added advantage [29]. PORT is indicated in stage II, III invasive disease as it decreases recurrence rates after CR from 28 % to 5 % [30]. Pollack reported an increase in 5 year disease free survival for stage II to stage IVA from 18 % to 62 % [31]. For advanced disease, chemotherapy with RT is recommended. CT based planning & 3D conformal technique (IMRT) to reduce normal tissue damage is required. A definitive dose of 60–70 Gy is given to patients with unresectable disease. For adjuvant treatment, a total dose of 45–50 Gy is used for clear or close margins;54 Gy for microscopically positive resection margin. In patients with gross residual disease, a total dose of 60 Gy(1.8 to 2 Gy/fraction per day) is recommended. Extensive elective nodal irradiation is not recommended as thymomas do not commonly metastasise to regional lymph nodes.
Role of Chemotherapy
Combination chemotherapy have shown good response rates & have been used in both adjuvant & neoadjuvant settings in treatment of advanced invasive, metastastatic & recurrent thymic tumors. Although 6 different combination regimens are provided, cisplatin/doxorubicin based regimens seem to yield the best outcome. Following neoadjuvant therapy complete surgical resection should be attempted where ever feasible and limited pleural metastases can be resected individually, whilst those with extensive pleural diseases may require chemotherapy or combination of chemotherapy and radio therapy.
Somatostatin Analogues
The high expression of somatostatin receptors by thymomas and their avid uptake of radiolabelled octreotide led to the possible therapeutic option of octreotide with predisolone and can lead to objective response [32]. Its role in thymic carcinomas has not been evaluated specifically and role in NETs has been reported in small series & case reports.
Molecular Targetted Therapy
A number of molecular targets have been identified in thymic tissues. These are EGFR expression in 80 % thymomas and 50 % thymic carcinomas, c-kit in 73 % thymic cancers & 5 % thymomas. Gefitinib, cetuximab, dasatinib are being evaluated and case reports and small series have shown some therapeutic benefit [33].
Follow Up
Follow up comprises of clinical examination, CT scanning, blood tests and nuclear imaging including PET/CT or octreotide scan on 6 monthly basis and continued for at least 10 years since late metastases have been reported upto this time following initial surgery(36).
Prognostic Factors
Positive prognostic markers include low grade tumors, low mitotic activity, no capsular invasion, no metastatic spread or lymph node involvement and complete resectability of the tumour at surgery. Poor prognostic factors include presence of associated endocrinopathies, incomplete resection of tumor, high grade tumors. The prognosis in patients with primary thymic NETs remains poor due to aggressive nature and high incidence of recurrence following surgery.
Conclusion
Tumors of thymus are heterogenous group of tumors ranging from relatively benign thymomas to highly aggressive carcinomas. Surgery continues to be the mainstay of treatment and complete resection of the tumour remains the most important prognostic factor. Multimodality therapy seems an appropriate approach to stage III and IV thymomas. Thymic NETs follow an aggressive clinical course. Novel therapeutic approaches are being evaluated.
References
Nishino M, Ashiku SK, Kocher ON, Thurer RL et al (2006) The thymus: a comprehensive review. Radiographics 26:335–348
Miller JF (2002) The discovery of thymus function and of thymus derived lymphocytes. Immunol Rev, 185 July: 7–14
Miller JF (2004) Events that led to the discovery of T cell development and function-a personal recollection. Tissue Antigens, 63 June: 509–17
Engels EA, Pfeiffer RM (2003) Malignant thymoma in United States: demographic pattern in incidence and association with subsequent malignancies. Int J Cancer, 105 July: 546–51
Duwe BV, Sterman DH, Musani AL (2005) Tumors of the mediastinum. Chest, 128 October: 2893–909
Suster S, Rosai J (1990) Histology of the normal thymus. Am J Surg Pathol, 14 March: 284–303
Suster S, Rosai J (1991) Thymic carcinoma-a clinicopathologic study of 60 cases. Cancer 67:1025
Devita, Hellman, Rosenberg. Principles and practice of oncology 8th edition, chapter 38: 973–986
Chaer R, Massad MG, Evans A, Snow NJ, et al. (2002) Primary neuroendocrine tumors of the thymus. Ann Thorac Surg, 74 Nov: 1733–40
Wright CD (2007) Management of thymomas. Crit Rev Oncol Hematol, June 12
Suster S (2006) Diagnosis of thymoma. J Clin Pathol, 59 Dec: 1238–44
Detterbeck FC (2006) Clinical value of WHO classification system of thymomas. Ann Thorac Surg 81:2328
Verley JM, Hollman KH (1985) Thymoma-A comparative study of clinical stage, histologic features and survival in 200 cases. Cancer, 55 March: 1074–86
Blumberg D, Port JL (1995) Thymomas: a multivariate analysis of factors predicting survival. Ann Thorac Surg, October: 908–13
Patterson GA (1992) Thymomas. Semin Thorac Cardiovasc Surg 4:39
Magois E, Guigay J, et al. (2007) Multimodal treatment of thymic cancers. Lung Cancer, July: 3
Eng TY, Fuller CD, Jagirdar J, et al. (2004) Thymic cancers: state of art review. Int J Radiat Oncol Biol Phys, 59 July: 654–64
Moran CA, Suster S (2000) Neuroendocrine tumors of thymus-a clinicopathological analysis of 80 cases. Am J Clin Pathol, 114 July: 100–10
Drachman DB (1994) Myasthenia gravis. N Engl J Med, 330 June: 1797–810
Evoli A, Minicuci GM et al (2007) Paraneoplstic syndromes associated with thymomas. J Neurol 254:756–762
Masoaka A, Monden Y, Nakahara K (1981) Follow up study of thymomas with special reference to their clinical stage. Cancer, 48 Dec: 2485–92
Koga K, Matsuno Y, Noguchi M, et al. (1994) A review of 79 thymomas: modification of staging system. Pathol Int, May: 359–67
El Bawab H, Al Sugair AA et al (2007) Role of PET scan in thymic pathology. Eur J Cardiothorac Surg 31:731
NCCN guidelines for thymomas and thymic cancers. Version 1.2011
Rea F, Maruli G, Bortolloti L et al (2006) Experience with the da Vinci robotic system for thymectomy. In patients with MG, report of 33 cases. Ann Thorac Surg 81:455
Liu HC, Chen YJ et al (2006) Debulking surgery for advanced thymomas. Eur J Surg Oncol 32:100
Giaccone G (2005) Treatment of malignant thymomas. Curr Opin Oncol, March: 140–46
Maggi G, Casodio C et al (1991) Thymomas: results of 241 operated cases. Ann Thorac Surg 51:152
Cowen D, Mornex RF, Bachelot (1995) Thymoma: result of a multicentric retrospective series of 149 non metastatic irradiated patients and review of literature. Radiother Oncol 34–9
Ciernik IF, Meier U, Lutolf UM (1994) Prognostic factors and outcome of incompletely resected invasive thymomas following radiation therapy. J Clin Oncol 12:1484
Pollach A, Komaki R, Cox JD (1992) Thymoma: treatment and prognosis. Int Radiol Oncol Biol Phys 23:1027
Gal AA, Kornstein MJ, Cohen C (2001) NETs of thymus: a clinopathological and prognostic study. Ann Thorac Surg, Oct: 1179–82
Ionescu DN, Sasatomi E, Ciepley K (2005) Protein expression and gene amplification of EGFR in thymomas. Cancer 103(3):167
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bushan, K., Sharma, S. & Verma, H. A Review of Thymic Tumors. Indian J Surg Oncol 4, 112–116 (2013). https://doi.org/10.1007/s13193-013-0214-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13193-013-0214-2