Abstract
Introduction
The purpose of this study is to describe a case where methylene blue improved hemodynamics in a poisoned patient.
Case Report
This is a single case report where a poisoned patient developed vasodilatory shock following ingestion of atenolol, amlodipine, and valsartan. Shock persisted after multiple therapies including vasopressors, high-dose insulin, hemodialysis, and 20% intravenous fat emulsion. Methylene blue (2 mg/kg IV over 30 min) was administered in the ICU with temporal improvement as measured by pulmonary artery catheter hemodynamic data pre- and post-methylene blue administration. Within 1 h of methylene blue administration, systemic vascular resistance improved (240 dyn s/cm5 increased to 1204 dyn s/cm5), and vasopressor requirements decreased with maintenance of mean arterial pressure 60 mmHg.
Discussion
Methylene blue may improve hemodynamics in drug-induced vasodilatory shock and should be considered in critically ill patients poisoned with vasodilatory medications refractory to standard therapies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Methylene blue has been identified as a potential treatment for vasodilatory shock refractory to standard treatment measures. This may include drug-induced vasodilatory shock as can be seen with calcium channel blocker and other vasodilator poisoning. Amlodipine is a dihydropyridine calcium channel blocker and antagonizes the movement of calcium ions into vascular smooth muscle cells. Severe dihydropyridine poisoning may be particularly difficult to manage, as it causes both cardiogenic and vasodilatory shock; the peripheral selectivity of dihydropyridines is often lost in overdose [1]. Valsartan is an angiotensin receptor II blocker (ARB). Hypotension can occur in toxicity due to vasodilation and attenuated sympathetic activity [2]; toxicity is likely enhanced when combined with other drugs that have vasodilator properties, such as calcium channel blockers [3]. Significant vasodilatory shock refractory to standard treatments can occur—this underscores the importance of identifying and promoting efficacious therapies for drug-induced vasodilatory shock.
Methylene blue is best known for its use as a reducing agent in the treatment of methemoglobinemia but is increasing in use as a vasoconstrictor given the literature on administration in vasodilatory shock. Increases in systemic vascular resistance and mean arterial pressure have been consistently described following methylene blue administration for shock due to non-poisoning causes, including vasoplegic syndrome after coronary artery bypass surgery [4] and sepsis [5–7]. Methylene blue, from a mechanistic standpoint, is an attractive therapy for drug-induced vasodilatory shock, though published literature includes only a small number of human case reports and animal studies [8–11]. The human reports primarily describe the successful use of methylene blue in vasodilatory shock caused by dihydropyridine calcium channel blockers, though successful treatment of shock from quetiapine [9] and valsartan [12] with methylene blue has also been described. A recently published animal study found significant increases in pulse, mean arterial pressure, and median survival time after administration of methylene blue in an experimental model of amlodipine-induced shock in rats [11]. Upon review of the human cases, however, we find no cases where hemodynamic data from invasive monitoring was available during the administration of methylene blue to more accurately describe its physiologic effects in humans with drug-induced vasodilatory shock.
We present a case where methylene blue was administered to a patient poisoned with cardioactive medications followed by objective improvements in hemodynamic parameters measured via a pulmonary artery catheter (PAC).
Case Report
A 61-year-old man with a history of hypertension, diabetes mellitus type 2, and depression was found in his garage in a running vehicle after he took an overdose of 500 mg atenolol, 200 mg amlodipine, and 375 mg valsartan reportedly several hours prior. He presented to the emergency department via paramedics. The patient was alert and oriented, vital signs reported to be within normal limits, and initial laboratory values were normal with the exceptions of a serum creatinine of 2 mg/dL and a carboxyhemoglobin blood concentration of 15 % determined by laboratory cooximetry. Despite a systolic blood pressure of 110 mmHg, he received 3 g calcium gluconate and 5 mg glucagon. Due to acute rise in creatinine from baseline, he received 2 L normal saline. He was admitted to the intensive care unit secondary to concern for potential hemodynamic instability from his ingestion. Within 8 h of presentation, dopamine was started for developing hypotension, and he required 10 mcg/kg/min to maintain a mean arterial blood pressure (MAP) > 55 mmHg and heart rate 50–60 beats per minute (bpm). He continued to receive intravenous fluids and was started on a calcium gluconate infusion 1 g/h.
Over the next several hours, catecholamines, specifically phenylephrine titrated to 300 mcg/min, epinephrine titrated to 0.1 mcg/kg/min, and vasopressin (0.04 units/min), were added for refractory shock, MAP < 50 mmHg. Dopamine was discontinued at the time of epinephrine initiation. Approximately 24 h from time of presentation, he was started on high-dose insulin, quickly titrated to maximum dose of 10 u/kg/h. Oliguric renal failure, lactic acidosis (lactate 4.5 mmol/L), and pulmonary edema developed through this period in addition to increasingly altered mental status and lethargy, necessitating intubation and mechanical ventilation approximately 30 h after presentation.
Due to continued poor clinical status following intubation, MAP 50–55 mmHg, and heart rate 70–80 bpm with minimal urine output < 25 mL/h, a bolus of 20 % intravenous fat emulsion (1.5 mL/kg) was given as rescue therapy for cardiotoxic poisoning; however, minimal change in blood pressure or heart rate was noted. The patient was also placed on continuous veno-venous hemodialysis (CVVHD) shortly after intralipid therapy for management of volume overload and worsening kidney function. Norepinephrine (titrated to 0.1 mcg/kg/min) was added to the existing resuscitation infusions to maintain MAP 60 mmHg. Due to refractory shock (unable to maintain MAP > 60 mmHg) continuing 40 h after presentation, an echocardiogram was obtained and a PAC was inserted.
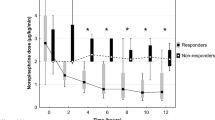
Methylene blue was then administered (2 mg/kg IV over 30 min) due to echocardiogram and PAC data consistent with vasodilatory shock: ejection fraction 60–65 %, cardiac output (CO) 10.5 L/min, mean pulmonary artery pressure (MPAP) 33 mmHg, pulmonary capillary wedge pressure (PCWP) 24 mmHg, cardiac index 5.2 L/min/m2, and systemic vascular resistance (SVR) 240 dyn · s/cm5. These measurements were obtained 30 min prior to methylene blue administration. Within 1 h of the methylene blue loading dose, the SVR improved to 1204 dyn · s/cm5, PCWP decreased to 18 mmHg, CO decreased to 6.9 L/min, cardiac index documented as 3.3 L/min/m2, and MPAP remained 33 mmHg. Vasopressor requirements decreased within 2 h following methylene blue loading dose and initiation of infusion (0.75 mg/kg/h)—phenylephrine was discontinued and epinephrine infusion was decreased by 50 %. Methylene blue infusion was discontinued following this as MAP was maintained at 65 mmHg (Fig. 1). There were no adverse effects noted—specifically no methemoglobinemia (1.2 % laboratory blood cooximetry, measured 2 h following administration of methylene blue), hemolysis, or signs of serotonin syndrome were observed during the hospital course.
Summary of vasopressor infusion and hemodynamic data throughout resuscitation, including during methylene blue infusion. Within 1 h of methylene blue administration (commenced 47 h after arrival), systemic vascular resistance improved 240 dyn · s/cm5 increased to 1204 dyn · s/cm5), and vasopressor requirements decreased with maintenance of mean arterial pressure 60 mmHg
The patient continued to improve hemodynamically over the next 72 h and was taken off CVVHD, high-dose insulin, and vasopressors gradually. Vasopressin was the last infusion to be discontinued. He was later discharged from the hospital without significant sequelae.
Discussion
Calcium channel blocker poisoning continues to have morbidity and mortality despite advances in critical care [13]. Co-ingestion of other cardiovascular drugs, such as beta blockers and ARB (such as valsartan), are additive to the effects on blood pressure and heart rate. ARB prevent vasoconstriction and attenuate sympathetic activity. Calcium channel blockers also block the responses of vascular smooth muscle to angiotensin II [14], and prolonged severe hypotension has been reported following combined amlodipine and valsartan ingestion [3].
Methylene blue is a novel adjunct for drug-induced vasodilatory shock. Its pharmacologic mechanism of effect and previous descriptions in sepsis literature [15–17] support its use in vasodilatory shock from calcium channel blocker poisoning. Septic shock is associated with excess nitric oxide production which in turn stimulates soluble guanylyl cyclase (cGMP) to increase endothelial smooth muscle relaxation [15]. Methylene blue inhibits guanylyl cyclase, resulting in less production of cGMP and subsequently decreased endothelial smooth muscle relaxation [17]. Though this mechanism is potentially beneficial in the treatment of any poison causing vasodilatory shock, it is particularly appealing for the treatment of severe amlodipine poisoning. Multiple animal studies have found amlodipine is associated with an increased concentration of nitric oxide following its administration [18, 19]. Thus, methylene blue may be beneficial in cases of amlodipine-induced shock refractory to standard vasopressor therapy.
Existing case reports for use of methylene blue in vasodilatory drug-induced shock describe improvements in clinical parameters of blood pressure, pulse rate, and acidemia shortly after (∼1 h) administration of methylene blue for shock recalcitrant to other interventions including high-dose insulin, vasopressors, calcium, and transvenous pacing [8–10, 12]. Invasive hemodynamic data is well documented in cases of refractory vasodilatory shock treated with methylene blue in sepsis and coronary artery bypass surgery [4, 5, 7]. However, these are not well documented in previous cases of refractory vasodilatory shock treated with methylene blue. The case presented here is unique in that objective improvements in hemodynamics measured via pulmonary artery catheter data are documented during methylene blue administration. Methylene blue administration was associated with a dramatic increase in SVR and subsequent reduction in catecholamine requirements. The observed changes in hemodynamics are similar to those documented in studies on the use of methylene blue in sepsis [17].
No apparent adverse effects were noted in this case nor have they been reported in previous cases in which methylene blue was used for drug-induced vasodilatory shock [8, 10]. Risks from use of methylene blue are mainly described in case reports in which methylene blue has been used for other indications. When used for methemoglobinemia, methylene blue is a reducing agent; however, it also may act as a potent oxidizing agent. Case reports of paradoxical methemoglobinemia caused by methylene blue exist; however, the majority of these reports are in infants who received high doses of methylene blue in a short period of time [20–22]. A review of 11 human clinical trials that used methylene blue for the treatment of septic shock did not reveal any cases of clinically significant methemoglobinemia when using methylene blue in doses comparable to those used in this case [23]. When administering methylene blue, providers should be aware of case reports of hemolysis when methylene blue has been administered to patients with G6PD deficiency [24, 25]. Serotonin syndrome is also a potential risk when administering methylene blue in the presence of other serotonergic drugs, such as serotonin reuptake inhibitors, due to inhibition of monoamine oxidase A [26, 27].
Limitations in the clinical applicability of this case include the presence of co-ingestants and the inability to discern whether the patient would have improved without the use of methylene blue. Drug concentrations were not available to ascertain contribution to toxicity. Other mechanisms that could have accounted for the change in systemic vascular resistance include effect of other medications being administered at the time (vasopressors or high-dose insulin), hemodialysis, drug elimination, or catheter-related measurement errors. However, the temporal relationship to the improvements in hemodynamic parameters and clinical measures of perfusion strongly suggest methylene blue was of clinical benefit.
Methylene blue may be considered in cases of drug-induced vasodilatory shock refractory to standard therapies. However, further studies on the specific agents where this therapy may be effective and indications for its use are required.
References
Schoffstall JM, Spivey WH, Gambone LM, Shaw RP, Sit SP. Effects of calcium channel blocker overdose-induced toxicity in the conscious dog. Ann Emerg Med. 1991;20:1104–8.
Prasa D, Hoffmann-Walbeck P, Barth S, Stedtler U, Ceschi A, Färber E, et al. Angiotensin II antagonists—an assessment of their acute toxicity. Clin Toxicol Phila Pa. 2013;51:429–34.
Smith SW, Ferguson KL, Hoffman RS, Nelson LS, Greller HA. Prolonged severe hypotension following combined amlodipine and valsartan ingestion. Clin Toxicol. 2008;46:470–4.
Yiu P, Robin J, Pattison CW. Reversal of refractory hypotension with single-dose methylene blue after coronary artery bypass surgery. J Thorac Cardiovasc Surg. 1999;118:195–6.
Preiser J-C, Lejeune P, Roman A, Carlier E, De Backer D, Leeman M, et al. Methylene blue administration in septic shock: a clinical trial. Crit Care Med. 1995;23:259–64.
Cheng X, Pang CC. Pressor and vasoconstrictor effects of methylene blue in endotoxaemic rats. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:648–53.
Daemen-Gubbels CR, Groeneveld PH, Groeneveld AB, van Kamp GJ, Bronsveld W, Thijs LG. Methylene blue increases myocardial function in septic shock. Crit Care Med. 1995;23:1363–70.
Jang DH, Nelson LS, Hoffman RS. Methylene blue in the treatment of refractory shock from an amlodipine overdose. Ann Emerg Med. 2011;58:565–7.
Fisher J, Taori G, Braitberg G, Graudins A. Methylene blue used in the treatment of refractory shock resulting from drug poisoning. Clin Toxicol Phila Pa. 2014;52:63–5.
Aggarwal N, Kupfer Y, Seneviratne C, Tessler S. Methylene blue reverses recalcitrant shock in β-blocker and calcium channel blocker overdose. BMJ Case Rep. 2013;2013. doi: 10.1136/bcr-2012-007402.
Jang DH, Donovan S, Nelson LS, Bania TC, Hoffman RS, Chu J. Efficacy of methylene blue in an experimental model of calcium channel blocker-induced shock. Ann Emerg Med. 2015;65:410–5.
Nabbi R, Riess ML, Woehlck HJ. Angiotensin-receptor-blocker-induced refractory hypotension responds to methylene blue. Acta Anaesthesiol Scand. 2012;56:933–4.
Mowry JB, Spyker DA, Cantilena LR, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol Phila Pa. 2014;52:1032–283.
Andrawis NS, Craft N, Abernethy DR. Calcium antagonists block angiotensin II-mediated vasoconstriction in humans: comparison with their effect on phenylephrine-induced vasoconstriction. J Pharmacol Exp Ther. 1992;261:879–84.
Andresen M, Dougnac A, Díaz O, Hernández G, Castillo L, Bugedo G, et al. Use of methylene blue in patients with refractory septic shock: impact on hemodynamics and gas exchange. J Crit Care. 1998;13:164–8.
Kwok ESH, Howes D. Use of methylene blue in sepsis: a systematic review. J Intensive Care Med. 2006;21:359–63.
Pasin L, Umbrello M, Greco T, Zambon M, Pappalardo F, Crivellari M, et al. Methylene blue as a vasopressor: a meta-analysis of randomised trials. Crit. Care Resusc. J. Australas. Acad Crit Care Med. 2013;15:42–8.
Lenasi H, Kohlstedt K, Fichtlscherer B, Mülsch A, Busse R, Fleming I. Amlodipine activates the endothelial nitric oxide synthase by altering phosphorylation on Ser1177 and Thr495. Cardiovasc Res. 2003;59:844–53.
Zhang X, Hintze TH. Amlodipine releases nitric oxide from canine coronary microvessels an unexpected mechanism of action of a calcium channel-blocking agent. Circulation. 1998;97:576–80.
Allegaert K, Miserez M, Lerut T, Naulaers G, Vanhole C, Devlieger H. Methemoglobinemia and hemolysis after enteral administration of methylene blue in a preterm infant: relevance for pediatric surgeons. J Pediatr Surg. 2004;39:E35–7.
Albert M, Lessin MS, Gilchrist BF. Methylene blue: dangerous dye for neonates. J Pediatr Surg. 2003;38:1244–5.
Blass N, Fung D. Dyed but not dead—methylene blue overdose. Anesthesiology. 1976;45:458–9.
Paciullo CA, McMahon Horner D, Hatton KW, Flynn JD. Methylene blue for the treatment of septic shock. Pharmacotherapy. 2010;30:702–15.
Foltz LM, Dalal BI, Wadsworth LD, Broady R, Chi K, Eisenhauer E, et al. Recognition and management of methemoglobinemia and hemolysis in a G6PD-deficient patient on experimental anticancer drug Triapine. Am J Hematol. 2006;81:210–1.
Brewer GJ. Rediscovery of the susceptibility of G6PD deficient persons to methemoglobinemia from oxidant drugs, and to hemolysis from methylene blue. Am J Hematol. 2007;82:87–8.
Gillman PK. Methylene blue implicated in potentially fatal serotonin toxicity. Anaesthesia. 2006;61:1013–4.
Gillman PK. Methylene blue and serotonin toxicity: definite causal link. Psychosomatics. 2010;51:448–9.
Conflict of Interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Laes, J.R., Williams, D.M. & Cole, J.B. Improvement in Hemodynamics After Methylene Blue Administration in Drug-Induced Vasodilatory Shock: A Case Report. J. Med. Toxicol. 11, 460–463 (2015). https://doi.org/10.1007/s13181-015-0500-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-015-0500-1