Abstract
Clinicians have limited experience with assessment and treatment of overdose from newer anticonvulsant medications. The aim of this investigation was to evaluate clinical effects of newer anticonvulsant overdose, determine if a relationship exists between dose and clinical effect, and if a particular agent appears more toxic in overdose. This was a retrospective study using electronic poison center data, evaluating clinical outcomes from newer anticonvulsant overdose. The Toxicall™ database from January 1, 2002 to December 31, 2011 was queried using key words: “gabapentin,” “lamotrigine,” “levetiracetam,” “tiagabine,” “topiramate,” “zonisamide,” “pregabalin,” and “oxcarbazine.” Polypharmacy overdose and children less than 15 years of age were excluded. Charts were reviewed by two abstractors for pharmaceutical, self-reported dose, clinical effect score, and clinical signs, symptoms, and vital signs recorded in the chart. Ordinal logistic regression was used to evaluate the relationship between drug type, dose, age, and sex to clinical effect score. Out of 501 cases identified, 347 met the final inclusion criteria. There were 116 gabapentin, 67 lamotrigine, 15 levetiracetam, 15 tiagabine, 56 topiramate, 23 pregabalin, and 55 oxcarbazepine cases. Overdose of newer anticonvulsants frequently results in altered mental status. Seizures may be more common with tiagabine, lamotrigine, and oxcarbazepine. There was one death reported from intentional overdose of topiramate. An information index was created to rank drug toxicity based on reported signs and symptoms for each overdose. There was no significant effect of dose on severity of outcome (β = 0.12, p = 0.23). However, the risk of a more severe outcome score was significantly increased with tiagabine relative to other drugs (β = 2.8, p = 0.001). Lamotrigine ranked highest in terms of toxicity (HT = 1.66) and number of interventions performed (HI = 1.17), and levetiracetam the lowest (HT = 0.98; HI = 0.88). We could not identify a dose-effect in these data which likely reflects the limitations of self-reported doses. Despite limitations of these data, the risk of more severe outcome scores appear to be higher with tiagabine overdose while lamotrigine overdose appears to result in more reported signs, symptoms, and interventions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Clinicians have limited experience with assessment and treatment of overdose from newer anticonvulsant medications. Unlike the traditional anticonvulsants (phenytoin, carbamazepine, valproic acid, and phenobarbital), serum drug levels are not readily available for newer agents. In 2011, there were over 31,000 cases of “other” anticonvulsant drug exposures reported to regional poison centers with 6,481 evaluated in healthcare facilities. Unfortunately these data do not specifically delineate all newer anticonvulsants [10]. While case reports provide some correlation with clinical effects in overdose, publication bias may favor severe clinical outcomes and skew perceptions of toxicity. Such a bias has the potential to overestimate the anticipated effects following overdose.
Despite the potential for bias, to date, case reports provide us the most information about clinical effects in overdose of the newer antiepileptics. Lamotrigine has been reported to cause seizures and electrocardiographic conduction delay [7, 9, 11, 13, 15, 24, 26–28]. Levetiracetam has been reported to cause respiratory depression with decreased muscle tone and diminished deep tendon reflexes in an adult overdose [4]. There are reports of levetiracetam causing pruritus and decreased muscle tone in pediatric overdoses [3]. Gabapentin toxicity has been reported in hemodialysis patients. In these cases, gabapentin was noted to cause tremors, altered mental status, and respiratory depression requiring intubation [17, 29]. Tiagabine has been noted to cause convulsive and non-convulsive status epilepticus in patients with and without a prior seizure history [4, 16, 24, 25]. Topiramate has been reported to cause ataxia and hallucinations in pediatric overdose [20], as well as altered mental status, somnolence, vertigo, agitation, coma, metabolic acidosis, and seizures in adults with no prior seizure history [2, 8, 12, 32]. There are several reviews of poison center data of newer anticonvulsant overdose, specifically topiramate, lamotrigine, and tiagabine, that reveal a wide variety of potentially serious, life-threatening effects such as coma, seizures, and respiratory depression [21, 22]. One poison center review of gabapentin adverse effects described drowsiness, dizziness, tachycardia, ataxia, and hypotension; however, all cases were managed in an outpatient setting [18]. These studies provide some understanding of individual anticonvulsants in overdose yet no direct comparison of newer anticonvulsants has been made.
The aim of this investigation was to evaluate the clinical effects of newer anticonvulsant overdose, determine if there is a relationship between dose and clinical effect, and determine if there is a particular agent(s) which appears more toxic in overdose.
Methods
Study Design
This was a retrospective cross-sectional chart review using electronic poison center data, evaluating clinical outcomes from newer anticonvulsant overdose. Institutional Review Board approval was obtained.
Setting and Population
The Virginia Poison Center provides toxicology consultation in the central Virginia region with an annual call volume of approximately 27,000. The poison center electronic database Toxicall™ was queried from January 1, 2002 to December 31, 2011 for hospitalized patients using the key words: “gabapentin,” “lamotrigine,” “levetiracetam,” “tiagabine,” “topiramate,” “zonisamide,” “pregabalin,” and “oxcarbazepine.” Chart acquisition was performed by specialists in poison information who are unaware of the study design. Poison center charts were abstracted onto a database with no patient identifiers.
Study Protocol
Charts were reviewed and abstracted onto a spreadsheet by two trained abstractors who were blinded to the purpose of the study. Inclusion criteria included all cases of anticonvulsant overdose referred to a healthcare facility. Patients were excluded if they were not treated in a healthcare facility. Cases involving children less than 15 years old were excluded to mitigate inaccuracies converting dose to multiples of maximum daily dose. Poly-substance ingestions were excluded after chart abstraction due to concerns about confounding the clinical outcomes. Clinical data were abstracted to a Microsoft Excel® database (Microsoft Corporation, Redmond, WA) and included the following: age, gender, estimated dose, time of ingestion, time to presentation, and past medical history. Ingested dose, when available, was converted to multiples of maximum daily dose by dividing estimated dose by the maximum daily dose to facilitate comparison between pharmaceuticals. Maximum daily doses used included: gabapentin 3,600 mg, lamotrigine 400 mg, levetiracetam 3,000 mg, tiagabine 56 mg, topiramate 400 mg, and zonisamide 600 mg. The effect severity score mirrors the categories used by the American Association of Poison Control Centers to document outcomes. Briefly, the effect score increases with severity of the outcome (0 = no effect, 1 = minor effect, 2 = moderate effect, 3 = major effect, 4 = death). Examples of the severity score are available online (http://www.aapcc.org/data-system/). All clinical effects, laboratory abnormalities, and medical interventions available were recorded. The abstractors received training on identification of relevant data to include in the analysis. This included a key, which listed descriptions of elements to be imported in each cell, example cases, and standard units of measurement, and abbreviations to be used. A 5 % sample of charts was randomly assigned for both abstractors for the purpose of determining inter-rater reliability.
Measures
The primary endpoint was the determination of the poison center composite severity score. Clinical outcomes were originally scored by raters in three categories: neurological effects (1 = seizures, 2 = altered mental status (AMS), 3 = coma, 4 = delirium/hallucinations/agitation); cardiovascular effects (1 = hypotension, 2 = hypertension); and miscellaneous (1 = hyperthermia, 2 = rhabdomyolysis, 3 = other, listed specifically in a separate column). To incorporate information in the latter category, to eliminate as many coding discrepancies between raters as possible, and for ease of analysis, patient data were re-coded as presence/absence (1/0) in the following categories: seizures; CNS effects (including headache, AMS, confusion, agitation, delirium, lethargy, or coma); neurological/pupillary response (mydriasis, nystagmus); neuromuscular (tremors, fasciculations, ataxia, asterixis, dystonia, myoclonus); GI (emesis, diarrhea, nausea, vomiting); dermatological; cardiac dysrhythmia (tachycardia, bradycardia); blood pressure effects (hypotension, hypertension); metabolic effects (hyperthermia, rhabdomyolysis, electrolyte imbalances, acidosis).
Data Analysis
Descriptive statistics (means, standard deviations, median, interquartile range (IQR), proportions) were used to summarize demographic data, clinical outcomes, and clinical effect scores. To evaluate the association between outcome severity and categorical predictor variables (drug type, age, sex), data were modeled by cumulative logit ordinal regression on a 4-level ordinal scale response (0 = no effect, 1 = minor effect, 2 = moderate effect, 3 = severe effect). As only one death was recorded, this category was eliminated from analysis. The ordinal logistic regression models the relationship between the cumulative logits of Y and the predictor variables X j as logit Y ij = α i + b j X j , where α i is the intercept for i = 1, 2, 3, and quantifies the “shift” of the regression of each outcome severity category compared to the “baseline” response of no effect, and the coefficient b j quantifies the strength of the relationship between the outcome severity response and each predictor variable. The odds ratio was computed as exp(b j ). To determine an approximate dose–response relationship for each drug, cumulative logit ordinal regression was performed on log-transformed estimated doses. Data were analyzed in SAS v. 9.3 [1].
Ranking of drug toxicity was based on the presence of clinical signs and symptoms, and quantified by the Shannon information index H′ calculated for each drug. Clinical outcome signs and symptoms in nine categories (seizures, altered mental status/CNS changes, pupillary response, neuromuscular, GI, dermatological, cardiac, hypotension, metabolic) were scored for presence or absence as 1 or 0, respectively. The composite outcome score was calculated as the proportional occurrence of signs and symptoms P i summed across each clinical category occurring for each ith patient, and was estimated as P i = n i /N, where n i is the total count of signs and symptoms present for each patient across all categories and N is the total number of individuals. The information index was then calculated as H′ = −ΣP i ⋅ln(P i ). The standard deviation of H′ is calculated as the square root of the variance, where Var (H′) ≈ [Σn i ⋅ln(n i )2 − [Σn i ⋅ln(n i )]2/N]/N 2 + (S − 1)/2 N 2 [23]. Drug rankings were assessed for equality of variances by Levene’s test, then compared by ANOVA on the summary statistics [19]; differences between rankings were assessed with Dunn method. These analyses were performed in JMP Pro 9.0.2 (SAS Inc., Cary, NC).
We determined inter-rater reliability on the original clinical scoring categories by randomly selecting 5 % of charts (n = 40) for re-abstraction by both abstractors. The kappa statistic κ was used to calculate inter-rater reliability for the paired rater data for gender, presence/absence of clinical signs and symptoms, and common interventions. Analyses were performed in SAS ver. 9.3, PROC FREQ (SAS Inc., Cary, NC).
Results
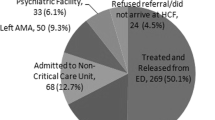
Out of 501 hospital-based cases identified, 104 pediatric and 50 co-ingestion cases were excluded, leaving 347 cases which met the final inclusion criteria. Patient summary data are presented in Table 1. There were 116 gabapentin, 67 lamotrigine, 15 levetiracetam, 15 tiagabine, 56 topiramate, 23 pregabalin, and 55 oxcarbazepine cases. There was one death reported from intentional overdose of topiramate. Patients were predominantly young (median age 30 years; IQR 20, 44) and female (69.5 %). Estimated dose in milligrams and standardized units (dose/maximum daily dose) is listed in Table 2.
Outcome Severity
The majority of patients (73 %) had no or minimal clinical outcome severity effects (Table 1). There was no statistically significant effect of drug type (OR 1.065; 95 % Wald CI 0.987, 1.150; p = 0.104), age (OR 1.012, 95 % Wald CI 0.998, 1.026; p = 0.084), or sex (OR 0.851; 95 % Wald CI 0.557, 1.298; p = 0.453) on outcome severity score.
Dose Response
With the lowest outcome severity, only lamotrigine had a statistically significant relationship between dose and outcome severity (Table 3).
Toxicity Rankings
There were statistically significant differences in toxicity rankings between drugs (Levene’s test F = 3.635, p = 0.0016; Welch ANOVA F = 96.076, p < 0.0001). Lamotrigine, followed by topiramate, ranked highest, and levetiracetam ranked lowest. However, the low ranking of levetiracetam could be partially attributed to the small sample size (n = 12) and resulting lack of information about signs and symptoms in most clinical categories evaluated (Table 4). For all drugs evaluated, CNS signs and symptoms were most prevalent (40 % of all cases). Dermatological signs and symptoms were least commonly recorded (1 %).
Inter-Rater Reliability
The reliability coefficients κ with corresponding 95 % CIs and ratings distributions for both observers are shown in Table 5. The κ values for clinical outcome judgments ranged from 0 to 1. Although differences between raters were minor for categories with relatively “hard” clinical outcomes (such as cardiac, blood pressure, and tremors), the neurological categories in particular were subject to the greatest scoring discrepancies. Capture of lethargy, ataxia, dizziness, and altered mental status were most likely to differ between raters, and showed the poorest agreement (Table 5).
Discussion
The newer antiepileptic agents in overdose are generally well tolerated with only one fatality reported to this poison center database. Levetiracetam appears to have the lowest toxicity and number of interventions performed. The predominant finding of AMS (60 %) is consistent with other series of levetiracetam overdoses [6].
This study revealed tiagabine, a selective GABA reuptake inhibitor, to have the highest severity outcome score. This result could be related to seizures found in 20 % and altered mental status in 67 % of overdose patients, the highest proportion of any of the AEDs evaluated in this study (Table 2). In previous reports, tiagabine has been found to induce non-convulsive status epilepticus [5, 30].
Lamotrigine, a glutamate inhibitor, sodium channel blocker, and serotonin reuptake inhibitor, was the most toxic and required the greatest number of interventions performed during overdose. Reported clinical effects include ataxia, nystagmus, decreased level of consciousness, seizures, and intraventricular conduction delay in overdose [30]. One review of Poison Center data of 493 patients with single substance lamotrigine exposures reported about half of patients experiencing toxic side effects, the most prominent being lethargy (20.9 %), vomiting (11 %), nausea (5.1 %), ataxia (4.9 %), and dizziness/vertigo (4.5 %), and tachycardia (4.3 %). Medical outcome was reported as minor in 150 (30.4 %), moderate in 73 (14.8 %), and major in 13 (2.6 %) cases [14, 21]. Similar to our study, altered mental status was the most common finding (45 %), and nearly a third of patients experienced gastrointestinal symptoms [21].
Limitations
This was a retrospective poison center chart review that has inherent limitations in the quality of data. Information contained in poison center charts is generated passively through voluntary discussions with healthcare providers by poison center specialists, sometimes by providers peripherally involved in patient care. This process undoubtedly results in omission of complete vital signs, physical exam findings, signs, symptoms, and laboratory data. Exposure or self-reported overdose does not necessarily represent an actual overdose and can bias the data suggesting less severe outcomes. Dosing information was self-reported and subject to significant inaccuracies, and rarely confirmed with plasma concentrations. For example, large variations in dosages for each severity category in this study precluded finding statistically significant dose-effect relationships for drugs other than lamotrigine. Imputing incomplete data into the information index used in our study can result in significantly limited information and should only be hypothesis generating. Repeating the information index with prospectively collected data by a bedside toxicology service, such as the Toxicology Investigator Consortium [31], and confirmatory drug concentrations, would provide more clinically robust data.
The information index H′ is sensitive to the quality of the presence/absence data for clinical signs and symptoms. To be reliable, the signs and symptoms lists must be comprehensive, and all clinical categories must be surveyed and checked off for each patient. However, patient surveys may be inadequate because all signs and symptoms are not equally “detectable.” For example, infrequently observed signs and symptoms are easily missed by less-experienced providers, or may be overlooked if they have a lower priority for patient treatment. H′ is strongly affected by sample size, and the effects of sampling variability on the calculation of H′ are not completely known [23].
Since our inter-rater reliability score was lowest for neurological findings, the organ system most commonly affected by antiepileptic medications in overdose, a future study using standardized bedside toxicologist assessment [31] would be helpful.
Conclusion
Overdose of newer anticonvulsants frequently results in altered mental status. Seizures may be more common with tiagabine, lamotrigine, and oxcarbazepine. We could not identify a dose-effect in these data which likely reflects the limitations of self-reported doses. Risk of more severe outcome was higher with tiagabine overdose while lamotrigine overdose appears to result in more reported signs, symptoms, and interventions.
References
Allison, P. D., & Safari books online. (2012). Logistic regression using SAS (2nd ed.). Cary, NC: SAS Pub
Anand JS, Chodorowski Z, Wisniewski M (2007) Seizures induced by topiramate overdose. Clin Toxicol (Phila) 45(2):197
Awaad Y (2007) Accidental overdosage of levetiracetam in two children caused no side effects. Epilepsy Behav 11(2):247
Barrueto F Jr, Williams K, Howland MA, Hoffman RS, Nelson LS (2002) A case of levetiracetam (keppra) poisoning with clinical and toxicokinetic data. J Toxicol Clin Toxicol 40(7):881–884
Bauer J, Cooper-Mahkorn D (2008) Tiagabine: efficacy and safety in partial seizures—current status. Neuropsychiatr Dis Treat 4(4):731–736
Bodmer M, Monte AA, Kokko J, Yin S (2011) Safety of non-therapeutic levetiracetam ingestions—a poison center based study. Pharmacoepidemiol Drug Saf 20(4):366–369. doi:10.1002/pds.2113
Braga AJ, Chidley K (2007) Self-poisoning with lamotrigine and pregabalin. Anaesthesia 62(5):524–527
Brar B, Glazer JP, Franco K, Edwards T (2005) Acute mental status changes with topiramate. J Am Acad Child Adolesc Psychiatry 44(8):725
Briassoulis G, Kalabalikis P, Tamiolaki M, Hatzis T (1998) Lamotrigine childhood overdose. Pediatr Neurol 19(3):239–242
Bronstein AC, Spyker DA, Cantilena LR Jr, Rumack BH, Dart RC (2012) 2011 annual report of the american association of poison control centers' national poison data system (NPDS): 29th annual report. Clin Toxicol (Phila, Pa) 50(10):911–1164. doi:10.3109/15563650.2012.746424
Buckley NA, Whyte IM, Dawson AH (1993) Self-poisoning with lamotrigine. Lancet 342(8886–8887):1552–1553
Chung AM, Reed MD (2004) Intentional topiramate ingestion in an adolescent female. Ann Pharmacother 38(9):1439–1442
Dinnerstein E, Jobst BC, Williamson PD (2007) Lamotrigine intoxication provoking status epilepticus in an adult with localization-related epilepsy. Arch Neurol 64(9):1344–1346
Fleurat M, Smollin C (2012) Case files of the University of California San Francisco medical toxicology fellowship: lamotrigine toxicity. J Med Toxicol: Off J Am Coll Med Toxicol 8(1):52–58. doi:10.1007/s13181-012-0210-x
Herold TJ (2006) Lamotrigine as a possible cause of QRS prolongation in a patient with known seizure disorder. Cjem 8(5):361–364
Jette N, Cappell J, VanPassel L, Akman CI (2006) Tiagabine-induced nonconvulsive status epilepticus in an adolescent without epilepsy. Neurology 67(8):1514–1515
Jones H, Aguila E, Farber HW (2002) Gabapentin toxicity requiring intubation in a patient receiving long-term hemodialysis. Ann Intern Med 137(1):74
Klein-Schwartz W, Shepherd JG, Gorman S, Dahl B (2003) Characterization of gabapentin overdose using a poison center case series. J Toxicol Clin Toxicol 41(1):11–15
Larson DA (1992) Analysis of variance with just summary statistics as input. Am Stat 46(2):151–152. doi:10.1080/00031305.1992.10475872
Lin G, Lawrence R (2006) Pediatric case report of topiramate toxicity. Clin Toxicol (Phila) 44(1):67–69
Lofton AL, Klein-Schwartz W (2004) Evaluation of lamotrigine toxicity reported to poison centers. Ann Pharmacother 38(11):1811–1815
Lofton AL, Klein-Schwartz W (2005) Evaluation of toxicity of topiramate exposures reported to poison centers. Hum Exp Toxicol 24(11):591–595
Magurran AE (1988) Ecological diversity and its measurement. Princeton University Press; Croom Helm, Princeton
O'Donnell J, Bateman DN (2000) Lamotrigine overdose in an adult. J Toxicol Clin Toxicol 38(6):659–660
Ostrovskiy D, Spanaki MV, Morris GL 3rd (2002) Tiagabine overdose can induce convulsive status epilepticus. Epilepsia 43(7):773–774
Reimers A, Reinholt G (2007) Acute lamotrigine overdose in an adolescent. Ther Drug Monit 29(5):669–670
Schwartz MD, Geller RJ (2007) Seizures and altered mental status after lamotrigine overdose. Ther Drug Monit 29(6):843–844
Thundiyil JG, Kearney TE, Olson KR (2007) Evolving epidemiology of drug-induced seizures reported to a poison control center system. J Med Toxicol 3(1):15–19
Verma A, St Clair EW, Radtke RA (1999) A case of sustained massive gabapentin overdose without serious side effects. Ther Drug Monit 21(6):615–617
Wade JF, Dang CV, Nelson L, Wasserberger J (2010) Emergent complications of the newer anticonvulsants. J Emerg Med 38(2):231–237. doi:10.1016/j.jemermed.2008.03.032
Wax PM, Kleinschmidt KC, Brent J, ACMT ToxIC case registry investigators (2011) The toxicology investigators consortium (ToxIC) registry. J Med Toxicol: Off J Am Coll Med Toxicol 7(4):259–265. doi:10.1007/s13181-011-0177-z
Wisniewski M, Lukasik-Glebocka M, Anand JS (2009) Acute topiramate overdose—clinical manifestations. Clin Toxicol (Phila) 47(4):317–320
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wills, B., Reynolds, P., Chu, E. et al. Clinical Outcomes in Newer Anticonvulsant Overdose: A Poison Center Observational Study. J. Med. Toxicol. 10, 254–260 (2014). https://doi.org/10.1007/s13181-014-0384-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13181-014-0384-5