Abstract
The objective of this study was to assess the farming system of organic lowland paddy to water pollution compared with conventional farming system in Deli Serdang Regency. An environmental assessment in this research is limited to the water pollution caused by pesticides and chemical fertilizers. Water samples were analyzed, and the levels of nitrate and pesticides were taken of wetland and community wells. At the wetland, water samples were taken at the drains into the plot of rice field and the channel water out of the mapped fields. Water sampling was done every season and is upon subsequent fertilization. Each water composite was collected from ten sampling points which each consist of five sampling points of organic paddy farming system and five sampling points of conventional paddy farming system. The results showed that the level of pesticides in the drains coming out of paddy fields was higher than the levels of pesticides in the inlet and water in the community wells. The level of pesticides in organic cultivation was lower than conventional cultivation methods. In the dry season, the highest levels of nitrate obtained in the outflow water are significantly different, with inlet and well water, likewise the case with the rainy season. There was a tendency for increasing levels of nitrate in water, out water, and well water in the dry season compared to the rainy season.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
“Environmental pollution” is the entry or inclusion of living beings, substances, energy, or other components into the environment by human activities which causes its quality to lower into a specific level so that the environment cannot function as it should be (1997 Indonesian Regulation; UU RI 1997). The polluting substances have been identified to cause bad impacts to the living organisms or environments. Polluting substance (pollutant) is a chemical substance in the state of liquid, solid, or gas that originated from the nature itself but was triggered by human activities, whether directly or indirectly (anthropogenic origin) (Maghanga et al. 2013).
Deterioration can be caused by natural misuse or human activities. Examples of deterioration that was caused by natural misuse are volcanic eruptions, floods, abrasions, and earthquakes, while deterioration that was caused by human activities can be in the form of mining or pollution of air, water, and soil as a result of non-environmentally friendly chemicals usage continuously and in an increasing dosage. There is an overwhelming evidence showing that these chemical usages have a potential risk to humans and other lives, along with unwanted side effects to the environment (Gliessman 2010; Zhengfei et al. 2004; Wihardjaka and dan Abdurachman 2007; Wihardjaka 2011). Pesticide residues have a very harmful effect on human health in the long term, including causes of cancer, genetic changers or mutations, birth defects, damages in the circulatory and nervous system, and also disorders of the endocrine, reproductive, and immune systems (Chandrasekar 2005; Mahmood et al. 2015). On the other hand, certain pest species that was controlled with pesticide has become resistant, resurgent, and changing the status of plant pests (Hardjowigeno 2003; Sarkar et al. 2003).
One of the human activities that can cause environmental pollution is conventional faming system. Conventional or modern farming system is a system of farming that utilizes various products resulted from modern technologies, such as improved varieties, fertilizers, chemical pesticides, and herbicides. Application of conventional farming system can result in negative impacts on the environment, socioeconomic condition, and public health (Gliessman 2010). A massive number of pollution come from farming, in the form of fertilizer and pesticide usage for plants. The main cause of mistakes in fertilizer usage is inaccurate dosage and incorrect application.
Usage of fertilizer that contains excessive amount of nitrogen to plants can cause negative effects, which include increasing levels of pest and diseases resulted from imbalance of nutrition, reducing the fertilizer’s efficiency, and polluting the environment. Nitrogen categories are NO3−, NO2−, NH4+ (exchangeable and mineral-fixed), N2 and N2O2, and the most important, NO3−, NO2−, NH4+ (exchangeable form). NO3− and NO2−in high concentration can also affect human’s health. The maximum standard of nitrogen-nitrate in drinking water is 10 mg/L and 45 mg/L if it was nitrate. The main cause of an excessive phosphor concentration in soil and soil solution is the usage of fertilizer and domestic waste disposal. Some of the phosphor substances are going to be absorbed by the soil, and some others are going to be in the soil solution’s water and ready to be used by the plants. Phosphor in groundwater can be in the form of orthophosphate, with the solubility order being H2PO4− > HPO42− > PO43. The existence of P in soil and groundwater has negative effects resulted from its nature of being a nutrient barrier. If the concentration is too much, P, which is released (desorption) from the soil or no longer absorbed by soil, is going to get out to the surface along with the basic flow and creates eutrophication on an open waters (Notodarmojo 2005). Small concentration of nitrogen and phosphate, especially phosphate, in open waters can bolster the continuity of water plants lives, like algae.
Pollution that occurs in soil is going to result in groundwater pollution, because groundwater is inseparable from the area in which the soil belongs. The function of groundwater as a medium for the dissolved substances’ mobilization (e.g., polluting substance) is very important. Reactions between polluting substance with common particles found in ground are the reaction of substances dissolved in soil solution with particles in the soil. Vice versa, polluted soil is going to release the polluting substances under the desorption mechanism or even dissolving into the groundwater itself, which will then move together with the groundwater. This dynamic condition always happens between substances or cations in liquid phase and into solid phase, which is the soil’s mass itself (solid matrix). This explains how the width of contact between soil particles with water is an important factor in reactions of dissolving substances and groundwater aside from the characteristics of the polluting substance itself. The wider the contact is, the more intensive the reactions will be. Environmental pollution resulted from pesticide residues may seriously affect society’s health. This may also happen due to the drift of water flow (river, groundwater, and ocean) and movement of wind/air in application of pesticide which also enter other ecosystem components that are not the target of control at the first place. Pesticide residues can also move into food chains, which will dangerously affect the living organisms that consume them.
Pesticide residues in agricultural products are getting serious attention from both national and international organizations, which eventually increases public awareness of the negative effects of pesticides. Meanwhile, the regulations regarding environmental safety are also increasingly stricter and involving more issues regarding the usage of chemicals and antibiotics for agricultural protection. This results in barriers to trade and a negative impact on the country’s economy (Mahmood et al. 2015; Tarumingkeng 1997; Sugiharto 2007).
Environmental pollution due to the usage of pesticides and fertilizers occurs because of the residues left on the physical and biotic environment (Sahmrukh et al. 2001; MN Khan et al. 2018). Contamination can occur due to hazardous materials that were carried and spread by the wind, through the flow of water, and through the body of organisms exposed to the agrochemical material. The pollution caused by the pesticides and chemical fertilizers’ usage that are applied in irrigated land is largely spread in water irrigation, river, and, at last, to the sea (Kole et al. 2001; Sahmrukh et al. 2001; MN Khan et al. 2018).
Pollution from pesticide residues is very harmful for the environment and human health; therefore control and restriction of the use of pesticides as well as pollution reduction is necessary, especially toward exported commodities. Global policy restrictions of the use of synthetic pesticides led to the popularization of clean technology. In this regards, the efforts are made to overcome the negative effects of pesticides and prevent continued pollution (Hardjowigeno 2003; Notodarmojo 2005).
Based on the above issues, we are in need of a system that is able to improve the productivity of agricultural land without destroying the environment in the process, also economically profitable and socially acceptable. Organic farming is defined as a crop farming system without the application of chemical fertilizers and pesticides but supports the delivery and the addition of organic matter to improve soil fertility and organic pesticides to maintain plant health (Sutanto 2002). Organic farming aims to generate sustainable crop production by improving soil fertility using natural resources such as recycled agricultural waste. According to IFOAM (Taylor and Michael 2008), it is defined as a holistic system of agricultural production and integrated by means of optimizing the health and productivity of agro-ecosystems naturally, resulting in sufficient food and fiber, continuity, and quality. Farming systems give different reactions in different climates and locations, so it is important to evaluate the effect of organic farming on sol fertility in a variety of climates and different study sites. Therefore, the main aim of this study is to evaluate the levels of pollution caused by paddy farming, whether it is organically or not in Deli Serdang Regency, Indonesia.
Methodology
Sampling and pretreatment
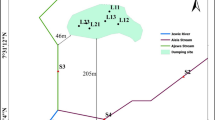
The method that was used with the object of study of this research was a survey, and the nature of the analysis was descriptive. This study’s analyzing unit was the water pollution level. Activities of this research were carried out in two seasons of year 2009–2010. The first one was at the rice cultivation in the dry season of 2009 (May–August 2009) and continued into the rainy season (September 2009–February 2010). The samples are based on a unit of land acquired from the review of the Deli Serdang map, which was divided into two districts: the Beringin and Pantai Labu District. Each district consists of five villages, in which each of them was actively distributing the organic farming activities for about 10 years. The five village areas were Karang Anyar Village and Sidodadi Village located in Beringin District; Binjai Bakung Village, Denai Lama Village, and Denai Kuala Village located in Pantai Labu District. Two paddy planting locations were selected from each village.
Water samples were taken from waterways entering organic and conventional paddy fields, drains coming out of organic and conventional paddy fields, and community wells. Samples were collected after fertilizer applications. Samples were collected in cleaned 500 ml brown borosilicate bottles, filled, and sealed with Teflon lined caps. The bottles were transferred to the laboratory in a big plastic cooler box and subsequently stored in a refrigerator at 4 °C immediately on reaching the laboratory. This helps to prevent biological degradation as well as the rates of possible physical and chemical reactions. Samples were filtered and analyzed within 48 h of refrigeration (D. Chapman 1992 (Chapman 1996; National Standardization Agency 2008)). From each sampling point, two samples were collected throughout the dry season and rainy season.
Determination of nitrates and pyrethroid pesticide residues (ά-cypermethrin)
Nitrate and pyrethroid pesticides were analyzed due to the survey results of local farmers, which showed that nitrate and pyrethroid pesticides were the most dominant type of pesticide used by them. At the wetlands, water samples were taken at the drains that went to the plot of the rice field and the water channel out of the mapped fields. Water sampling was done every season upon fertilization.
The analysis of nitrates was carried out using the advanced water quality laboratory series HACH-DR 2400 data logging spectrophotometer. This is a microprocessor-controlled LED-sourced filter photometer with in-built programs for measurements of various parameters in water including NO3–N at programmed wavelengths and reaction times. The method for nitrate analysis utilizes the quantitative reduction of nitrate in the sample to nitrite by cadmium and subsequent production of the amber-colored product. The production was done through the reaction of the nitrite with sulfuric acid and the coupling of the formed diazonium salt with gentisic acid that produces color with intensity proportional to the original NO3–N in the sample. Interference by nitrite was compensated by addition of bromine water followed by phenol (HACH Company, 1992). A 10-mL cell was filled with the sample, and bromine water added dropwise until the developed yellow color stabilized. This was followed by a drop of phenol solution to produce a colorless solution. A Nitra Ver 5 nitrate reagent powder pillow was added then shaken for 1 min and allowed to stand for 5 min to complete the reaction. A blank was made by filling a 10-mL cell, placed in the cell compartment, and used to zero the instrument. The prepared sample was then placed in the cell compartment and the concentration of the nitrate read in mg/L NO3–N (HACH Company, 1992).
In this study, pyrethroid (ά-sipermetrin) pesticide residues were analyzed using gas chromatography. The steps of sample preparation were extraction process, clean-up, and pesticide residue determination. Extraction was carried out with a homogenizer. The purpose of this extraction was to quantitatively separate the analyte from its carrier matrix (co-extractant). With the presence of this co-extractant, it could affect the extraction efficiency and clean-up. For the analysis of pesticide residues, Florisil was an adsorbent commonly used as a clean-up column. Florisil is a magnesium silicate compound, Mg2SiO3, which is produced from precipitation of sodium silicate with magnesium sulfate. In the clean-up process with this fluorine column, the co-extractants found in samples of agricultural commodities such as fats, phospholipids, pigments, waxes, and other impurities could be removed. In this study, pyrethroid (ά-sipermetrin) pesticide residues were analyzed using gas chromatography. The steps of sample preparation were extraction process, clean-up, and pesticide residue determination. Extraction was carried out with a homogenizer. The condition of the gas chromatography system in this study was as follows: injector temperature, 250 °C; splitless injection at 1 L volume with auto injector; gas flow rate N2, 22 mL/min; capillary column; Rtx-1 (30 m × 0.25 mm × 0.25 mm) with a crossbond stationary phase 100% dimethylpolisiloksan; column temperature, 230 °C; ECD; detector temperature, 250 °C. With a gas chromatography tool, chromatogram results would be obtained which would be used to confirm the identity of the analyzed compound. Retention time is the time needed for a solution to migrate (A.O.A.C. 2005; National Standardization Agency 2008; Hladik et al. 2009)1984; BSN 2008; Hladik. et al. 2009).
The collected data were statistically analyzed to answer the problem of organic farming’s influence on paddy plankton the environmental quality of the soil using ANOVA (Duncan 1955; Sudjana 1996).
Results and discussion
Pesticide contamination in water
Research showed that there was no interaction between pesticide on the water that came in, went out, or villagers’ well water and organic or conventional farming method. Then, planting season, water sample location, and farming methods also showed no interaction. Furthermore, no interaction was found even between two factors such as farming methods and planting season, farming methods and water sample location, and also planting season and water sample location.
The difference of pesticide levels were caused by the difference of water locations. The pesticide level rose when water flowed through plots of rice fields (Table 1). Pesticide levels in water that came out of the rice field are higher than the water that went into the rice field. Well water had also been polluted with pesticide, although the level was lower compared to water that got inside or outside the rice field. Consecutively, the biggest pyrethroid pesticide level was found in water that came out of the rice field, water that went into the rice field, and then water in villagers’ well.
As seen in Table 1, although there was no interaction happening, data showed that during dry season, the level of pesticide in water that went into the organic rice plots was higher compared to that in conventional ones. After going through the rice plots, there was a rise in the pesticide level. However, it is lower than the conventional one. During rainy season, in which the level of pesticide in water that went in was not that much of a difference, the rise of pesticide level with organic method, although small, but still lower compared to the conventional one. The transferring of contaminant happened through dissolving of water that went into the rice field, caused by complex combination of hydrogeology and other chemical factors that went with it.
According to the interview with farmers from conventional farming location, the high amount of pesticide residue was caused by the rising usage of pesticide in rainy season. This happened due to raindrops washing off the pesticide, so in order to be more effective, farmers sprayed more pesticide to the field. Aside from that, during rainy season, there would be more erosion happening in the ground, in which the water in irrigation flow would be washed off into the river so that insecticide contained within the soil would be polluting the river and living organisms that live in it.
Organic farming system can lower the water pollution level compared to conventional farming system, caused by the chemical-free methods. Pesticide level in well water during dry season was higher than the rainy season. During dry season, pesticide level in well water near the organic farm was lower than the well near the conventional rice field. During rainy season, there was no difference of pesticide level found in well water that was near the conventional rice field or organic rice field. This was caused by both of the organic and conventional rice fields belonging in the same overlay, which resulting in water that went into the organic rice field contaminated by pesticide from the conventional ones. Another cause would be unseparated irrigation in between organic and conventional rice field, resulting in pesticide residue found in water that went into the organic rice field.
Irrigation water in Pantai Labu District’s rice field originated from Pulo Naga water source, while Beringin District’s one came from Ramunia water source. Along the research, division of water happened periodically due to lack of debit found in those water sources. Average rainfall during the dry season of 2009 (May–August) was low, which was 178.75 mm. In contrary, during the rainy season of 2009–2010 (September 2009–February 2010), the average rainfall was high, which was 215.55 mm.
The use of pyrethroid pesticides tends to be constantly high regardless to the rules of pest control that can result in negative impacts, namely, pollution of soil which will reduce environmental quality and biodiversity. Therefore, it was highly hydrophobic, and soon there would be a mass transfer from the aqueous phase to suspension of soil or sediment particles. Pesticides that were examined in this study was α-cypermethrin.α-Cypermethrin is a nonpolar compound which can be absorbed on the surface of the ground and bound with soil particles (Harsanti 2008; Salgado 2013). α-Cypermethrin solubility in water is low, which is equal to 0.004 mg/L to the temperature of 25 °C. α-Cypermethrin is hydrophobic; therefore, it shows a low solubility in water. This caused the compounds to bind firmly on the ground so that the potential of these compounds to contaminate the ground water is low (Knisel 1993). Maximum residue limit of α-cypermethrin in drinking water had been regulated in the Minister of Health’s Regulation concerning the requirements for drinking water quality. The maximum level of pyrethroid concentration on water for drinking and farm irrigation was 0.3 mg/L (Republic of Indonesia's Ministry of Health 2010) (Regulation of the Minister of Health No. 492/Menkes/Per/IV/2010). According to the regulation and the calculation, it was found that pyrethroid pesticides’ concentration on surface level water and well water in the research location was still below the standard quality threshold.
Pollution of water by nitrate
Pollution of water by nitrate can be caused by farming activities such as overly usage of fertilizer and chemical pesticide. Nitrogen contained in fertilizer would undergo changes if bound in soil, e.g., in the form of ammonium (NH4+), nitrate (NO3), and/or nitrite (NO2). The mobile characteristic of nitrate caused it to easily cover by field’s reductive layer. Aside from that, some of it also evaporated into air (volatilization) and dissolved by abstersion or erosion.
Variety analysis result showed that there was interaction between three factors: farming methods, planting season, and water pickup location; not even between two factors of farming methods and planting season, nor between farming methods and water pickup location. The difference between nitrate levels was affected by planting season and location of where the water was taken from, whether it was in the flow of water coming into the rice field, flow of water out of the rice field, or villagers’ well. The level of nitrate in water was on the highest point in the water that came out of the rice field, compared to that in villagers’ well or flow that went into the rice field. Nitrate level was also affected by farming methods; organic farming method caused lower nitrate level compared to conventional one (Table 2). The increasing level was caused by season and farming methods. Highest point of increment was found in water that flowed through organic rice field in dry season and the lowest point in irrigation water that flowed through conventional rice field in rainy season. The increasing level of nitrate on water could be caused by polluted irrigation water that went it to water the field, then flowed from conventional rice field to organic rice field. The location of organic rice field that was side by side to conventional rice field and unseparated irrigation were the cause of nitrate contamination to water that came from scattered urea fertilizer, along with other chemical components. The increasing level of nitrate on water was affected by pesticide level on water and depended on the complex combinations of hydrology and chemical factors that attached with it.
A decrement of nitrate level on water happened during rainy season, whether it is to water that went in, water that came out, or to villagers’ well water. However, the biggest nitrate level was found on water that went out. Duncan double distance test toward nitrate level on water that went it, came out, and well water during dry or rainy season with organic or conventional farming method results can be seen in Table 3.
Variety test analysis showed that there was an interaction between planting season and water location toward nitrate level. Nitrate level difference was found in water that went in, came out, and well water in both of the planting seasons. During dry season, highest level of nitrate was found in water that went out, which has real difference with water that came in and well water, likewise on the rainy season. There was a tendency of nitrate level increment on water that went it, came out, and well water on dry season compared to the rainy season.
In organic rice field, pesticide and nitrate level on water that went into the organic rice plots were higher than conventional rice fields. After going through the rice field, pesticide level increment happened. However, the pesticide level increment in organic rice field was way lower compared to conventional one. During rainy season with little difference of pesticide in water that went in, increment of pesticide level in organic rice field was still lower compared to conventional one, albeit small in difference.
Research showed that some organic rice plots on the research location were located side by side with conventional rice plots and with no barrier in the form of pathway, trench, or tall plants around the organic field. This condition was indicated as one of the reasons of how pesticide and nitrate pollution can still be found in organic farm’s soil or water, which caused contamination of chemical component whether through irrigation or wind.
Based on the Indonesian Republic Government Regulation Number 82 Year 2001 about Water Quality Management and Water Pollution Control, the maximum level of nitrate concentration on water for drinking and farm irrigation, class I and II is 10 ppm, while class III and IV is 20 ppm. According to the regulation and the calculation, it was found that nitrate concentration on surface level water and well water in the research location was still below the standard quality threshold.
Nitrate and nitrite are very easy to blend with water and were found freely in nature. Nitrite can be easily oxidized into nitrate; therefore, nitrate is the most frequent compound to be found in both underground and surface level water. Pollution caused by nitrogen fertilizer, including ammonia anhydrate like organic waste of both animals and humans, and seepage from septic tank can increase the nitrate level in water. Compound that contains nitrate in water usually dissolve easily by migrating with underground water (Andreu and Picó 2004; Maghanga et al. 2013; Notodarmojo 2005). According to Maghanga et al. 2013, excessive nitrate that is caused by plant’s life will be carried away by water that seeps through soil, because soil does not have any mechanism to hold it. This caused relatively high nitrate level in soil water.
The increasing nitrate level on channels of water that came out of the rice field after going through the rice plot which was planted on dry and rainy season by organic and conventional farming methods can be seen in Table 4.
After going through the rice field, nitrate level on water increased. The increment was affected by season and farming method. The biggest increasing level can be found in organic rice field on dry season, while the smallest is on irrigation water that went through conventional rice field on rainy season. Increasing level of nitrate on water can be caused by polluted irrigation water that irrigated rice field, then went through conventional rice field into the organic one. According to Indradewa (2012), organic rice field that is located side by side with conventional rice field, with unseparated irrigation, can cause water contamination caused by scattered urea fertilizer and other chemical compounds.
Conventional rice farming uses a lot of urea fertilizer. Nitrogen content inside the fertilizer will undergo changes when located underground, e.g., in the form of ammonium (NH2), nitrate (NO3), or nitrite (NO2). Nitrogen that exists will evaporate into air (volatilization) and dissolve by abstersion or erosion. Nitrogen that evaporates into air has the potential to pollute the environment, while the ones that are dissolved by abstersion or erosion will pollute water bodies. Therefore, irrigation water had been polluted. Incorrect fertilizer distribution, like spreading it randomly, will cause many fertilizer to be carried away by the wind and fall into water bodies. This will cause ineffective fertilizing and enrichment of nitrogen in water bodies.
Conclusion
In general, it can be concluded that the system of organic rice cultivation in the Beringin and Pantai Labu District was causing lower pollution levels by α-cypermethrin pesticides and nitrate compared to the conventional one. Although the water in organic rice field was also contaminated by pesticide and nitrate, the level was still lower than those in conventional rice field. This is due to the organic rice field’s location that was side by side with the conventional rice fields without road, ditch, or plant barrier around the area, resulting in the contamination of chemicals from irrigation water or the wind.
Recommendation
The implementation of the system block area is recommended so that the organic rice field will not be contaminated by synthetic chemicals in the conventional rice field. Furthermore, separate irrigation for organic and conventional rice field is also recommended or building settling pond to reduce contamination of chemical compounds in the irrigation water.
References
A.O.A.C. Association of Official Analytical Chemyst (2005) Official methods of analysis of the Association of Official Analytical Chemist. The Association of Official Analytical Chemist.Inc., Arlington
Andreu V, Picó Y (2004) Determination of pesticides and their degradation products in soil: critical review and comparison of methods. TrAC Trends Anal Chem 23(10–11):772–789. https://doi.org/10.1016/j.trac.2004.07.008
Chandrasekar A (2005) Excess use of chemical pesticides causing huge damage to ecology in coastal Andhra. Business standard. Retrieved from http://www.business-standard.com/India/news/(15.06.13).
Chapman D (1992) Water quality assessment. A Guide to the Use of Biota, Sediments and Water. Chapman and Hall, London
Chapman D (Ed.) (1996) Water Quality Assessment. A Guide to the Use of Biota, Sediments and Water,in Environmental Monitoring-Second Edition. Chapman and Hall, London, UK
Duncan D (1955) Multiple range and multiple F tests. Biometrics 11:1–42 Google Scholar
Gliessman SR (2010) The frame work for conversion. In: Gliessman SR, Rosemerey M (eds) The Conversionto Sustainable Agriculture, Principles, Process and Practices. CRC Press, NewYork
Company HACH (1992) DR-2010 series data logging spectrophotometer:operations and procedures manual. Hach Company, Loveland
Hardjowigeno S (2003) Ilmu Tanah. Akademika Presindo, Jakarta
Harsanti ES (2008) Dampak penggunaan insektisida terhadap kualitas lingkungan fisik dan produk bawang merah serta perilaku petani dalam usahatani bawang merah (Desa Srigading Kecamatan Sanden,Kabupaten Bantul). Postgraduate Thesis, Gadjah Mada University
Hladik ML, Smalling KL, Kuivila KM (2009) Methods of analysis—determination of Pyrethroid insecticides in water and sediment using gas chromatography/mass spectrometry. U.S. Geological Survey, Reston
Indonesian Government Law No. 23 (1997) Concerning: environmental management. State Secretariat. Jakarta
Indradewa D (2012) Kontroversi isu pemanasan global dalam penyediaan pangan di Indonesia. Proceedings of the Anniversary of the Faculty of Agriculture, Gadjah Mada University, Yogyakarta:70–95
Kole RK, Banerjee H, Bhattachanyya A (2001) Monitoring of market fish samples for endosulfan and hexachlorocyclohexane residues in and around Calcutta. Bull Envirron Contam Toxicol 67:554–559
Knisel WG (1993) Groundwater loading effects of agricultural management systems, United States Department on Agriculture. Agricultural Research Servise, Tifton
Maghanga JK, Kituyi JL, Kisinyo PO, Ng’etich WK (2013) Impact of nitrogen fertilizer applications on surface water nitrate levels within a Kenyan tea plantation. Journal of chemistry 2013:1–4. https://doi.org/10.1155/2013/196516
Mahmood I, Sameen RI, Kanwal S, Gul A, Khalid RH (2015) Effects of pesticides on environment. In Plant,Soil and Microbes. Springer International:253–269. https://doi.org/10.1007/978-3-319-27455-3-13
MN Khan, M Mobin, ZK Abbas and SA Alamri (2018) Fertilizers and their contaminants in soils, Surface and Groundwater, Encyclopedia of the Anthropocene. https://doi.org/10.1016/B978-0-12-809665-9.09888-8
National Standardization Agency (2008) Air dan air limbah- Bagian 59: Metoda pengambilan contoh air limbah. SNI 6989.59:2008. Jakarta
Notodarmojo S (2005) Pencemaran Tanah dan Air Tanah. ITB, Bandung
Peraturan Menteri Kesehatan Republik Indonesia Nomor 492/Menkes/Per/IV/2010
Sarkar S, Singh SR, Singh RP (2003) The effect of organic and inorganic fertilizers on soil physical condition and the productivity of a rice-lentil cropping sequence in India. J Agric Science 140:419–425
Salgado VL (2013) Insectide mode of action technical training manual. https://www.researchgate.net/publication/275959530. BASF, Germany
Sahmrukh M, Corapcioglu MY and Hassona FAA (2001) Modelling the effect of chemical fertilizers on ground water quality in the Nile Valley Aquifer, Egypt. Ground Water 39 (1): 59-67. https://doi.org/10.1111/j.1745-6584.2001.tb00351.x
Standard dan Paten (BSN) Badan Standarisasi Nasional. 2008. Air dan air limbah- Bagian 59: Metoda pengambilan contoh air limbah. SNI 6989.59:2008.Jakarta.Badan Standardisasi Nasional
Sutanto R (2002) Penerapan Pertanian Organik Pemasyarakatan dan Pengembangannya. Kanisius, Jakarta
Sudjana (1996) Metoda Statistika. Tarsito, Bandung
Sugiharto E (2007) Studi analisis dampak residu pestisida pada komoditas pertanian di Propinsi Daerah Istimewa Yogyakarta dalam kemajuan terkini penelitian. Klaster Agro, Yogyakarta, pp 222–237
Tarumingkeng R (1997) Toksikologi Insektisida. Universitas Kristen Krida Wacana, Jakarta
Taylor ME, Michael DM (2008) Effects of agri-environment schemes in a long-term ecological time series.Agriculture. Ecosystems and Environment 130:9–15
Undang-Undang Republik Indonesia (1997) No. 23 tentang Pengelolaan Lingkungan Hidup, Jakarta, Kementerian Negara Lingkungan Hidup Republik Indonesia
Wihardjaka A, Abdurachman S (2007) Dampak pemupukan jangka panjang padi sawah tadah hujan terhadap emisi gas methana. Pusat Penelitian dan Pengembangan Tanaman Pangan, pp:200–205
Wihardjaka A (2011) Pengaruh jerami padi dan bahan penghambat nitrifikasi terhadap emisi gas rumah kaca (Metana dan Dinitrogen Oksida) pada ekosistem sawah tadah hujan di Kabupaten Pati, Jawa Tengah. Gadjah Mada University, Dissertation
Zhengfei G, Afons OL, Ada W, Ruud H (2004) Damage control inputs: a comparison of conventional and organic farming systems. Eur Rev Agric Econ 32(2):167–189
Acknowledgments
The authors acknowledge organic and conventional farmer groups in Beringin District and Pantai Labu in Deli Serdang Regency, North Sumatra Province.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Panjaitan, E., Sidauruk, L., Indradewa, D. et al. Impact of agriculture on water pollution in Deli Serdang Regency, North Sumatra Province, Indonesia. Org. Agr. 10, 419–427 (2020). https://doi.org/10.1007/s13165-020-00282-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13165-020-00282-7