Abstract
This study aims to understand the effects of recent tree encroachment on plant richness and diversity of Sphagnum-dominated bogs isolated in an agricultural landscape. A nested paired sampling design was used to compare plant species richness and beta diversity between open and forested habitats of 14 bogs in southern Québec, Canada. We evaluated the impact of tree encroachment at regional and local scales (between and within bogs, respectively). Tree basal area, canopy openness and stand age were evaluated in forested habitats. We used permutation paired sample t-tests to compare species richness between open and forested sites. Beta diversity was calculated as between-site similarities in composition, and differences were evaluated using tests for homogeneity in multivariate dispersion. Forested habitats had greater species richness than open habitats due to enrichment by facultative and non-peatland species as well as by mid- and shade-tolerant vascular plants. At both scales, this species enrichment was associated with flora differentiation (increase of beta diversity), although at regional scale, this was true for bryophytes only. Tree basal area had a positive influence on forested habitats species richness. These compositional changes are expected to increase similarity between bog flora and upland vegetation, and consequently decrease regional diversity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Woody encroachment, i.e., the increase of tree or shrub density, cover or biomass in naturally open ecosystems, has become a major concern worldwide. First reported in grasslands and savannas of arid and semi-arid biomes (Van Auken 2000; Eldridge et al. 2011), it has been documented increasingly in wetlands including salt marshes (Shirley and Battaglia 2006; Osland et al. 2013; Saintilan and Rogers 2015), floodplains (Warren et al. 2007; Bowman et al. 2008) wetland prairies (Bruce et al. 1995; Clark and Wilson 2001), fens (Peringer and Rosenthal 2011; Bart et al. 2016) and Sphagnum-dominated bogs (Frankl and Schmeidl 2000; Pellerin and Lavoie 2003a; Berg et al. 2009; Lee et al. 2017). In wetlands, woody encroachment has mostly been attributed to anthropogenic disturbances, notably drainage, modification in grazing or fire regimes, nitrogen deposition, elevated CO2 levels or climatic changes (e.g., Berendse et al. 2001; Pellerin and Lavoie 2003b; Middleton et al. 2006; Berg et al. 2009; Talbot et al. 2010; Saintilan and Rogers 2015; Lee et al. 2017). Irrespective of its causes, woody encroachment has been shown to alter resource levels (e.g., temperature, light, water and nutrient availability), threaten plant and animal diversity and profoundly modify ecosystem functions and services (e.g., Eldridge and Soliveres 2014; Lachance et al. 2005; Warren et al. 2007; Pasquet et al. 2015).
The impacts of woody encroachment on species abundance and richness in bogs are rather well understood. Tree invasion in bogs usually hampers Sphagnum growth and reduces their cover and richness (Laine et al. 1995; Ohlson et al. 2001; Limpens et al. 2003). For example, Ohlson et al. (2001) examined surface peat cores collected near 151 pines in a Norwegian bog and found that pine establishment caused the disappearance of Sphagnum mats in 39% of samples. The shift from open bog to bog woodland is also associated with a decrease in the cover and richness of other bog-specialist plant species (often heliophilic) and a concomitant increase of shade or drought-tolerant forest species (Laine et al. 1995; Gunnarsson et al. 2002; Lachance et al. 2005; Pellerin et al. 2009; Tousignant et al. 2010; Kapfer et al. 2011; Pasquet et al. 2015). For example, in 16 peatlands of southeastern Québec subjected to recent spruce and pine cover expansion, the presence of bog specialist plants was highly negatively associated with an increase in forest cover (R2 = 0.63; Lachance et al. 2005). Although an overall gain of species richness is often observed in bogs following woody encroachment (Pellerin et al. 2009; Woziwoda and Kopeć 2014; Dyderski et al. 2015), it has been hypothesized that the resulting flora is less valuable for conservation purposes due to its increased similarity with surrounding forest plant communities, which simplifies regional diversity (e.g., Laine et al. 1995; Calmé et al. 2002; Lachance et al. 2005; Pasquet et al. 2015). This could be of particular concern in temperate regions where bog plant communities and richness contrast sharply with surrounding forests (Ingerpuu et al. 2001; Moore 2002). Despite numerous studies published on the impacts of woody encroachment on bog flora, beta diversity has been overlooked.
Biotic homogenization (reduced beta diversity) is the process by which the similarity of species, traits or genetic composition of communities increases across space over time (McKinney and Lockwood 1999; Olden and Poff 2003). This process is usually the result of the simultaneous extinction of native, specialized species and introduction of exotic or ruderal native ones (McKinney and Lockwood 1999; Beauvais et al. 2016; Brice et al. 2017). Wetlands are expected to be particularly prone to homogenization following degradation, as wetland plants usually spread more widely than forest ones (Santamaría 2002; Ricklefs et al. 2008) and have been shown to contribute to the homogenization of state-level floras in North America (Santamaría 2002; Qian and Guo 2010). Wetlands are also highly susceptible to invasion by exotic species (Galatowitsch et al. 1999; Zedler and Kercher 2004). On the other hand, several wetland types are species poor, which could leave them more vulnerable to differentiation (increase of beta diversity) following environmental changes and subsequent species invasion (Olden and Poff 2003).
In this study, we investigated the effects of recent tree encroachment on plant richness and diversity of Sphagnum-dominated bogs isolated in an agricultural landscape in southern Québec (Canada), using a space-for-time substitution approach (Pickett 1989). More specifically, we evaluated whether tree encroachment induced biotic homogenization at regional and local scales (i.e., between and within bogs, respectively). We also explored how tree stand characteristics (tree basal area, canopy openness and age) influenced richness and diversity of forested habitats. We hypothesized local flora enrichment with tree encroachment, with the magnitude of the changes related to tree stand characteristics. More specifically, we predicted an increase of species richness in forested habitats resulting from the co-occurrence of peatland shade-intolerant and forest shade-tolerant species, and that this enrichment induces a biotic differentiation (increase of beta diversity) at the local scale. Inversely, at the regional scale, we predicted a biotic homogenization (decrease of beta diversity) due to the spread of a few generalist forest species throughout the study area. Finally, we predicted that the changes will be greater in older stands characterized by higher tree basal area and lower canopy openness.
Methods
Study Area
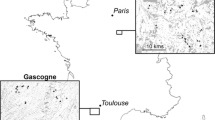
The study area is located in the western portion of the St. Lawrence Lowlands in southern Québec, and extends from 45°N to 47°N latitude and 71°W to 74°W longitude (Fig. 1). The area is enclosed by the Canadian Shield to the north and the Appalachian Mountains to the south and east. The Lowlands have a flat topography and are characterized by deep arable soils derived from glacial and marine deposits. The rich soils support intensive agriculture over large areas. More precisely, the landscape is composed of about 50% agricultural fields, 30% woodlands, 10% urbanized areas and 10% wetlands (Statistics Canada 2010). Bogs represent about 35% of wetlands (Poulin et al. 2016). The mean annual temperature of the study area fluctuates from 4.1 °C in the northeast to 6.7 °C in the southwest (Environment Canada 2011). Average precipitation ranges from a high of 1120 mm in the northeast to a low of 965 mm in the southwest, of which 23% and 17% respectively falls as snow (Environment Canada 2011).
Bogs of the study area are mostly dominated by ericaceous shrubs, Sphagnum mosses and Picea mariana thickets. Over the last few decades, 56,681 ha, or 19% of the region’s wetlands have been lost or disturbed by human activities (Poulin et al. 2016). The proportion of disturbed bogs is estimated to be equivalent, and tree encroachment has been reported in many of the remaining peatlands (Pellerin and Lavoie 2003a, Pasquet et al. 2015; Pellerin et al. 2016; Beauregard 2017). For instance, the reconstruction of tree encroachment in 28 temperate bogs of southern Québec showed a 138% increase of forest habitats (1353 ha) since ca. 1930 (Online Resource 1). In most of these bogs, tree encroachment (mostly Betula populifolia, Picea mariana, Larix laricina, and Pinus banksiana) began at the margins and spread over the expanse. According to multi-proxy historical reconstructions, tree encroachment in these sites resulted from the interaction of multiple factors, notably drainage (in situ and in the surrounding agricultural catchment) and dry climatic periods that both induced gradual drying of the peat surface as well as fires (Pellerin and Lavoie 2003a; Pasquet et al. 2015; Pellerin et al. 2016). Nitrogen deposition may also have exacerbated the phenomenon, but only in a few sites of the southernmost portion of the study area (Pasquet et al. 2015), as most sites are located in areas where these deposits are very low (Pellerin and Lavoie 2003a; Turunen et al. 2004; Pellerin et al. 2016).
Site Selection
Based on information in previous studies on tree encroachment in bogs (Online Resource 1) and recent Google Earth Digital Globe satellite imagery, we identified all peatlands in the study area with at least a quarter of their area characterized by dense forest cover (N = 35 bogs). To ensure we surveyed only bogs that have been recently colonized by trees (i.e., in the last 50 years), the evolution of tree cover in these bogs was assessed by comparing past aerial photographs (1960) to recent satellite imagery. Tree encroachment occurred mostly after 1960 in all of the 35 pre-selected bogs. We then selected only bogs in which present-day forest cover was dominated by coniferous trees (Picea mariana or Larix laricina), and eliminated all sites with signs of tree cutting or other intensive human disturbances (e.g., peat mining, cranberry farming) or that were inaccessible. From among the 20 remaining bogs, we selected 14 that were spaced widely across the study area (Fig. 1; Online Resource 2). These bogs ranged in size from 5 to 369 ha and did not show visible sign of human disturbances although some, or all of them, may be affected by indirect disturbances (e.g., change in the hydrology of the surrounding catchment, nitrogen deposition) or may have burnt in the past.
Sampling Design
A nested paired sampling design was used to compare plant species richness and beta diversity between open and forested habitats of the 14 selected bogs (Online Resource 3). The paired sampling design was used as a space-for-time substitution, assuming paired open and forested habitats had a similar flora before encroachment. Using the 1960s aerial photographs and the most recent Google Earth Digital Globe satellite imagery, we identified the sector with the seemingly densest recent forest cover for each bog. The coordinates of the central portion of these sectors were retrieved from Google Earth and relocated in the field using a GPS. We ensured the sampling quadrat was located in a homogeneous plant community with a cover of at least 50% of trees taller than 3 m and at least 25 m from any edge. The closest open habitat characterized by less than 5% cover of trees taller than 3 m, and also located at least 25 m from any edge, was then selected. We validated that its environmental characteristics (peat thickness, pH and electrical conductivity) were similar to those of the paired forested quadrat (MANOVA where bogs were treated as an error term: F(3,11) = 2.56; p = 0.108). Consequently, the main difference within pairs of quadrats was the extent of the tree cover. Distances between paired open and forested quadrats ranged from 246 to 1774 m.
In each habitat, we delineated a 625 m2 quadrat (25 m × 25 m) with nine nested plots of 25 m2 (5 m × 5 m) for sampling vascular plants and 18 nested circular subplots of 0.38 m2 (0.35 m radius) for bryophytes. The nine 25 m2 plots were systematically located in the quadrat and spaced equally 5 m apart, while two subplots were randomly located within each of the nine plots (Online Resource 3).
Field Sampling
Vegetation was sampled between June and August 2016. Percentage cover of each species was estimated using 7 classes: 1: <1%, 2: 1–5%, 3: 6–10%, 4: 11–25%, 5: 26–50%, 6: 51–75%, 7: >75%. Species nomenclature followed VasCan (Brouillet et al. 2017) for vascular plants and Faubert (2012, 2013, 2014) for bryophytes.
In the center of each forested quadrat, we evaluated tree basal area using a wedge prism (factor 2). We also estimated canopy openness (%) by averaging 36 readings (4 by plot) on a Lemmon’s model C spherical densiometer (Lemmon 1956). Finally, to estimate forest stand age, the ten trees with the largest diameter at breast height were cored as closely as possible to the collar using an increment borer. In laboratory, cores were dried, finely sanded, and annual growth rings were counted under a low-power (50×) binocular microscope.
Data Analysis
Species Richness
For each quadrat, total richness was assessed as the number of species in all plots and subplots. Vascular richness was evaluated using only plots, and bryophyte richness using subplots. The richness of vascular plants, bryophytes, and both plant groups together (total) was compared between open and forested quadrats using permutation paired sample t-tests (9999 permutations).
In addition, we took into account species’ habitat preferences by comparing richness of preferential, facultative and non-peatland species between open and forested quadrats. Species habitat preference follows Garneau (2001), Tousignant et al. (2010), Faubert (2012, 2013, 2014) and the New England Wild Flower Society (2017). Species that mostly grow in peatlands (either bogs or fens) were classified as “preferential”, those that can grow in either peatlands or other ecosystems as “facultative”, while species seldom found in peatlands were classified as “non-peatland species” (Online Resource 4). Linear mixed-effect models were used to compare the number of species between habitat types (open or forested) and between habitat preferences (preferential, facultative, non-peatland) as well as the interaction between both factors. Bogs were treated as a random factor, therefore allowing us to control for pairing between quadrats and dependence between the numbers of species of different preferences in the same quadrat. Because a significant interaction was found between habitat types and habitat preferences, we tested the individual effect of each factor with linear mixed-effect models for each level of the other factor and then conducted post hoc Tukey tests to further investigate if the number of species of each habitat preference differed for open and forested habitats. We verified that model residuals followed a normal distribution.
We also compared the richness of intolerant, mid-tolerant and shade-tolerant species between open and forested quadrats. Species tolerance follows Lapointe (2014), Brice et al. (2016) as well as USDA and NRCS (2018). We only took into account vascular species shade tolerance, as the information was not as reliable or available for all bryophyte species (Online Resource 4). The same linear mixed-effect model procedure described above was used.
Beta Diversity
Prior to evaluating beta diversity, we computed a site-by-species matrix for each group of species (total, vascular and bryophytes) at both spatial scales (regional and local). The site-by-species matrices at the regional scale used the quadrat as unit (28 units: 14 quadrats × 2 habitats) and each entry corresponds to the mean cover of a species in a quadrat (calculated from the median of cover classes in each plot). The site-by-species matrices at the local scale used the plot as unit (252 units: 9 plots × 14 quadrats × 2 habitats) and each entry corresponds to the mean cover of the two subplots (calculated from the median of cover classes in each subplot).
Differences in beta diversity between open and forested quadrats were analysed using a distance-based test for homogeneity of multivariate dispersions (PERMDISP; Anderson 2006). PERMDISP calculates the distance of each site to the centroid in ordination space (principal coordinates analysis) and then tests whether these distances are different between groups (i.e., open VS forested habitats) through permutation tests. More precisely, a site-by-site distance matrix (using Hellinger distance; Legendre and De Cáceres 2013) was first used to compute the centroid of each group of sites (two groups at the regional scale: open or forested habitats; 28 groups at the local scale: 14 quadrats × 2 habitats). Then, the distance of each site to its associated group centroid was calculated, and the dispersion of these distances (within-group variance) was used as an estimate of beta diversity (the greater the within-group variance, the higher the beta diversity). For the regional scale, the site distances to centroid were subjected to t-tests with 9999 permutations to determine whether dispersion differed between groups. For the local scale, the site distances to centroid were compared by fitting a linear mixed-effect model where the sites were treated as a random factor, therefore allowing us to control for pairing between plots of the same bog. We verified that model residuals followed a normal distribution.
To detect species shifts (turnover) between habitats at the regional scale, we tested for location differences between centroids using PERMANOVAs with a pseudo-F ratio (9999 permutations; Anderson 2001). Because this test is sensitive to differences in multivariate dispersions (Anderson and Walsh 2013), data visualization was used to support interpretation of the statistical test. The differences in multivariate dispersion and composition were illustrated in principal coordinates analysis ordinations (PCoA) based on their respective distance matrices.
Effects of Tree Stand Characteristics
We used multiple linear regressions to evaluate the relationships between tree stand characteristics (age of the forest stands, canopy openness and tree basal area) and total richness and beta diversity of forested quadrats. For beta diversity, we used site distance to group centroid (see above) as response variable. All explanatory variables were standardized to z-scores (μ = 0 and σ = 1). We ensured all model residuals followed a normal distribution.
All statistical analyses were performed using R 3.3.2 (R Core Team 2016). Permutation t-tests and permutation paired sampled t-tests were done using paired.perm.test() and perm.test(), respectively, from the broman package (Broman and Broman 2017). Linear mixed-effect models were fitted with lme() in nlme. Tukey tests were done with the lsmeans()function in the lsmeans library (Lenth 2016). Hellinger transformations were done using decostand(), multivariate dispersion analyses were performed using betadisper() and centroid locations were tested using adonis2(), all from the vegan package (Oksanen et al. 2017). Multiple linear regressions were performed with the lm()function in stats (R Core Team 2016).
Results
Species Richness
Overall, 117 species (87 vascular, 30 bryophytes) were inventoried in the 14 bogs. Of these, 58 species (45 vascular, 13 bryophytes) were found in open habitats and 100 (72 vascular, 28 bryophytes) in forested habitats, while 41 species (30 vascular, 11 bryophytes) where shared between the two habitats. Only two exotic species were found (Frangula alnus and Sorbus aucuparia; Brouillet et al. 2017). Mean total and vascular richness were significantly higher in forested habitats than in open habitats, while mean bryophyte richness was similar between both habitats (Fig. 2).
Total, vascular and bryophyte species richness for open and forested habitats in bogs characterized by recent tree encroachment, southern Québec (Canada). Provided are mean species richness (black dot), median (line), 25–75% quartiles (boxes) and ranges (whiskers). Different letters indicate a significant difference (p ≤ 0.05) determined by paired permutation t-tests
The numbers of preferential, facultative and non-peatland species varied differently between open and forested habitats (Fig. 3; Online Resource 5). There were more preferential species in open than in forested habitats, but when only vascular species were considered, open and forested habitats remained similar. On the other hand, facultative and non-peatland species were always more abundant in forested than in open habitats. Within open habitats, the number of species generally decreased from preferential to facultative to non-peatland species, although, for bryophytes, facultative and non-peatland species were almost absent. Within forested habitats, the numbers of preferential and facultative species were similar and both were higher than the number of non-peatland species when considering the total flora and vascular plants. For bryophytes, the numbers of preferential and non-peatland species were similar and both were higher than the number of facultative species.
Richness of preferential, facultative and non-peatland species in open and forested habitats for the total species pool, vascular plants and bryophytes in bogs characterized by recent tree encroachment, southern Québec (Canada). Provided are mean species richness (black dot), median (line), 25–75% quartiles (boxes) and ranges (whiskers). Different lower case letters indicate a significant difference (α = 0.05; linear mixed-effect models) between open and forested habitats, whereas different upper case letters indicate a significant difference (α = 0.05; Tukey’s test) within open or forested habitats
The numbers of shade-intolerant, mid-tolerant and shade-tolerant vascular species varied significantly between open and forested habitats (Fig. 4; Online Resource 6). There were more intolerant species in open than in forested habitats, while mid-tolerant and tolerant species were more abundant in forested than in open habitats. Within open habitats, there were more intolerant species than mid-tolerant and tolerant ones. Within forested habitats, the number of tolerant species was greater than that of mid-tolerant species, while the number of intolerant species was transitional between both. Overall, the number of species associated with forested habitats, either shade-tolerant, facultative or non-peatland species, was more variable between quadrats than the number of peatland species (larger box plots; Figs. 3 and 4).
Richness of shade-intolerant, mid-tolerant and shade-tolerant vascular species in open and forested habitats in bogs characterized by recent tree encroachment, southern Québec (Canada). Provided are mean species richness (black dot), median (line), 25–75% quartiles (boxes) and ranges (whiskers). Different lower case letters indicate a significant difference (α = 0.05; linear mixed-effect models) between open and forested habitats, whereas different upper case letters indicate a significant difference (α = 0.05; Tukey’s test) within open or forested habitats
Beta Diversity
At the regional scale, beta diversity of the total species pool and of vascular species was similar between open and forested habitats, but was greater in forested than in open habitats for bryophytes (Table 1a; Fig. 5), meaning that bryophyte communities were more different from quadrat to quadrat in forested than in open habitats. Furthermore, a clear species turnover occurred between open and forested habitats, as centroid locations were significantly different for all groups of species (Table 1c; Fig. 5). At the local scale, beta diversity was lower in open than in forested habitats for the total species pool and for vascular species, but no difference was found for bryophytes (Table 1b; Fig. 6).
Influence of habitat type (open = black circles; forested = grey triangles) on beta diversity of total, vascular and bryophyte species at the regional scale in bogs characterized by recent tree encroachment, southern Québec (Canada). Beta diversity was measured as the distance of sites to their group centroid, here represented on the first two axes of a PCoA and using boxplots of the sites-to-centroid distance. Provided are mean distance to centroid (black dot), median (line), 25–75% quartiles (boxes) and ranges (whiskers). Different letters indicate a significant difference (p ≤ 0.05) determined by t-tests. Circles are ellipses of standard deviation
Influence of habitat type on beta diversity of total, vascular and bryophyte species at the local scale in bogs characterized by recent tree encroachment, southern Québec (Canada). Beta diversity was measured as the average distance of sites to their group centroid, here represented using boxplots of the sites-to-centroid distance. Provided are mean distance to centroid (black dot), median (line), 25–75% quartiles (boxes) and ranges (whiskers). Different letters indicate a significant difference (p ≤ 0.05) determined by linear mixed-effect models
Relationship between Tree Stand Characteristics and Diversity
Mean tree stand age was 64 years (SE = 20 years), with the oldest stand estimated to be 106 years old. Canopy openness was 20% on average (SE = 17%), and average basal area was 14 m2 (SE = 5 m2). Total species richness was only positively related to tree basal area (t(10) = 2.354; p = 0.04), meaning that the number of species increased with tree biomass. No other significant relation was found between richness or beta diversity and tree stand characteristics (Online Resource 7).
Discussion
In this study, we predicted that tree encroachment in temperate Sphagnum-dominated bogs isolated in an agricultural landscape would engender floral differentiation (increase of beta diversity) within peatlands (local scale) following enrichment by non-peatland species, whereas we expected homogenization between peatlands (regional scale) due to the spread of generalist forest species across sites. As expected, forested habitats had greater species richness than open habitats, mostly related to enrichment by facultative and non-peatland species as well as by mid- and shade-tolerant vascular plants. At the local scale, this species enrichment was, as predicted, associated with floral differentiation, because beta diversity (measured as variance of compositional turnover within group) was higher in forested than in open habitats. On the other hand, and contrary to our prediction, tree encroachment did not foster homogenization at a regional scale. Bryophyte beta diversity at this scale was even higher among forested habitats than open habitats. Finally, and also contrary to expectation, tree stand characteristics had no influence on forested quadrat richness or beta diversity, with the exception of tree basal area, which was positively correlated to species richness.
Tree Encroachment and Species Richness
The comparison of species richness between open and forested habitats suggests that tree encroachment fosters plant species richness in bogs. This finding concurs with that of previous studies conducted in peatlands (e.g., Pellerin et al. 2009; Woziwoda and Kopeć 2014; Dyderski et al. 2015), but contrasts with studies on grasslands, in which woody encroachment mostly induces species impoverishment (Price and Morgan 2008; Ratajczak et al. 2012; Guido et al. 2017). Grasslands represent one of the richest habitats on earth (Wilson et al. 2012), and most species composing their extremely rich graminoid communities have high light requirements and are therefore often excluded following tree encroachment (Lett and Knapp 2003). Contrary to grasslands, Sphagnum-dominated bogs are species-poor ecosystems (Wheeler 1993; Vitt et al. 1995; Warner and Asada 2006), due to high abiotic stressors such as a high water table as well as low mineral and nutrient content (Wheeler 1993; Zoltai and Vitt 1995); historical legacy through evolutionary time scale represents another potential explanation (Pärtel 2002). Trees likely lessen these strong environmental filters, and foster the establishment of terrestrial species while engendering the loss of light-demanding and waterlogging-tolerant typical bog species. In this regard, it has been shown that tree encroachment in drained peatlands often accelerates soil dehydration through interception of precipitation and increased evapotranspiration (Frankl and Schmeidl 2000; Sarkkola et al. 2010; Holmgren et al. 2015). It may also increase soil nutrient availability by litter fall, and reduce solar radiation at the soil level (Limpens et al. 2003; Wertebach et al. 2014; Paradis and Rochefort 2017; Ratcliffe et al. 2017). All these environmental changes have been shown to be detrimental to Sphagnum growth (Laine et al. 1995; Ohlson et al. 2001; Limpens et al. 2003), but to have positive effects on tree seedling establishment and growth (Ohlson et al. 2001; Eppinga et al. 2009; Holmgren et al. 2015), which could further facilitate a persistent shift towards forested communities (Heijmans et al. 2013).
According to our results, the increased richness following tree encroachment was associated with the increase of facultative and non-peatland species richness, mostly for vascular plants but also for bryophytes, while it either did not affect (vascular plants) or lowered (bryophytes) the richness of peatland preferential species (see Fig. 3). Furthermore, the enrichment of vascular species was linked to an increase of mid-tolerant and shade-tolerant ones, despite a concomitant decrease of shade-intolerant species (see Fig. 4). This loss of peatland endemics and heliophilic plants was also observed in previous studies (e.g., Gunnarsson et al. 2002; Pellerin et al. 2009; Kapfer et al. 2011; Pasquet et al. 2015). For example, in one bog of the same study area, but characterized by Betula populifolia instead of coniferous encroachment, Pasquet et al. (2015) demonstrated that non-peatland species were 15 and five times more abundant in old (≥ 30 years) and new bog woodlands than in open Sphagnum-dominated habitats, respectively. As well, in a Sphagnum-dominated bog in southern Sweden, all of the ten species negatively impacted by the increase in trees and dwarf shrubs over 54 years were bog species, whereas forest species increased in frequency (Kapfer et al. 2011). Of the species that experienced a shift of their optimum value, 67% simultaneously shifted towards lower light values, which represents an increase in shade-tolerant species. This species turnover likely indicates that flora of peatlands subjected to tree encroachment tend to become more similar to upland ecosystems, which could induce regional floral homogenization. Although this phenomenon has frequently been mentioned (e.g., Laine et al. 1995; Calmé et al. 2002; Lachance et al. 2005; Pasquet et al. 2015), it remains to be documented.
Tree Encroachment and Beta Diversity
As with species richness, beta diversity at the local scale (within peatlands) was higher in forested than in open habitats and was still mostly associated with vascular species (see Fig. 6). At the regional scale, we found no evidence of reduced beta-diversity (see Fig. 5). Bryophyte beta diversity was even higher for forested than for open habitats, suggesting a differentiation of bryophyte communities from one bog to another with tree encroachment.
Species turnover and changes in species richness are the two main mechanisms inducing changes in beta diversity (Olden and Poff 2003; Legendre 2014). In our study, both mechanisms are likely involved, as species richness was higher in forested than in open habitats and because there was an important turnover from preferential to facultative and non-peatland species following tree encroachment, as well as from shade-intolerant to shade-tolerant species. Tree encroachment likely induced more variable environmental conditions within the open Sphagnum-dominated bogs, which have in turn fostered species richness and turnover. In our case, light availability and microhabitat diversity were certainly more variable in forest than in open habitats. In fact, the tree canopy is unlikely to develop uniformly, which has allowed the co-occurrence of light-demanding bog species and shade-tolerant forest species in forested habitats (see Fig. 4). Furthermore, the presence of trees in peatlands likely created new niches via stump bases, the aerial part of root systems or decaying woody debris, as such microhabitats have indeed been associated with species richness in swamps (Pollock et al. 1998; Flinn et al. 2008). As well, the fact that woody debris increases with the volume of living trees in a stand (Siitonen et al. 2000) could explain the positive relationship obtained between species richness and basal area. Actually, most of the bryophyte species observed only on forested sites (e.g., Brachythecium plumosum, Hypnum pallescens, Plagiothecium laetum, Tetraphis pellucida) are known to be associated with decaying wood debris, stumps or forest litter (Faubert 2013, 2014). Although microhabitat variability is clearly evident in open bogs with the development of hummocks and hollows microtopography, Sphagnum communities are usually quite similar among bogs at a scale larger than the hummock-hollow gradient (Poulin et al. 2002; Vitt 2006). This was the case in our study sites, where open quadrats were usually dominated by the same four species (Sphagnum capillifolium, S. fuscum, S. rubellum or S. magellanicum). Although both phenomena are likely involved in the differentiation observed at both spatial scales, we believe that light variability was mostly responsible for the differentiation of vascular plants at the local scale, and that microhabitat variability mostly induced the differentiation of bryophytes at the regional scale.
The absence of biotic homogenization at the regional scale may be related, in part, to a time lag between ecosystem changes and plant community response (e.g., Lindborg and Eriksson 2004; Helm et al. 2006; Savage and Vellend 2015). In fact, although tree encroachment began, on average, 60 years ago in the studied peatlands, the high tree canopy established more recently on most sites (about 20 years ago according to aerial photographs). This might explain the absence of a significant relationship between stand age and either species richness or beta diversity, at both local and regional scales. In fact, tree growth in peatlands that are not intentionally drained for forestry purposes is slow and very variable in area, due, among other factors, to the high water table and poor soil aeration (Lieffers and Rothwell 1986; Macdonald and Lieffers 1990). Consequently, tree encroachment may not have been impacting bogs for long enough for the changes in their magnitude to be observable, a phenomenon referred to as “extinction debt” (Tilman et al. 1994).
Issues Relevant to Space-for-Time Substitution Studies
In this study, we investigated the effects of recent tree encroachment on plant richness and diversity of bogs using a space-for-time substitution methodology. This method has been criticized for not taking into account the historical level of similarity between the communities studied and the possibility that it may reflect a spatial rather than temporal species turnover (Rooney et al. 2007). In our study, for example, plant communities of sites affected by tree encroachment may have been already different from those in the portions of the sites still open, or trees may have been more susceptible to establishing in a certain type of open bog. However, previous studies that resampled historical plots in bogs of southern Québec did not find that tree encroachment tended to occur in certain plant communities (Pellerin et al. 2009; Pasquet et al. 2015). On the other hand, encroachment often began at the margins, where peat deposits are thinner (Pellerin and Lavoie 2003a; Pasquet et al. 2015), or near drainage ditches (Pellerin et al. 2016). Here, we found no significant difference between peat thickness, pH and conductivity of paired forested and open quadrats. Recently, it has been demonstrated that space-for-time substitution may underestimate the effects of disturbances on species diversity and community turnover compared to a before/after sampling approach, but that both approaches often reveal similar tendencies (França et al. 2016). Because our results are consistent with those of other studies on the impacts of tree encroachment on plant communities of bogs (e.g., Frankl and Schmeidl 2000; Gunnarsson et al. 2002; Lachance et al. 2005; Pellerin et al. 2009; Pasquet et al. 2015), we believe our results are valid but may represent a conservative assessment of changes.
Conclusion
In conclusion, our study showed that tree encroachment may induce important changes in richness and diversity in temperate bogs isolated in an agricultural landscape. Most of the observed changes were associated with an increase in facultative and non-peatland shade-tolerant species in forested habitats, at the expanse of peatland shade-intolerant species. This species shift could result in increased similarity between bogs and upland vegetation, and therefore decrease regional diversity. The observed compositional change could also have major impacts on the diversity of other trophic groups as Sphagnum-dominated bogs provide refuge for a variety of endemic birds, invertebrates and protists (Warner and Asada 2006). This is especially true for bogs of temperate regions, where plant and bird assemblages contrast more with the surrounding landscape than bogs of boreal regions (Moore 2002; Calmé et al. 2002). In this sense, our work highlights the importance of establishing conservation buffer zones in peatlands isolated in an anthropized matrix when designing reserves. These buffers should benefit from clear legislation aiming at reducing drainage or land conversion, which in turn affect the water balance of bogs. This is particularly important as tree encroachment is increasing worldwide, and because tree growth could generate a feedback loop leading to further tree encroachment (Frankl and Schmeidl 2000; Eppinga et al. 2009; Ireland and Booth 2012), inducing a persistent alternative stable state.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Anderson MJ (2006) Distance-based tests for homogeneity of multivariate dispersions. Biometrics 62:245–253. https://doi.org/10.1111/j.1541-0420.2005.00440.x
Anderson MJ, Walsh DCI (2013) PERMANOVA, ANOSIM, and the Mantel test in the face of heterogeneous dispersions: What null hypothesis are you testing? Ecological Monographs 83:557–574. https://doi.org/10.1890/12-2010.1
Bart D, Davenport T, Yantes A (2016) Environmental predictors of woody plant encroachment in calcareous fens are modified by biotic and abiotic land-use legacies. Journal of Applied Ecology 53:541–549. https://doi.org/10.1111/1365-2664.12567
Beauregard, P (2017) Dynamique du bouleau gris (Betula populifolia) dans les tourbières ombrotrophes de la Montérégie (Québec). Dissertation, Université Laval
Beauvais M-P, Pellerin S, Lavoie C (2016) Beta diversity declines while native plant species richness triples over 35 years in a suburban protected area. Biological Conservation 195:73–81. https://doi.org/10.1016/j.biocon.2015.12.040
Berendse F, Van Breemen N, Rydin H et al (2001) Raised atmospheric CO2 levels and increased N deposition cause shifts in plant species composition and production in Sphagnum bogs. Global Change Biology 7:591–598. https://doi.org/10.1046/j.1365-2486.2001.00433.x
Berg EE, Hillman KM, Dial R, DeRuwe A (2009) Recent woody invasion of wetlands on the Kenai Peninsula Lowlands, south-central Alaska: a major regime shift after 18000 years of wet Sphagnum–sedge peat recruitment. Canadian Journal of Forest Research 39:2033–2046. https://doi.org/10.1139/X09-121
Bowman DMJS, Riley JE, Boggs GS et al (2008) Do feral buffalo (Bubalus bubalis) explain the increase of woody cover in savannas of Kakadu National Park, Australia? Journal of Biogeography 35:1976–1988. https://doi.org/10.1111/j.1365-2699.2008.01934.x
Brice M-H, Pellerin S, Poulin M (2016) Environmental filtering and spatial processes in urban riparian forests. Journal of Vegetation Science 27:1023–1035. https://doi.org/10.1111/jvs.12425
Brice M-H, Pellerin S, Poulin M (2017) Does urbanization lead to taxonomic and functional homogenization in riparian forests? Diversity and Distributions 23:828–840. https://doi.org/10.1111/ddi.12565
Broman KW, Broman AT (2017) broman: Karl Broman’s R Code. R package version 0.67–4. Available at https://CRAN.r-project.org/package=broman
Brouillet L, Coursol F, Meades SJ et al (2017) VASCAN, the Database of Vascular Plants of Canada. Available at http://data.canadensys.net/vascan/. Accessed 28 Mar 2017 https://doi.org/10.3897/phytokeys.25.3100
Bruce KA, Cameron GN, Harcombe PA (1995) Initiation of a new woodland type on the Texas coastal prairie by the Chinese Tallow Tree (Sapium sebiferum (L.) Roxb.). Bulletin of the Torrey Botanical Club 122:215–225. https://doi.org/10.2307/2996086
Calmé S, Desrochers A, Savard J-PL (2002) Regional significance of peatlands for avifaunal diversity in southern Québec. Biological Conservation 107:273–281. https://doi.org/10.1016/S0006-3207(02)00063-0
Clark DL, Wilson MV (2001) Fire, mowing, and hand-removal of woody species in restoring a native wetland prairie in the Willamette valley of Oregon. Wetlands 21:135–144. https://doi.org/10.1672/0277-5212(2001)021[0135:FMAHRO]2.0.CO;2
Dyderski MK, Gdula AK, Jagodziński AM (2015) Encroachment of woody species on a drained transitional peat bog in ‘Mszar Bogdaniec’ nature reserve (Western Poland). Folia Forestalia Polonica 57:160–172. https://doi.org/10.1515/ffp-2015-0016
Eldridge DJ, Soliveres S (2014) Are shrubs really a sign of declining ecosystem function? Disentangling the myths and truths of woody encroachment in Australia. Australian Journal of Botany 62(7):594–608. https://doi.org/10.1071/BT14137
Eldridge DJ, Bowker MA, Maestre FT et al (2011) Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis: Synthesizing shrub encroachment effects. Ecology Letters 14:709–722. https://doi.org/10.1111/j.1461-0248.2011.01630.x
Environment Canada (2011) Canadian Climate Normals 1981–2010. Available at http://climate.weather.gc.ca/climate_normals/index_e.html. Accessed 12 Oct 2017
Eppinga MB, Rietkerk M, Wassen MJ, Ruiter PCD (2009) Linking habitat modification to catastrophic shifts and vegetation patterns in bogs. Plant Ecology 200:53–68. https://doi.org/10.1007/s11258-007-9309-6
Faubert J (2012) Flore des bryophytes du Quebec-Labrador : Volume 1. Anthocérotes et hépatiques. Société québécoise de bryologie, Saint-Valérien, Québec
Faubert J (2013) Flore des bryophytes du Québec-Labrador : Volume 2, Mousses, première partie. Société québécoise de bryologie, Saint-Valérien, Québec
Faubert J (2014) Flore des bryophytes du Québec-Labrador : Volume 3, Mousses, seconde partie. Société québécoise de bryologie, Saint-Valérien, Québec
Flinn KM, Lechowicz MJ, Waterway MJ (2008) Plant species diversity and composition of wetlands within an upland forest. American Journal of Botany 95:1216–1224. https://doi.org/10.3732/ajb.0800098
França F, Louzada J, Korasaki V, Griffiths H, Silveira JM, Barlow J (2016) Do space-for-time assessments underestimate the impacts of logging on tropical biodiversity? An Amazonian case study using dung beetles. Journal of Applied Ecology 53:1098–1105. https://doi.org/10.1111/1365-2264.12657
Frankl R, Schmeidl H (2000) Vegetation change in a South German raised bog: Ecosystem engineering by plant species, vegetation switch or ecosystem level feedback mechanisms? Flora 195:267–276. https://doi.org/10.1016/S0367-2530(17)30980-5
Galatowitsch SM, Anderson NO, Ascher PD (1999) Invasiveness in wetland plants in temperate North America. Wetlands 19:733–755. https://doi.org/10.1007/BF03161781
Garneau M (2001) Statut trophique des taxons préférentiels et des taxons fréquents mais non préférentiels des tourbières naturelles du Québec-Labrador. In: Payette S, Rochefort L (eds) Écologie des tourbières du Québec-Labrador. Presses Université Laval, Québec, pp 523–532
Guido A, Salengue E, Dresseno A (2017) Effect of shrub encroachment on vegetation communities in Brazilian forest-grassland mosaics. Perspectives in Ecology and Conservation 15:52–55. https://doi.org/10.1016/j.pecon.2016.11.002
Gunnarsson U, Malmer N, Rydin H (2002) Dynamics or constancy in Sphagnum dominated mire ecosystems? A 40-year study. Ecography 25:685–704. https://doi.org/10.1034/j.1600-0587.2002.250605.x
Heijmans MMPD, van der Knaap YAM, Holmgren M, Limpens J (2013) Persistent versus transient tree encroachment of temperate peat bogs: effects of climate warming and drought events. Global Change Biology 19:2240–2250. https://doi.org/10.1111/gcb.12202
Helm A, Hanski I, Pärtel M (2006) Slow response of plant species richness to habitat loss and fragmentation. Ecology Letters 9:72–77. https://doi.org/10.1111/j.1461-0248.2005.00841.x
Holmgren M, Lin C-Y, Murillo JE et al (2015) Positive shrub–tree interactions facilitate woody encroachment in boreal peatlands. Journal of Ecology 103:58–66. https://doi.org/10.1111/1365-2745.12331
Ingerpuu N, Vellak K, Kukk T, Pärtel M (2001) Bryophyte and vascular plant species richness in boreo-nemoral moist forests and mires. Biodiversity & Conservation 10:2153–2166. https://doi.org/10.1023/A:1013141609742
Ireland AW, Booth RK (2012) Upland deforestation triggered an ecosystem state-shift in a kettle peatland. Journal of Ecology 100:586–596. https://doi.org/10.1111/j.1365-2745.2012.01961.x
Kapfer J, Grytnes J-A, Gunnarsson U, Birks HJB (2011) Fine-scale changes in vegetation composition in a boreal mire over 50 years. Journal of Ecology 99:1179–1189. https://doi.org/10.1111/j.1365-2745.2011.01847.x
Lachance D, Lavoie C, Desrochers A (2005) The impact of peatland afforestation on plant and bird diversity in southeastern Québec. Écoscience 12:161–171. https://doi.org/10.2980/i1195-6860-12-2-161.1
Laine J, Vasander H, Laiho R (1995) Long-term effects of water level drawdown on the vegetation of drained pine mires in southern Finland. Journal of Applied Ecology 32:785–802. https://doi.org/10.2307/2404818
Lapointe M (2014) Plantes de milieux humides et de bord de mer du Québec et des Maritimes. Michel Quintin, Montréal, Québec
Lee A, Fujita H, Kobayashi H (2017) Effects of drainage on open-water mire pools: open water shrinkage and vegetation change of pool plant communities. Wetlands 37:741–751. https://doi.org/10.1007/s13157-017-0907-3
Legendre P (2014) Interpreting the replacement and richness difference components of beta diversity. Global Ecology and Biogeography 23:1324–1334. https://doi.org/10.1111/geb.12207
Legendre P, De Cáceres M (2013) Beta diversity as the variance of community data: dissimilarity coefficients and partitioning. Ecology Letters 16:951–963. https://doi.org/10.1111/ele.12141
Lemmon PE (1956) A spherical densiometer for estimating forest overstory density. Forest Science 2:314–320. https://doi.org/10.1093/forestscience/2.4.314
Lenth RV (2016) Least-Squares Means: The R Package lsmeans. Journal of Statistical Software 69:1–33. https://doi.org/10.18637/jss.v069.i01
Lett MS, Knapp AK (2003) Consequences of shrub expansion in mesic grassland: Resource alterations and graminoid responses. Journal of Vegetation Science 14:487–496. https://doi.org/10.1111/j.1654-1103.2003.tb02175.x
Lieffers VJ, Rothwell RL (1986) Effects of depth of water table and substrate temperature on root and top growth of Picea mariana and Larix laricina seedlings. Canadian Journal of Forest Research 16:1201–1206. https://doi.org/10.1139/x86-214
Limpens J, Berendse F, Klees H (2003) N deposition affects N availability in interstitial water, growth of Sphagnum and invasion of vascular plants in bog vegetation. The New Phytologist 157:339–347. https://doi.org/10.1046/j.1469-8137.2003.00667.x
Lindborg R, Eriksson O (2004) Historical landscape connectivity affects present plant species diversity. Ecology 85:1840–1845. https://doi.org/10.1890/04-0367
Macdonald SE, Lieffers VJ (1990) Photosynthesis, water relations, and foliar nitrogen of Picea mariana and Larix laricina from drained and undrained peatlands. Canadian Journal of Forest Research 20:995–1000. https://doi.org/10.1139/x90-133
McKinney ML, Lockwood JL (1999) Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends in Ecology & Evolution 14:450–453. https://doi.org/10.1016/S0169-5347(99)01679-1
Middleton BA, Holsten B, van Diggelen R (2006) Biodiversity management of fens and fen meadows by grazing, cutting and burning. Applied Vegetation Science 9:307–316. https://doi.org/10.1111/j.1654-109X.2006.tb00680.x
Moore PD (2002) The future of cool temperate bogs. Environmental Conservation 29:3–20. https://doi.org/10.1017/S0376892902000024
New England Wild Flower Society (2017) Go Botany. Available at https://gobotany.newenglandwild.org/. Accessed 29 Sep 2017
Ohlson M, Økland RH, Nordbakken J-F, Dahlberg B (2001) Fatal interactions between Scots Pine and Sphagnum mosses in bog ecosystems. Oikos 94:425–432. https://doi.org/10.1034/j.1600-0706.2001.940305.x
Oksanen J, Blanchet FG, Friendly M et al (2017) vegan: Community Ecology Package. R package version 2:4–6 Available at https://CRAN.rproject.org/packag=broman
Olden JD, Poff NL (2003) Toward a mechanistic understanding and prediction of biotic homogenization. The American Naturalist 162:442–460. https://doi.org/10.1086/378212
Osland MJ, Enwright N, Day RH, Doyle TW (2013) Winter climate change and coastal wetland foundation species: salt marshes vs. mangrove forests in the southeastern United States. Global Change Biology 19:1482–1494. https://doi.org/10.1111/gcb.12126
Paradis É, Rochefort L (2017) Management of the margins in cutover bogs: ecological conditions and effects of afforestation. Wetlands Ecology and Management 25:177–190. https://doi.org/10.1007/s11273-016-9508-9
Pärtel M (2002) Local plant diversity patterns and evolutionary history at the regional scale. Ecology 83:2361–2366. https://doi.org/10.1890/00129658(2002)083[2361:LPDPAE]2.0.CO;2
Pasquet S, Pellerin S, Poulin M (2015) Three decades of vegetation changes in peatlands isolated in an agricultural landscape. Applied Vegetation Science 18:220–229. https://doi.org/10.1111/avsc.12142
Pellerin S, Lavoie C (2003a) Reconstructing the recent dynamics of mires using a multitechnique approach. Journal of Ecology 91:1008–1021. https://doi.org/10.1046/j.1365-2745.2003.00834.x
Pellerin S, Lavoie C (2003b) Recent expansion of jack pine in peatlands of southeastern Québec: A paleoecological study. Écoscience 10(2):247–257. https://doi.org/10.1080/11956860.2003.11682772
Pellerin S, Mercure M, Desaulniers AS, Lavoie C (2009) Changes in plant communities over three decades on two disturbed bogs in southeastern Québec. Applied Vegetation Science 12:107–118. https://doi.org/10.1111/j.1654-109X.2009.01008.x
Pellerin S, Lavoie M, Boucheny A, Larocque M, Garneau M (2016) Recent vegetation dynamics and hydrological changes in bogs located in an agricultural landscape. Wetlands 36:159–168. https://doi.org/10.1007/s13157-015-0726-3
Peringer A, Rosenthal G (2011) Establishment patterns in a secondary tree line ecotone. Ecological Modelling 222:3120–3131. https://doi.org/10.1016/j.ecolmodel.2011.05.025
Pickett STA (1989) Space-for-time substitution as an alternative to long-term studies. In: Likens GE (ed) Long-Term Studies in Ecology. Springer, New York, pp 110–135. https://doi.org/10.1007/978-1-4615-7358-6_5
Pollock MM, Naiman RJ, Hanley TA (1998) Plant species richness in riparian wetlands–a test of biodiversity theory. Ecology 79:94–105. https://doi.org/10.1890/0012-9658(1998)079[0094:PSRIRW]2.0.CO;2
Poulin M, Careau D, Rochefort L, Desrochers A (2002) From satellite imagery to peatland vegetation diversity: How reliable are habitat maps? Conservation Ecology 24(6):651–665. https://doi.org/10.5751/ES-00446-060216
Poulin M, Pellerin S, Cimon-Morin J et al (2016) Inefficacy of wetland legislation for conserving Quebec wetlands as revealed by mapping of recent disturbances. Wetlands Ecology and Management 24:651–665. https://doi.org/10.1007/s11273-016-9494-y
Price JN, Morgan JW (2008) Woody plant encroachment reduces species richness of herb-rich woodlands in southern Australia. Austral Ecology 33:278–289. https://doi.org/10.1111/j.1442-9993.2007.01815.x
Qian H, Guo Q (2010) Linking biotic homogenization to habitat type, invasiveness and growth form of naturalized alien plants in North America. Diversity and Distributions 16:119–125. https://doi.org/10.1111/j.1472-4642.2009.00627.x
R Core Team (2016) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria
Ratajczak Z, Nippert JB, Collins SL (2012) Woody encroachment decreases diversity across North American grasslands and savannas. Ecology 93:697–703. https://doi.org/10.1890/11-1199.1
Ratcliffe JL, Creevy A, Andersen R et al (2017) Ecological and environmental transition across the forested-to-open bog ecotone in a west Siberian peatland. Science of the Total Environment 607–608:816–828. https://doi.org/10.1016/j.scitotenv.2017.06.276
Ricklefs RE, Guo Q, Qian H (2008) Growth form and distribution of introduced plants in their native and non-native ranges in Eastern Asia and North America. Diversity & Distributions 14:381–386. https://doi.org/10.1111/j.1472-4642.2007.00457.x
Rooney TP, Olden JD, Leach MK, Rogers DA (2007) Biotic homogenization and conservation prioritization. Biological Conservation 134:447–450. https://doi.org/10.1016/j.biocon.2006.07.008
Saintilan N, Rogers K (2015) Woody plant encroachment of grasslands: a comparison of terrestrial and wetland settings. New Phytologist 205:1062–1070. https://doi.org/10.1111/nph.13147
Santamaría L (2002) Why are most aquatic plants widely distributed? Dispersal, clonal growth and small-scale heterogeneity in a stressful environment. Acta Oecologica 23:137–154. https://doi.org/10.1016/S1146-609X(02)01146-3
Sarkkola S, Hökkä H, Koivusalo H et al (2010) Role of tree stand evapotranspiration in maintaining satisfactory drainage conditions in drained peatlands. Canadian Journal of Forest Research 40:1485–1496. https://doi.org/10.1139/X10-084
Savage J, Vellend M (2015) Elevational shifts, biotic homogenization and time lags in vegetation change during 40 years of climate warming. Ecography 38:546–555. https://doi.org/10.1111/ecog.01131
Shirley LJ, Battaglia LL (2006) Assessing vegetation change in coastal landscapes of the northern Gulf of Mexico. Wetlands 26:1057–1070. https://doi.org/10.1672/0277-5212(2006)26[1057:AVCICL]2.0.CO;2
Siitonen J, Martikainen P, Punttila P, Rauh J (2000) Coarse woody debris and stand characteristics in mature managed and old-growth boreal mesic forests in southern Finland. Forest Ecology and Management 128:211–225. https://doi.org/10.1016/S0378-1127(99)00148-6
Statistics Canada (2010) St-Laurent Lowlands ecoregion. Available at http://www.statcan.gc.ca/pub/16-002-x/2010002/tbl/11285/tbl001-eng.htm. Accessed 20 Sep 2017
Talbot J, Richard PJH, Roulet NT, Booth RK (2010) Assessing long-term hydrological and ecological responses to drainage in a raised bog using paleoecology and a hydrosequence. Journal of Vegetation Science 21:143–156. https://doi.org/10.1111/j.1654-1103.2009.01128.x
Tilman D, May RM, Lehman CL, Nowak MA (1994) Habitat destruction and the extinction debt. Nature 371:65–66. https://doi.org/10.1038/371065a0
Tousignant M-Ê, Pellerin S, Brisson J (2010) The relative impact of human disturbances on the vegetation of a large wetland complex. Wetlands 30:333–344. https://doi.org/10.1007/s13157-010-0019-9
Turunen J, Roulet NT, Moore TR, Richard PJH (2004) Nitrogen deposition and increased carbon accumulation in ombrotrophic peatlands in eastern Canada. Global Biochemical Cycles. https://doi.org/10.1029/2003GB002154
USDA and NRCS (2018) The PLANTS Database. Available at https://www.plants.usda.gov. Accessed 23 Mar 2018
Van Auken OW (2000) Shrub invasions of North American semiarid grasslands. Annual Review of Ecology and Systematics 31:197–215. https://doi.org/10.1146/annurev.ecolsys.31.1.197
Vitt DH (2006) Functional characteristics and indicators of boreal peatlands. In: Wieder RK, Vitt DH (eds) Boreal Peatland Ecosystems. Springer, Berlin, pp 9–24. https://doi.org/10.1007/978-3-540-31913-9_2
Vitt DH, Li Y, Belland RJ (1995) Patterns of bryophyte diversity in peatlands of continental Western Canada. The Bryologist 98:218–227. https://doi.org/10.2307/3243306
Warner BG, Asada T (2006) Biological diversity of peatlands in Canada. Aquatic Sciences 68:240–253. https://doi.org/10.1007/s00027-006-0853-2
Warren RJ, Rossell IM, Moorhead KK, Dan Pittillo J (2007) The influence of woody encroachment upon herbaceous vegetation in a southern Appalachian wetland complex. The American Midland Naturalist 157:39–51. https://doi.org/10.1674/0003-0031(2007)157[39:TIOWEU]2.0.CO;2
Wertebach T-M, Hölzel N, Kleinebecker T (2014) Birch encroachment affects the base cation chemistry in a restored bog. Ecohydrology 7:1163–1171. https://doi.org/10.1002/eco.1447
Wheeler BD (1993) Botanical diversity in British mires. Biodiversity and Conservation 2:490–512. https://doi.org/10.1007/BF00056744
Wilson JB, Peet RK, Dengler J, Pärtel M (2012) Plant species richness: the world records. Journal of Vegetation Science 23:796–802. https://doi.org/10.1111/j.1654-1103.2012.01400.x
Woziwoda B, Kopeć D (2014) Afforestation or natural succession? Looking for the best way to manage abandoned cut-over peatlands for biodiversity conservation. Ecological Engineering 63:143–152. https://doi.org/10.1016/j.ecoleng.2012.12.106
Zedler JB, Kercher S (2004) Causes and consequences of invasive plants in wetlands: opportunities, opportunists, and outcomes. Critical Reviews in Plant Sciences 23:431–452. https://doi.org/10.1080/07352680490514673
Zoltai SC, Vitt DH (1995) Canadian wetlands: Environmental gradients and classification. Plant Ecology 118:131–137. https://doi.org/10.1007/BF00045195
Acknowledgements
This research received financial support from the Natural Sciences and Engineering Research Council of Canada (Discovery grant to S. Pellerin: RGPIN-2014-05367 and M. Poulin RGPIN-2014-05663) and the Québec Centre for Biodiversity Science. We are grateful to all landowners, including the Government of Québec, Nature Action and Transport Canada, who allowed us to conduct field sampling on their lands. Our thanks to numerous field assistants, P. Legendre and S. Daigle for statistical advices and K. Grislis for linguistic revision.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(DOCX 119 kb)
Rights and permissions
About this article
Cite this article
Favreau, M., Pellerin, S. & Poulin, M. Tree Encroachment Induces Biotic Differentiation in Sphagnum-Dominated Bogs. Wetlands 39, 841–852 (2019). https://doi.org/10.1007/s13157-018-1122-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-018-1122-6