Abstract
Neonicotinoid insecticides have been reported to occur widely in surface waters, including those of wetlands within the Prairie Pothole Region (PPR). In the US portion of the PPR, the US Fish and Wildlife Service has established Waterfowl Production Areas (WPAs) in an effort to enhance waterfowl production. Most WPAs have an area of protected upland surrounding wetlands that can act as a buffer to reduce the transport of contaminants, including pesticides. We assessed the extent that neonicotinoid insecticides occurred in the ponded water of wetlands within WPAs located along a gradient of agricultural influence throughout west-central Minnesota. Of the five neonicotinoids we tested for, two were not detected. However, at least one of the other three, imidacloprid, clothianidin and thiamethoxam, were detected in 29% of our wetland water samples. Additionally, both the occurrence and total concentrations of neonicotinoids were higher in sites with higher surrounding crop use. Neonicotinoid insecticides, if persistent for long periods of time, have the potential to affect aquatic-invertebrate communities within PPR wetlands. Our research indicates that areas often perceived as protected may still be at risk to neonicotinoid contamination, emphasizing the importance of maintaining effective grassland buffers around wetlands.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Anthropogenic stressors to aquatic environments, including inputs from agrochemicals, can have detrimental impacts to these important resources. Wetlands provide areas of high biodiversity and provide vital ecological functions, for example, through groundwater recharge and the provisioning of food resources and habitat for a wide range of fish and wildlife species (Houlahan et al. 2006; Erwin 2009). The increased reliance on chemical fertilizers and pesticides has contributed to a growing concern about potential environmental impacts, including effects on aquatic ecosystems, including wetlands (McLaughlin and Mineau 1995; Stehle and Schulz 2015). In addition to impacting aquatic insects directly, insecticides unintentionally introduced into wetlands could have larger impacts to the ecosystem, for example, through cascading effects throughout the food web and the disruption of ecological services (Donald et al. 1999).
The Prairie Pothole Region (PPR) encompasses an estimated 777,000 km2 area in North America, extending from central Alberta to central Iowa, and, in the United States alone, contains nearly 2.6 million hectares of wetlands (Dahl 2014). This ecologically important region is responsible for 40–60% of the duck production in North America and provides critical habitat for many other wetland dependent species (Guntenspergen et al. 2002). Over the last decade, this area has undergone a significant change in land-use practices, with many farms shifting towards large-scale operations, relying heavily on the use of insecticides to limit crop damage and improve agricultural yields (Meehan et al. 2011).
The increasing reliance on insecticides can be partially attributed to the introduction and rapid adoption of neonicotinoid insecticides. This class of insecticide is one of the most widely used globally and accounts for nearly 26% of the global insecticide market (Sparks 2013). First developed in the 1980s and brought to market in the early 1990s, neonicotinoids are now licensed for use in over 120 countries worldwide. Valued for their versatility and broad-spectrum toxicity, neonicotinoids are most commonly used as seed treatments, whose use has increased rapidly in the US and other areas of the world in recent decades (Cox Jr et al. 1998; Jeschke et al. 2011; Douglas and Tooker 2015). Common crops treated with neonicotinoids include corn (Zea mays L.), soybean (Glycine max L.), and wheat (Triticum spp.). Facilitated by the high water-solubility of neonicotinoids, the compounds are systemically taken up into plant tissues, providing protection to the young germinating plant (Simon-Delso et al. 2015). However, studies have shown that less than 20% of the active ingredient may be taken up by the plant, with the rest potentially persisting in surrounding soils (Sur and Stork 2003). With relatively long half-lives in soil that can exceed 1000 days and high water-solubility, there is potential for these compounds to persist in the environment and be transported to surrounding water bodies via groundwater, surface runoff or subsurface tile drainage (Goulson 2013; Van Dijk et al. 2013).
With concern for the persistence and potential impact to the environment, there has been increasing interest in examining the fate and distribution of neonicotinoids across the landscape. Recent studies have shown that aquatic systems situated in agricultural regions are susceptible to contamination by neonicotinoid insecticides (Anderson et al. 2013; Main et al. 2014; Hladik et al. 2014). Concentrations of these compounds have been found to occur in rivers, streams, lakes and wetlands receiving surface water from agricultural fields (Struger et al. 2017). Previous work from the prairies of Canada have shown that wetlands devoid of buffer vegetation are of higher probability to become exposed to concentrations of neonicotinoids than wetlands surrounded by perennial vegetation (Main et al. 2015). While wetlands located directly in agricultural fields within the PPR have been shown to contain neonicotinoids (Main et al. 2014; Evelsizer and Skopec 2016), it is unclear the level of contamination in more protected areas. The purpose of our study was to describe the distribution and concentration of five common neonicotinoids (imidacloprid, thiamethoxam, clothianidin, acetamiprid and thiacloprid) in wetlands found on Waterfowl Production Areas (WPAs) in west central Minnesota. WPAs managed by the U.S Fish and Wildlife Service (USFWS), provide important waterfowl brood-rearing habitat and serve as protection areas containing a mixture of grassland and wetland habitats. Our study provides additional insight into the fate and distribution of neonicotinoid compounds across the landscape and provides an indication of the water quality of wetlands located in wildlife areas such as WPA’s. Documenting the occurence of neonicotinoids in WPAs of the PPR will ultimately lead to an improved understanding of the fate and potential effects neonicotinoids may have on protected aquatic ecosystems in this and other regions. Such an improved understanding will allow natural resource managers, conservation groups as well as researchers to develop strategies and policies to improve wetland water quality and wildlife habitat in agriculturally intensive regions.
Methods
Study Area
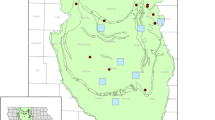
Our study was conducted within several counties located in the western portion of Minnesota’s PPR (Fig. 1). Sampled wetlands were located on WPAs managed by the USFWS Morris Wetland Management District, a unit of the National Wildlife Refuge System. To limit variation in landform geomorphology and precipitation, our study focused on wetlands located within the North Central Glaciated Plains ecological region (ECOMAP 1993).
Site Selection
In 2017, 40 wetlands within the Morris Wetland Management District that spanned a gradient of intensity of agricultural use were randomly selected and sampled for neonicotinoids. Ponded-water permanence was determined using vegetation indicators as described by Stewart and Kantrud (1971). Wetlands were selected from all seasonally ponded and semipermanently ponded wetlands in a WPA that were between 0.8 and 10 ha in size. In this region, corn and soybean are the dominant commercial crops, the production of which typically involves the use of neonicotinoid pesticides, primarily as seed treatments (Hladik et al. 2014; Douglas and Tooker 2015). To estimate a wetland’s susceptibility to potential neonicotinoid contamination, we compiled data on crop cover from 2012 to 2015 from the US Department of Agriculture (USDA) National Agricultural Statistics Service (NASS)‘s Cropland Data Layer (USDA 2012, 2013, 2014, 2015) and generated a 500-m buffer around each basin using GIS software (ArcGIS 10.4). Compiled land-use data were calculated through the Geospatial Modeling Environment (GME) which uses the open-source statistical software R and ArcGIS to sum the individual raster cells of each land-use type (Beyer 2015; R Core Team 2018). Output from the software provided estimates of the percent cropland within each 500-m buffered area, which was used to generate a low (<25%), moderate (25–75%) and high (>75%) rating of surrounding cropping intensity for each wetland.
Field Methods
Water samples were collected from each wetland on three separate occasions so that sample timing matched important agricultural activities taking place on the landscape. Since previous studies (Hladik et al. 2014; Main et al. 2014) have shown that neonicotinoid levels tend to be the highest during the early spring and summer months, our three sampling events were targeted to occur these times. Our first sampling took place in April, between snowmelt and planting activities to account for the potential runoff of neonicotinoids in snowmelt (Main et al. 2017). Following updates from the Minnesota Crop Progress and Condition report (USDA 2017), we conducted our second sampling event near the end of May when 94% of corn and 74% of the soybean crop had been successfully planted. Our final sampling event occurred during the early part of the growing season, in the month of June. Water samples were pesticide analyses were collected at ~10-cm water depth at each sampling location beyond emergent vegetation. Samples were stored at 4 °C in the field and subsequently frozen at the lab until analysis.
Chemical Analysis
Wetland water samples were analyzed for neonicotinoids at the Mississippi State Chemical Laboratory, Mississippi State University (Starkville, Mississippi) by liquid chromatography coupled with tandem mass spectrometry detection (LC/MS/MS). Quantifications were performed using external calibration standards using certified standard reference material. Samples were analyzed for five neonicotinoid compounds: imidacloprid, thiamethoxam, clothianidin, acetamiprid and thiacloprid. The detection limit for all neonicotinoids was 0.002 ppb. Means concentrations of each compound were calculated using data only from wetlands when compounds were detected, i.e., non-detections were not included.
Results
Chemical analyses of the collected water samples indicated widespread distribution of neonicotinoids within the wetland management district. Of the five neonicotinoid compounds we tested for, three (clothianidin, thiamethoxam, imidacloprid) were detected. Overall 50% (20/40) of the wetlands that were sampled for neonicotinoids tested positive for at least one compound throughout the multiple sampling events. Of the 120 wetland water samples collected and analyzed during our study, at least one compound was detected in 35 (29%). Of these 35, 12 (34%) contained two or all three neonicotinoid compounds. The mean total neonicotinoid concentration of samples with detectable concentrations was 14.7 ng/L and the maximum concentration was 60 ng/L (Table 1). Only clothianidin was present during each of the three sampling events while the other two were detected in only two of the sampling periods (Table 2). Overall, concentrations of the three detected compounds were relatively similar throughout the multiple sampling events (Fig. 2). Clothianidin was the most commonly occurring neonicotinoid in water samples (present in 24% of our samples) but had the lowest mean and maximum concentration (mean: 8.6 ng/L; maximum: 37 ng/L) compared to the other detected compounds, imidacloprid (mean: 13.1 ng/L; maximum 38 ng/L) and thiamethoxam (mean: 10.6 ng/L; maximum: 60 ng/L).
Sample timing and the amount of cultivated crop near each basin played a role in the number of detections and the concentration levels found within a water sample (Table 2). Data collected during the separate sampling events indicate the detection frequency of neonicotinoids was highest during our post planting survey event (Fig. 3.) Roughly 49% (17/35) of the positive detections were from this time, with the planting phase accounting for another 46% (16/35) of the positive detections. Pre-planting detections were very low, with a detection frequency of only 5% (2/35). Total neonicotinoid concentrations also followed a similar pattern. Peak concentrations of neonicotinoids were highest during the post-planting and planting phase with mean concentrations of 15.6 and 15.3 ng/L, respectively. The mean concentration of 2.0 ng/L found during the pre-planting phase was much lower.
Summary of (a) detection concentrations and (b) detection frequencies of total neonicotinoids in relation to planting activity and agricultural intensity from water samples collected from wetlands during the spring and summer of 2017. Low (<25%), moderate (25–75%) and high (>75%) categories represent cropped area within a 500-m buffer surrounding each wetland (based on crop cover data from 2012 to 2015). Shapes represent individual sites for which neonicotinoid compounds were detected
Land use within the buffer area also influenced the occurence and concentration of neonicotinoids in the samples. Areas classified with high cropping intensity had the greatest detection frequency with a 70 and 80% detection rate during the planting and post planting phases (Fig. 3b). These areas also were found to have our highest concentration levels with mean values of 15.9 and 17.8 ng/L, also in relation to the planting and post-planting survey events. Areas under moderate cropping intensity still had a number of detections both during the planting and post-planting phases, with a detection frequency of 40%, throughout both time periods. Mean concentrations during the planting and post-planting surveys were similar to the levels found within the high-intensity regions with mean concentrations of 16.5 and 15.1 ng/L. Overall, we identified a positive correlation between land use and total neonicotinoid concentrations within our study wetlands, rs = .39, p = 0.02 (Fig. 4).
Discussion
To our knowledge, this is the first study that specifically assessed the occurrence of neonicotinoid insecticides on federally managed and protected WPAs in west central Minnesota. Detected levels of neonicotinoids in rivers, streams and drainage ditch systems (Starner and Goh 2012; Hladik and Kolpin 2016; Struger et al. 2017), and in a series of studies focusing solely on surface waters of wetlands in Canada’s PPR (Main et al. 2014, 2015, 2016) have shown these insecticides to be frequently detected, but highly variable from region to region. While the majority of the research sites studied by Main et al. (2014, 2015, 2016) were located directly in agricultural fields, our research sites were located within WPAs, but our results indicate that wetlands found on protected habitats such as WPA’s are not immune to contamination by neonicotinoids. Overall 29% of our water samples had detectable levels of neonicotinoid pesticides. In addition, half of the wetlands tested positive for a least one compound throughout the three sampling events.
Although the sites sampled during our study spanned a gradient of influence from surrounding agricultural activities, the study locations were situated in protected areas, with at least a portion of their upland habitat consisting of uncultivated grassland vegetation. As our sites were less disturbed, consequently the neonicotinoid concentrations we report in this study are lower than previous reported values in agriculturally dominated wetlands within the PPR and other aquatic ecosystems that have been surveyed for neonicotinoids previously in central North America (Main et al. 2014; Hladik and Koplin 2016). Main et al. (2014) found concentrations of four neonicotinoid compounds in wetlands within cropped fields to have a mean concentration of 91.7 ng/L and a maximum concentration as high as 3110 ng/L, compared to our findings of an average and maximum total neonicotinoid concentration of 14.7 ng/L and 60 ng/L, respectively. Drained wetlands in the PPR of Iowa also showed detectable levels of neonicotinoids (Evelsizer and Skopec 2016) at levels exceeding both our study and the study conducted by Main et al. (2014). Since the wetlands studied by Evelsizer and Skopec (2016) were no longer functioning as intact wetlands and subject to traditional farming practices, it was not unexpected to find levels of concentrations an order of magnitude greater than values reported here. The difference between our results and those of others studying wetlands in crop fields suggest that catchments containing a significant portion of perennial cover surrounding the wetland may result in lower concentrations of pesticide contaminants. While our study area contains many WPAs spread across the region, a large majority of the area consists of field crop agriculture dominated by corn, soybean, wheat, and sugar beets. The intensity of agricultural use within the surrounding watershed can have a major influence on the occurrence of neonicotinoids, and, in comparison to our low-intensity sites, we observed high detections and concentration of neonicotinoids in areas receiving moderate (25–75%) to high (<75%) cropping intensity within our 500-m buffer distance to the wetland (Fig. 4). Other studies of wetlands have found similar results, with an increased presence of contaminants between buffered and non-buffered wetlands (Osborne and Kovacic 1993; Riens et al. 2013).
As observed in other studies of neonicotinoid distribution across North America (Hladik et al. 2014; Evelsizer and Skopec 2016), the three most common active ingredients; imidacloprid, clothianidin and thiamethoxam were the compounds detected during our survey. Transport of neonicotinoids into wetlands via snowmelt has been shown to be a major driver of detectable concentrations of active compounds in wetland surface waters prior to spring planting activities (Main et al. 2016). However, this was not particularity evident at our sites with only 2 of the 40 basins having a detectable concentration prior to planting. This may have been due to the early loss of snow from the landscape during the spring of 2017, while wetlands were still frozen, resulting in less transport of pesticides during spring thaw. As the ground was still frozen when the majority of snow melted, meltwater may not have percolated through soil and neonicotinoids present in the soil from surrounding agricultural activities may not have been readily transported into our wetlands during snowmelt. Our second and third samplings, which followed periods of precipitation, resulted in our highest observed detections and concentration of neonicotinoids. Precipitation events coinciding with planting activities during the spring and early summer has also been a common mechanism for the transport of neonicotinoids to nearby surface waters with previous studies observing a similar pattern (Hladik et al. 2014; Struger et al. 2017). Struger et al. (2017) found a positive correlation between active ingredients and the sampling day following rainfall events, highlighting the importance of sample timing in an effort to assess peak runoff events. Additional research efforts should be focused on developing a better understanding of how persistent these chemicals are, and to what extent they remain in wetlands over the growing season.
Agricultural drainage was evident at several of our survey sites, which could potentially help explain the transport of neonicotinoids onto some of the WPAs. While surface-water runoff can be a major driver, sub-surface tile drainage can also contribute to the delivery of neonicotinoids to nearby wetlands, especially if the drain outlet discharges directly into the wetland or nearby ditch leading to the wetland. Neonicotinoid use throughout the region is primarily in the form of seed treatments, and, when subjected to seasonal rains in the spring and early summer, compounds have been shown to directly move into tile systems providing a preferential flow of neonicotinoid contaminated water to nearby sites (Wettstein et al. 2016; Chrétien et al. 2017). Small streams and agricultural ditch systems can provide another exposure route of neonicotinoid contaminated water (Starner and Goh 2012; Struger et al. 2017). Many of these small systems, which can be primarily fed by agricultural runoff, can route water throughout an area and interconnect wetlands across the landscape. These streams and drains can empty or travel through WPAs subjecting organisms to repeated pulses of multiple active ingredients that can result in cumulative impacts on organisms (Maloney et al. 2017). Throughout our study, we observed several of our study sites containing mixtures of neonicotinoids, with 34% of our detected samples having at least two compounds.
In addition to the frequent occurrence of neonicotinoid compounds within protected WPAs, comparison of concentration data from our three survey periods to published aquatic benchmark values indicate that wetlands found on WPAs can contain concentrations that exceed the suggested chronic toxicity benchmark set for imidacloprid. The current benchmarks set by the U.S. Environmental Protection Agency are 385 and 10 ng/L (USEPA 2018) for acute and chronic toxicity with similar thresholds of 200 and 8.3 ng/L, respectively, set by the European Water Framework Directive (Smit et al. 2015). Additional research is needed to evaluate whether these pesticides are having significant impacts on these protected wetlands.
Neonicotinoids are thought to be linked to the declines of a variety of organisms, with much attention on bees and other native pollinator species (Krupke et al. 2012; Hallmann et al. 2014; Hladik et al. 2016). However, in wetlands and other surface waters experiencing contamination by neonicotinoids, non-target organisms such as aquatic insect species also have the potential to be exposed to both acute and chronic concentrations, affecting emergence success and sex ratios (Cavallaro et al. 2017). Though, it should be noted that many of the studies testing impacts to aquatic insects were conducted ex situ, with a limited amount of field studies measuring the chronic and acute impact in natural field conditions. The waterfowl species that use WPAs rely heavily on the availability of aquatic insects during times of breeding and migratory activities (Danell and Sjöberg 1977). Long-term exposure of neonicotinoid concentrations exceeding 35 ng/L have been shown to have chronic effects on aquatic invertebrate populations that are sensitive to pesticide contamination (Morrissey et al. 2015). While only 7 of the 40 wetlands we sampled where found to contain concentrations above this critical value, it does provide evidence that areas considered protected can still be impacted by the transport of neonicotinoids. Our research, as well as that of others (e.g., Main et al. 2015), suggests maintaining buffers of grassland habitat can be an effective way to reduce neonicotinoid concentrations in wetlands of the PPR, but the design and effectiveness of buffers may vary.
Conclusion
Our sampling of prairie-pothole wetlands throughout western Minnesota identified the presence of three of the most commonly used neonicotinoids in the region (USGS 2014). Out of the five active ingredients tested, clothianidin, imidacloprid and thiamethoxam were found to occur in sampled wetlands during the 2017 sampling season. The results found in this study corroborate other studies conducted in similar regions of North America, confirm the widespread distribution of these compounds within the environment (Starner and Goh 2012; Main et al. 2014; Hladik and Koplin 2016), and extend their identified distribution to areas often considered to be protected. As expected, land-use intensity was positively correlated with detected concentrations of these pesticides at our study sites, with higher concentrations of neonicotinoids found in areas with a higher percentage of the surrounding watershed used for crop production, suggesting that sites with large intact buffers may provide protection form agrichemicals such as neonicotinoids.
Not only did several of our wetlands exceed the guidelines for individual chronic pesticide thresholds, a number of our sites also tested positive for multiple active ingredients. However, little is known about the potential toxicity of mixtures of neonicotinoids on aquatic organisms, with recent research (Maloney et al. 2017) indicating that simple additivity is not adequate to determine toxicity. While we did not test for other commonly used agrochemicals, others have shown that chemicals such as fertilizers, herbicides and other insecticides can also be common in wetlands sounded by agricultural production (Riens et al. 2013; Evelsizer and Skopec 2016). Evaluating such effects to aquatic organisms can be a challenge to scientists. However, we suggest that further research continue to examine the fate of agrochemicals in prairie-wetland ecosystems and develop an understanding of their impacts to aquatic ecosystems.
References
Anderson TA, Salice CJ, Erickson RA, McMurry ST, Cox SB, Smith LM (2013) Effects of landuse and precipitation on pesticides and water quality in playa lakes of the southern high plains. Chemosphere 92:84–90. https://doi.org/10.1016/j.chemosphere.2013.02.054
Beyer H (2015) Geospatial modelling environment. Available via http://www.spatialecology.com/gme/index.htm. Accessed 15 Aug 2017
Cavallaro MC, Morrissey CA, Headley JV, Peru KM, Luber K (2017) Comparative chronic toxicity of imidacloprid, clothianidin, and thiamethoxam to Chironomus dilutus and estimation of toxic equivalency factors. Environmental Toxicology and Chemistry 36:372–382. https://doi.org/10.1002/etc.3536
Chrétien F, Giroux I, Thériault G, Gagnon P, Corriveau J (2017) Surface runoff and subsurface tile drain losses of neonicotinoids and companion herbicides at edge-of-field. Environmental Pollution 224:255–264. https://doi.org/10.1016/j.envpol.2017.02.002
Cox RR Jr, Hanson MA, Roy CC, Euluss NH, Johnson DH, Butler MG (1998) Mallard duckling growth and survival in relation to aquatic invertebrates. Journal of Wildlife Management 62:124–133. https://doi.org/10.2307/3802270
Dahl TE (2014) Status and trends of wetlands in the conterminous United States 2004 to 2009. US Department of the Interior, US Fish and Wildlife Service, Washington
Danell K, Sjöberg K (1977) Seasonal emergence of chironomids in relation to egglaying and hatching of ducks in a restored lake (northern Sweden). Wildfowl 28:129–135
Donald DB, Syrgiannis J, Hinter F, Weiss G (1999) Agricultural pesticides threaten the ecological integrity of norhtern prairie wetlands. Science of the Total Environment 231:173–181
Douglas MR, Tooker JF (2015) Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. field crops. Environmental Science & Technology 49:5088–5097. https://doi.org/10.1021/es506141g
ECOMAP (1993) National hierarchical framework of ecological units. USDA Forest Service, Washington, DC
Erwin KL (2009) Wetlands and global climate change: the role of wetland restoration in a changing world. Wetlands Ecology and Management 17:71–84. https://doi.org/10.1007/s11273-008-9119-1
Evelsizer V, Skopec M (2016) Pesticides, including neonicotinoids, in drained wetlands of Iowa’s prairie pothole region. Wetlands 38:221–232. https://doi.org/10.1007/s13157-016-0796-x
Goulson D (2013) REVIEW: an overview of the environmental risks posed by neonicotinoid insecticides. Journal of Applied Ecology 50:977–987. https://doi.org/10.1111/1365-2664.12111
Guntenspergen G, Peterson S, Leibowitz SG, Cowardin L (2002) Indicators of wetland condition for the prairie pothole region of the United States. Environmental Monitoring and Assessment 78:229–252
Hallmann CA, Foppen RPB, Turnhout CAMV et al (2014) Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511:341–343. https://doi.org/10.1038/nature13531
Hladik ML, Kolpin DW (2016) First national-scale reconnaissance of neonicotinoid insecticides in streams across the USA. Environment and Chemistry 13:12–20. https://doi.org/10.1071/en15061
Hladik ML, Kolpin DW, Kuivila KM (2014) Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environmental Pollution 193:189–196. https://doi.org/10.1016/j.envpol.2014.06.033
Hladik ML, Vandever M, Smalling KL (2016) Exposure of native bees foraging in an agricultural landscape to current-use pesticides. Science of the Total Environment 542:469–477. https://doi.org/10.1016/j.scitotenv.2015.10.077
Houlahan JE, Keddy PA, Makkay K, Findlay CS (2006) The effects of adjacent land use on wetland species richness and community composition. Wetlands 26:79–96. https://doi.org/10.1672/0277-5212(2006)26[79:teoalu]2.0.co;2
Jeschke P, Nauen R, Schindler M, Elbert A (2011) Overview of the status and global strategy for neonicotinoids. Journal of Agricultural and Food Chemistry 59:2897–2908. https://doi.org/10.1021/jf101303g
Krupke CH, Hunt GJ, Eitzer BD, Andino G, Given K (2012) Multiple routes of pesticide exposure for honey bees living near agricultural field. PLoS One 7(1):e29268s. https://doi.org/10.1371/journal.pone.0029268
Main AR, Headley JV, Peru KM, Michel NL, Cessna AJ, Morrissey CA (2014) Widespread use and frequent detection of neonicotinoid insecticides in wetlands of Canada’s Prairie Pothole Region. PLoS One 9(6):e101400. https://doi.org/10.1371/journal.pone.0092821
Main AR, Michel NL, Headley JV, Peru KM, Morrissey CA (2015) Ecological and landscape drivers of neonicotinoid insecticide detections and concentrations in Canada’s prairie wetlands. Environmental Science & Technology 49:8367–8376. https://doi.org/10.1021/acs.est.5b01287
Main AR, Michel NL, Cavallaro MC, Headley JV, Peru KM, Morrissey CA (2016) Snowmelt transport of neonicotinoid insecticides to Canadian prairie wetlands. Agriculture, Ecosystems and Environment 215:76–84. https://doi.org/10.1016/j.agee.2015.09.011
Main AR, Fehr J, Liber K, Headley JV, Peru KM, Morrissey CA (2017) Reduction of neonicotinoid insecticide residues in prairie wetlands by common wetland plants. Science of the Total Environment 579:1193–1202. https://doi.org/10.1016/j.scitotenv.2016.11.102
Maloney EM, Morrissey CA, Headley JV, Peru KM, Liber K (2017) Cumulative toxicity of neonicotinoid insecticide mixtures to Chironomus dilutus under acute exposure scenarios. Environmental Toxicology and Chemistry 36:3091–3101. https://doi.org/10.1002/etc.3878
Mclaughlin A, Mineau P (1995) The impact of agricultural practices on biodiversity. Agriculture, Ecosystems and Environment 55:201–212. https://doi.org/10.1016/0167-8809(95)00609-v
Meehan TD, Werling BP, Landis DA, Gratton C (2011) Agricultural landscape simplification and insecticide use in the Midwestern United States. Proceedings of the National Academy of Sciences 108:11500–11505. https://doi.org/10.1073/pnas.1100751108
Morrissey CA, Mineau P, Devries JH, Sanchez-Bayo F, Liess M, Cavallaro MC, Liber K (2015) Neonicotinoid contamination of global surface waters and associated risk to aquatic invertebrates: a review. Environment International 74:291–303. https://doi.org/10.1016/j.envint.2014.10.024
Osborne LL, Kovacic DA (1993) Riparian vegetated buffer strips in water-quality restoration and stream management. Freshwater Biology 29:243–258. https://doi.org/10.1111/j.1365-2427.1993.tb00761.x
R Core Team (2018) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/
Riens JR, Schwarz MS, Mustafa F, Hoback WW (2013) Aquatic macroinvertebrate communities and water quality at buffered and non-buffered wetland sites on federal waterfowl production areas in the Rainwater Basin, Nebraska. Wetlands 33:1025–1036. https://doi.org/10.1007/s13157-013-0460-7
Simon-Delso N, Amaral-Rogers V, Belzunces LP, Bonmatin JM, Chagnon M, Downs C, Furlan L, Gibbons DW, Giorio C, Girolami V, Goulson D, Kreutzweiser DP, Krupke CH, Liess M, Long E, McField M, Mineau P, Mitchell EAD, Morrissey CA, Noome DA, Pisa L, Settele J, Stark JD, Tapparo A, van Dyck H, van Praagh J, van der Sluijs JP, Whitehorn PR, Wiemers M (2015) Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environmental Science and Pollution Research 22:5–34. https://doi.org/10.1007/s11356-014-3470-y
Smit CE, Posthuma-Doodeman CJAM, Vlaardingen PLAV, Jong FMWD (2015) Ecotoxicity of imidacloprid to aquatic organisms: derivation of water quality standards for peak and long-term exposure. Human and Ecological Risk Assessment 21:1608–1630. https://doi.org/10.1080/10807039.2014.964071
Sparks TC (2013) Insecticide discovery: an evaluation and analysis. Pesticide Biochemistry and Physiology 107:8–17. https://doi.org/10.1016/j.pestbp.2013.05.012
Starner K, Goh KS (2012) Detections of the neonicotinoid insecticide imidacloprid in surface waters of three agricultural regions of California, USA, 2010–2011. Bulletin of Environmental Contamination and Toxicology 88:316–321. https://doi.org/10.1007/s00128-011-0515-5
Stehle S, Schulz R (2015) Agricultural insecticides threaten surface waters at the global scale. PNAS 112:5750–5755. https://doi.org/10.1073/pnas.1500232112
Stewart, RE, Kantrud HA (1971) Classification of natural ponds and lakes in the glaciated prairie region. Bureau of Sport Fisheries and Wildlife, Resource Publication 92. Washington
Struger J, Grabuski J, Cagampan S, Sverko E, McGoldrick D, Marvin CH (2017) Factors influencing the occurrence and distribution of neonicotinoid insecticides in surface waters of southern Ontario, Canada. Chemosphere 169:516–523. https://doi.org/10.1016/j.chemosphere.2016.11.036
Sur R, Stork A (2003) Uptake, translocation and metabolism of imidacloprid in plants. Bulletin of Insectology 56:35–40
USDA National Agricultural Statistics Service Cropland Data Layer (2012) Published crop-specific data layer. Available via https://nassgeodata.gmu.edu/CropScape/ Accessed May 01 2016
USDA National Agricultural Statistics Service Cropland Data Layer (2013) Published crop-specific data layer. Available via https://nassgeodata.gmu.edu/CropScape/ Accessed May 01 2016
USDA National Agricultural Statistics Service Cropland Data Layer (2014) Published crop-specific data layer. Available via https://nassgeodata.gmu.edu/CropScape/ Accessed May 01 2016
USDA National Agricultural Statistics Service Cropland Data Layer (2015) Published crop-specific data layer. Available via https://nassgeodata.gmu.edu/CropScape/ Accessed May 01 2016
USDA National Acricultural Statistics Service - Minnesota Crop Progress and Condition Report (2017) Published Crop Progress and Condition Reports. Available via https://www.nass.usda.gov/Statistics_by_State/Minnesota/Publications/Crop_Progress_&_Condition/index.php. Accessed April 01 - July 01 2017
USEPA (2018) Aquatic life benchmarks and ecological risk assessments for registered pesticides. U.S. Environmental Protection Agency. Available via https://www.epa.gov/pesticide-science-and-assessing-pesticide-risks/aquatic-life-benchmarks-and-ecological-risk. Accessed May 01 2018
USGS (2014) National water-quality qssessment (NAWQA) program annual P\pesticide use maps. Available via https://waterusgsgov/nawqa/pnsp/usage/maps/. Accessed Sept 15 2017
Van Dijk TC, Staalduinen MAV, Sluijs JPVD (2013) Macro-invertebrate decline in surface water polluted with imidacloprid. PLoS One 8(5):e62374. https://doi.org/10.1371/journal.pone.0062374
Wettstein FE, Kasteel R, Delgado MFG et al (2016) Leaching of the neonicotinoids thiamethoxam and imidacloprid from sugar beet seed dressings to subsurface tile drains. Journal of Agricultural and Food Chemistry 64:6407–6415. https://doi.org/10.1021/acs.jafc.6b02619
Acknowledgments
We thank Sara Vacek and Josh Eash for their assistance and technical support with sample collection for our study. Additionally, we thank the staff at the Morris Wetland Management district for the use of facilities and the hosting of sampling within their district. We would also like to thank Dawn Macdonald and Vince Capeder for their assistance in troubleshooting equipment while in the field. Funding for analyses of our wetland water samples were provided by the U.S. Fish and Wildlife Service. Findings and conclusions in this study are those of the authors and do not necessarily represent the views of the U.S. Fish and Wildlife Service.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, N., Sweetman, J. Distribution and Concentration of Neonicotinoid Insecticides on Waterfowl Production Areas in West Central Minnesota. Wetlands 39, 311–319 (2019). https://doi.org/10.1007/s13157-018-1090-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-018-1090-x