Abstract
Ecological facilitation (mutualism and commensalism) appears to be a strong force shaping biotic communities, and may be more likely in stressful and dynamic environments like wetlands. We examined a specific type of mutualism, ‘protective nesting associations,’ between herons and egrets (Ardeidae) and American alligators (Alligator mississippiensis). We predicted that wading birds would be attracted to sites with alligators. A survey of potential nesting sites in the Everglades showed strong nonrandom association, with wading birds never nesting without alligators. At previously unoccupied nesting colony sites, we experimentally manipulated apparent densities of alligators and birds using alligator and bird decoys. Small day-herons (little blue herons (Egretta caerulea), tricolored herons (Egretta tricolor), and snowy egrets (Egretta thula)) were significantly more numerous at sites with both alligator and bird decoys than other treatments. These findings together support the hypothesis that wading birds actively choose predator-protected nesting locations based in part on information from both conspecifics and alligators, and suggest that the mechanism supporting this habitat choice is primarily due to nest protection benefits the alligators inadvertently provide. We propose that this interaction is strong and could be geographically widespread, and suggest that it may be critical to shaping management and conservation of wetland function.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Positive ecological interactions (e.g. facilitation, mutualism, commensalism) have emerged as a strong force in structuring ecological communities (Stachowicz 2001; Bruno et al. 2003; Altieri et al. 2007; Silliman et al. 2011; van der Zee et al. 2016), and perhaps in driving evolutionary processes (Kikvidze and Callaway 2009; Kiers et al. 2010). Empirical and theoretical evidence suggests that ecological facilitation may be more common in stressful and dynamic environments like wetlands than in relatively more stable terrestrial or marine environments (Callaway 2007). While much of the facilitation literature has been dominated by examples of plant interactions (reviewed in: Brooker et al. 2008), there are a growing number of reports of positive interactions between animal species (Nummi and Hahtola 2008; Prugh and Brashares 2012; Moe et al. 2014; Harvey et al. 2016). Here, we provide evidence of positive ecological interactions among wetland birds and alligators, the latter of which may function as nest protectors.
Nest predation is generally a strong selective force in the evolution of avian nesting behavior and life history (Martin 1993; Ibáñez-Álamo et al. 2015). Protective nesting associations occur when one species places its nest by active choice near that of another, more formidable species that drives away predators of the first species simply by defending its own territory. Examples include red-backed shrikes (Lanius collurio) serving to drive off predators of barred warblers (Sylvia nisoria, Polak 2014), and territorial peregrine falcons (Falco peregrinus) nesting near seabirds serve to decrease predation by bald eagles (Haliaeetus leucocephalus, Hipfner et al. 2011). Most nest protector species fall into one of four categories: (1) powerful or aggressive birds nesting solitarily or in loose aggregations, (2) colonies of pugnacious birds, (3) crocodilians or (4) colonies of aggressive or annoying social insects (Haemig 2001a; Sergio et al. 2008). Descriptive studies of protective nesting associations are relatively common (Quinn and Ueta 2008), but most studies have not examined whether the association occurs by active choice and whether there is a benefit to the association (but see Morosinotto et al. 2012; Haemig 2001b). Crocodilians could be used by birds as nest protectors because crocodilians are known to prey on predators of bird nests such as snakes and mammals (Bondavalli and Ulanowicz 1999) and crocodilians sometimes guard their own nests (Kushlan and Kushlan 1980). American alligators (Alligator mississippiensis) have been anecdotally reported to make islands in South Carolina “predator-secure” for nesting boat-tailed grackles (Quiscalus major; Post and Seals 1991; Post 1998a), least bitterns (Ixobrychus exilis; Post 1998b), and common moorhens (Gallinula chloropus; Post and Seals 2000) by deterring mammalian predators such as raccoons (Procyon lotor). Robinson (1985) suggested that black caimans (Melanosuchus niger) and yellow-rumped caciques (Cacicus cela) may have a similar association. While these studies noted or implied an association, they provided no formal test of association. In Ghana, Hudgens (1997) found that blue-billed malimbes (Malimbus nitens) nested much closer to African dwarf crocodile (Osteolamus tetraspis) nest sites than would be expected from a random distribution. Further, the mechanisms by which the protectee may have recognized and aggregated to the protector have not been demonstrated.
Long-legged wading birds (herons, egrets, ibises, storks, spoonbills; Pelecaniformes and Ciconiiformes) nesting colonially near alligators or in alligator habitat in the southeastern United States of America present a good opportunity to study potential mutualism between a nest protector and a symbiont. Birds, mammals, and snakes commonly prey on wading bird nests and may be one of the most important factors affecting choice of nesting location (Frederick and Collopy 1989; Coulter and Bryan 1995; Tsai et al. 2016). Although long-legged wading birds are often colonial nesters, there is almost no group or individual nest protection behavior (Rodgers 1987). Mammalian predators that can climb trees can destroy many nests in a short period. Further, mammalian predators are often nocturnal and can pose a real threat to attending adults. Even a single night of intrusion by raccoons may lead to abandonment of the entire colony (Rodgers 1987; Kelly et al. 1993). This suggests that swamping (satiation) of mammalian nest predators through synchronous breeding is an unlikely benefit of coloniality for this group of birds.
Wading birds appear to avoid nest predation by selecting inaccessible nesting sites, such as islands surrounded by water (Frederick and Collopy 1989; Erwin et al. 1995; Tsai et al. 2016). However, nest sites in shallowly inundated wetlands are often accessible to nest predators such as raccoons that swim readily and move long distances in search of food in aquatic habitats. In areas outside the range of alligators, raccoons have been known to make open-water crossings of up to 950 m (Hartman and Eastman 1999), and readily move among widely separated offshore islands (200 m) to prey on nests and eggs of waterbirds (Ellis et al. 2007). Water depth in many wetlands is generally shallow (0–3 m) (Loveless 1959; Mitsch and Gosselink 1993) and vegetation within wetlands often provides resting substrate for swimming mammals. This suggests that expanses of open water alone are not likely to function as a deterrent to raccoon use of wetlands and island archipelagos.
The threat of predation by crocodilians could be a strong force deterring raccoons from moving about in wetlands (Jenni 1969; Post 1998a). This is supported by the observation that raccoons occur commonly as prey items of large (>1.8 m total length) alligators (Giles and Childs 1949; Barr 1997; Shoop and Ruckdeschel 1990; Rice 2004).
Conversely, alligators may be less successful at capturing prey in very shallow water and several studies have suggested that the nest success of long-legged wading birds declines when nest trees are no longer inundated, due to increased predation by mammals (Ruckdeschel and Shoop 1987; Frederick and Collopy 1989; Rodgers 1987; Post and Seals 1991; Kelly et al. 1993; Coulter and Bryan 1995). One interpretation of this evidence is that the movement behavior of semi-aquatic small mammals may be severely limited by the threat of predation by alligators. These hypothesized relationships suggest that presence of alligators should be an indicator of safer nesting conditions for long-legged wading birds.
From this information, we predicted that wading birds should be attracted to visual evidence of alligators when choosing colony sites. We tested this prediction in two ways. First, we measured the degree of association between alligators and nesting wading birds on a large sample of tree islands that constituted potential nesting habitat. Second, we experimentally increased the apparent density of alligators at potential colony islands using decoys. Wading birds often breed colonially (Crozier and Gawlik 2003; Heath and Frederick 2003) and this aggregative cue might also be an important part of nest site selection. For this reason, we also included wading bird decoys in our experimental treatments. Our prediction was that treatments involving alligator decoys (alligator alone, or alligator + wading bird decoys) would have a stronger response in both occupancy and numbers of nesting birds in the colony site than either birds alone or no decoys at all.
Methods
Study Area
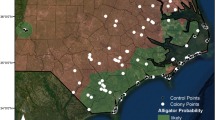
Study sites were located in Water Conservation Area-3A (WCA-3A) of Dade and Broward Counties, Florida (Fig. 1). WCA-3A is a large (cf 400 km2) impounded area of seasonally flooded (0–3 m depth) sawgrass (Cladium jamaicense) and wet prairie dotted with small tree islands. On slightly elevated tree islands dominated by willow (Salix caroliniana), little blue herons (Egretta caerulea), tricolored herons (Egretta tricolor), and snowy egrets (Egretta thula) (collectively hereafter, Egretta herons) tend to nest in aggregations of two to 200 individuals (Loveless 1959; Frederick 1995). Although there are numerous types of tree islands in the Everglades (Loveless 1959), Egretta herons nest almost exclusively on willow-dominated tree islands (Frederick and Collopy 1988, 1989) that are usually created by the action of alligators excavating and maintaining a small pond or depression (alligator “hole”) used for reproduction and refuge during low water levels (Mazzotti and Brandt 1994). Nesting is during the dry season (January through May).
For experimental manipulation, we selected 40 small willow-dominated tree islands within a 150 km2 section of marsh, all of which had an alligator hole. Islands ranged in size from 50 m to 200 m in their largest dimension, similar to sites used previously by nesting Egretta herons in WCA-3A (P. Frederick, unpublished database). We compared areas of selected islands with the Mann-Whitney U test. Based on systematic annual ground and aerial surveys, all sites used for experimental work had been previously unoccupied by nesting wading birds for at least 16 consecutive years (P. Frederick unpublished database).
Decoy Experiment Design
We manipulated apparent densities of alligators using decoys, with whole tree islands as the unit of treatment. Wading birds are known to be attracted to white bird decoys (Crozier and Gawlik 2003; Heath and Frederick 2003), and we included bird decoys in treatments to increase the likelihood that Egretta herons visited sites with and without the otherwise very cryptic alligator decoys. We used four different treatments: 10 alligator decoys, 18 bird decoys, 10 alligator plus 18 bird decoys, and no decoys. Decoy treatments were randomly assigned to each of 40 tree island sites. Nighttime spotlight surveys during this time indicated that all tree islands had live alligators associated with them. Rather than manipulating presence or absence of alligators, these treatments were therefore intended to create some islands with super-normal alligator stimulus.
Alligator decoys were cast using polyurethane spray insulation (Foam It Green®, Guardian Energy Technologies, Inc., Riverwoods, Illinois) from a silicone mold of a dead 2.3 m alligator. The mold was of the dorsal half of the alligator only, making the decoys appear as if they were floating at the surface of the water. The alligator decoys were larger than 1.8 m, the minimum size at which alligators begin to breed, defend territories, and be a significant predation threat to a raccoon (Giles and Childs 1949; Joanen and McNease 1980; Klause 1984; Shoop and Ruckdeschel 1990). Alligator decoys were painted in realistic colors using sprayed latex paint.
At each island with bird decoys, we presented three commercially available great egret decoys (Flambeau, Inc.®, Middlefield, Ohio) and 15 modified lawn flamingo decoys (Garden Plast, Inc., Accra, Ghana). The pink flamingo decoys were painted white with a pneumatic spray gun and flamingo heads were replaced by a polyurethane-cast piece similar to a small, white wading bird’s head and bill structure. Modified flamingo decoys have a proven ability to initially attract small wading birds to foraging areas (Crozier and Gawlik 2003).
Bird decoys were placed at mid-canopy height, approximately 1–2 m apart in willow trees surrounding the alligator hole at each treatment site in February 2011, 1–3 weeks prior to the initiation of breeding by Egretta herons. Alligator decoys were positioned in the water at the edge of the tree island. No-decoy treatment islands were entered and explored by us in the same fashion as other treatments.
Detecting Responses to Decoys
We used the maximum number of Egretta herons detected during any survey as an indicator of interest in the treatment by birds that might potentially nest. The use of counts of individual birds instead of nests is justified because 1) in small islands where all nests can be seen during the incubation phase, counts of birds and nests were nearly identical; 2) islands surveyed were very small (50 to 200 m in largest dimension), providing high confidence that all birds would be seen when disturbed by the approach of the boat (Frederick et al. 1996), 3) herons and egrets do not use colonies for feeding (Rodgers 1987) and 4) counting nests by walking through colonies can cause nest abandonment and alter the attractiveness of the colony (Tremblay and Ellison 1979). Juveniles (distinguishable by plumage) were only rarely sighted at colonies and were not included in counts.
During response surveys, we approached each site as closely as possible with an airboat. All flushed birds visible from the exterior of the island were noted and we kept our visits to less than 5 min. This method of counting individuals flushed by the airboat is the same method used in the previous 16 years by P. Frederick to survey these islands. Drought and low water conditions in 2011 limited accessibility by airboat to an increasing number of islands as the breeding season progressed. Surveys were conducted bi-weekly beginning 2 March and continued until 26 April, when only two of the 40 sites were accessible via airboat (N = 105 site visits). Two sites were surveyed all five times, nine sites were surveyed four times, twelve sites were surveyed three times, six sites were surveyed twice, and eleven sites were surveyed just once. Decoy presence and condition were examined opportunistically and all were confirmed to be present and in good condition when they were removed in July 2011.
On the final survey of all experimental sites (morning of 27 April 2011), we used a helicopter (Bell 206B JetRanger III) instead of airboats as the survey platform. The survey was timed to coincide with the point at which most heron/egret eggs had hatched, but no young had fledged. This increased our chances of at least one adult bird being present at the nest and therefore visible from the helicopter. The helicopter hovered at approximately 125 m above ground level after approaching the island from the east. From the left side of the aircraft, one observer counted, identified, and recorded all wading birds flushed from the island. The other observer took photographs using a Canon EOS 50D with a 28–135 mm image stabilizing lens. We believe that this method is comparable to ground survey counts by airboat in small wading bird detection ability.
Alligator-Wading Bird Spatial Overlap
We also examined the relationship of wading bird nesting with alligator presence by tallying both at a random sample of tree islands in WCA 3. These islands were independent of those islands that were manipulated, and the survey was done in 2015, four years after the manipulations. We used two observers in a helicopter (Bell JetRanger II hovering at 125 m to visually detect presence of adults, young, and fledglings) and evidence of recent alligator activity (alligator seen, or fresh alligator tracks/trail around the alligator hole) in 73 tree islands between 0900 and 1100 on 30 April and 1 May 2015.
Analysis
Decoy experiment: We used a two-tailed Chi-squared test of equal proportions to detect departures from an even distribution of the maximum number of birds nesting at each site, pooled by treatment group. Alpha was equal to 0.05. A Chi-squared test was used rather than other statistical tests because our data were discrete, non-normal and contained a large proportion of zeros. To further examine bird response, we performed post-hoc pairwise Chi-squared comparisons between all treatments. A Bonferroni adjustment was applied to maintain the family-wise error rate. We divided alpha (0.05) by the number of pairwise comparisons (6), to obtain the adjusted alpha, 0.0083.
Additionally, we compared colony occupancy rather than number of birds as a response variable to the experimental treatments using a Chi-squared test of equal proportions.
Alligator-bird association: We used a two-tailed Chi-squared contingency test to detect possible association between bird nesting and alligator presence.
Results
The experimental islands were typical of Egretta nesting sites, ranging from approximately 450 m2 to 5000 m2 (mean = 1200 m2, measured by ARCGIS, using satellite imagery). We found no significant pairwise differences in mean areas of colonies used in our four treatments (P > 0.10 in all cases, Mann-Whitney U test).
Bird Response to Decoys
The distribution of numbers of Egretta herons found in surveys relative to decoy treatment was significantly different from an even distribution (Chi square: Χ 2 3 = 72.45, P = 0.0001) (Fig. 2).
Pairwise comparisons (Table 1) showed the number of birds attracted to the alligator + bird treatment was higher than that of all other groups (P < 0.0001). There was no significant difference between numbers of birds attracted to the bird decoy treatment and the no-decoy treatment (Chi square: Χ 2 1 = 1.012, P = 0.31). Birds were less attracted to the alligator decoy treatment than the bird decoy treatment (Chi square: Χ 2 1 = 18.328, P < 0.0001).
The number of treatment islands of any type that birds were attracted to was not significantly different from an even distribution (Chi square: Χ 2 3 = 2.00, P = 0.57) (Fig. 3).
Of 73 tree islands surveyed in 2015, we detected alligators and birds together at 43 islands, alligators alone at 20 islands, birds alone at zero islands, and neither birds nor alligators at 6 islands (Chi square = 8.96, p = 0.028).
Discussion
In our aerial surveys, the association between alligators and nesting birds was highly nonrandom, and we found no instances in which birds nested without alligators present. Since there were appreciable numbers of tree islands that had alligators but not birds, the distribution of birds appeared to be uneven across alligator-occupied sites. Since the avian species we studied typically nest colonially, a clumped distribution is perhaps to be expected. This evidence suggests that wading birds actively avoid areas without alligators. We interpret the evidence from the decoy experiment to mean that wading birds were attracted to nesting colonies at least partly based on visual information about alligators, but only when visual evidence of other Egretta herons were present. Wading birds were not attracted to alligator decoys or wading bird decoys when either was presented alone. Similarly, Dusi (1985) attempted to attract little blue herons and cattle egrets (Bubulcus ibis) to a small island with homemade decoys and recordings of each species. Although seven different species of wading birds visited the island briefly, none nested during that 3-year study. This reinforces our finding that presence of bird decoys alone was not enough to cause birds to select the site for nesting. The combination of both decoy types appeared to be necessary to create an environment attractive enough for herons to initiate nesting in novel locations. This use of dual or multiple social information sources may be similar to the process by which other protective nesting associations between colonial species and nest protectors occur (Quinn et al. 2003; Hudgens 1997; Robinson 1985).
The alligator decoys alone could have been non-attractive to herons for several reasons. Herons may have simply not seen the alligator decoys, which were camouflaged to the point that it was sometimes difficult for humans to detect them at 2 m range. Without the long-range stimulus of the highly visible white bird decoys, it is possible that birds never visited the alligator-only islands and therefore the intended stimulus was not detected. Alternatively, birds may have chosen nesting sites based on presence information from both other wading birds and alligators. One plausible scenario is that birds were initially attracted by bird decoys to examine sites, and positive feedback was further stimulated by noticing alligator decoys at close range. Our experimental design did not allow us to distinguish between these hypothesized mechanisms.
We did not find a significant response to decoy treatments based on presence/absence of birds in colonies. In keeping with the survey information (above), Egretta herons are highly social, aggregative nesters. With a sample size of ten islands per treatment, our experiment may have had relatively low power to detect an effect of occupancy, due to the birds’ social nesting behavior. While occupancy would certainly offer the most convincing test of response, we believe that numerical responses also constitute one measure of the attraction that we had predicted.
This study suggests the existence of an apparent preference for nesting near alligators that may extend to other bird/ crocodilian associations. The association may arise from alligator attraction to birds, bird attraction to alligators, or both. The evidence presented here that herons were attracted by a combination of birds and alligators to novel nesting locations suggests that these highly mobile birds are probably using alligators as one of the cues of high quality nesting habitat. The evidence therefore suggests that birds are active choosers in this association. Outside of the range of alligators, raccoons and other semi-aquatic mammalian nest predators are known to move among widely separated offshore islands (200 m) surrounded by deep water to prey on nests and eggs of waterbirds (Ellis et al. 2007). Our results suggest that wading bird nests could therefore be more vulnerable to mammalian predation in parts of their range where alligators are not present. This results in the prediction that outside of the range of alligators, colonial birds should be more reliant on long distance from mainland, tall or difficult to climb trees, or other, non-crocodilian animals that prey on potential nest predators in their choices of nest sites.
The evidence presented here suggests that wading birds choose nesting locations in part based on social information from both other wading birds and alligators, affording a strong example of ecological facilitation between wetland animal species. We predict that the mechanism supporting this habitat choice is primarily due to the nest protection benefits that alligators inadvertently provide, and the nutritional boost that alligators receive from wading bird chick carcasses (Nell and Frederick 2016; Nell et al. 2016). High predation rates have been demonstrated when water is not present under nests and alligators cannot move effectively (Post and Seals 1991; Frederick and Collopy 1989; Burtner 2011). It is unclear what part of the recognition of interspecific presence information comes about through experiential learning, and what part may be genetically determined. In either case, we suggest that recognition of nest habitat quality through interspecific cues can be a productive way to understand habitat selection, and may have strong management and conservation implications in wetland habitats (Sergio et al. 2008).
While positive ecological interactions have been increasingly emphasized in community ecology (Bronstein 2009), the majority of known examples involve plants or other sessile, habitat-forming organisms like corals as one of the species (Cavieres and Badano 2009). Animal-animal interactions are less well understood, yet may be of special interest because mobile animals can choose where to settle, and can adjust behavior to maximize the positive aspects of interactions with another species. The alligator-bird interaction appears to be strongly positive for both species (Nell et al. 2016), in part because both species groups can use cues and are mobile enough to co-locate. It remains unclear, however, whether stressful conditions (e.g. food limitation in an extremely oligotrophic wetland like the Everglades) are necessary for the evolution of positive animal-animal interactions, and whether such interactions usually function to expand the realized niche in such situations (Crotty and Bertness 2015).
References
Altieri AH, Silliman BR, Bertness MD (2007) Hierarchical organization via a facilitation cascade in intertidal cordgrass bed communities. American Naturalist 169:195–206
Barr B (1997) Food habits of the American alligator, Alligator mississippiensis, in the southern Everglades. Dissertation, University of Miami
Bondavalli C, Ulanowicz R (1999) Unexpected effects of predators upon their prey: the case of the American alligator. Ecosystems 2:49–63
Bronstein JL (2009) The evolution of facilitation and mutualism. Journal of Ecology 97:1160–1170
Brooker RW, Maestre FT, Callaway RM, Lortie CL, Cavieres LA, Kunstler G, Liancourt P, Tielborger K, Travis JMJ, Anthelme F, Armas C, Coll L, Corcket E, Delzon S, Forey E, Kikvidze Z, Olofsson J, Pugnaire FI, Quiroz CL, Saccone P, Schiffers K, Seifan M, Touzard B, Michalet R (2008) Facilitation in plant communities: the past, the present, and the future. Journal of Ecology 96:18–34
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends in Ecology & Evolution 18:119–125
Burtner B (2011) Symbiosis between long-legged wading birds (Ciconiiformes) and alligators (Alligator mississippiensis)? Testing the 'Nest Protector Hypothesis.’ MS Thesis, University of Florida
Callaway RM (2007) Positive interactions and interdependence in plant communities. Springer, Dordrecht
Cavieres LA, Badano EI (2009) Do facilitative interactions increase species richness at the entire community level? Journal of Ecology 97:1181–1191
Coulter MC, Bryan AL (1995) Factors affecting reproductive success of wood storks (Mycteria americana) in east-central Georgia. Auk 112:237–243
Crotty SM, Bertness MD (2015) Positive interactions expand habitat use and the realized niches of sympatric species. Ecology 96:2575–2582
Crozier GE, Gawlik DE (2003) The use of decoys as a research tool for attracting wading birds. Journal of Field Ornithology 74:53–58
Dusi JL (1985) Use of sounds and decoys to attract herons to a colony site. Colonial Waterbirds 8:178–180
Ellis JC, Shulman MJ, Jessop H, Suomala R, Morris SR, Seng V, Wagner M, Mach K (2007) Impact of raccoons on breeding success in large colonies of great black-backed gulls and herring gulls. Waterbirds 30:375–383
Erwin RM, Hatfield JS, Wilmers TJ (1995) The value and vulnerability of small estuarine islands for conserving metapopulations of breeding waterbirds. Biological Conservation 71:187–191
Frederick PC (1995) Wading bird nesting success studies in the water conservation areas of the Everglades, 1992–1995. South Florida water Management District. West Palm Beach, Florida
Frederick PC, Collopy MW (1988) Reproductive ecology of wading birds in relation to water conditions in the Florida Everglades. Florida cooperative fish and Wildlife research institute unit, School for Research and Conservation, University of Florida. Technical report number 30
Frederick PC, Collopy MW (1989) The role of predation in determining reproductive success of colonially nesting wading birds in the Florida Everglades. Condor 91:860–867
Frederick PC, Towles T, Sawicki RJ, Bancroft GT (1996) Comparison of aerial and ground techniques for discovery and census of wading bird (Ciconiiformes) nesting colonies. Condor 98:837–841
Giles LW, Childs VL (1949) Alligator management of the Sabine National Wildlife Refuge. Journal of Wildlife Management 13:16–28
Haemig P (2001a) Symbiotic nesting of birds with formidable animals: a review with applications to biodiversity conservation. Biodiversity Conservation 10:527–540
Haemig P (2001b) Predation risk alters interactions among species: competition and facilitation between ants and nesting birds in a boreal forest. Ecology Letters 2:178–184
Hartman LH, Eastman DS (1999) Distribution of introduced raccoons Procyon lotor on the queen Charlotte Islands: implications for burrow-nesting seabirds. Biological Conservation 88:1–13
Harvey JA, Ode PJ, Malcicka M, Gols R (2016) Short-term seasonal habitat facilitation mediated by an insect herbivore. Basic and Applied Ecology 17:447–454
Heath JA, Frederick PC (2003) Trapping white ibises with rocket nets and mist nets in the Florida Everglades. Journal of Field Ornithology 74:187–192
Hipfner JM, Morrison KW, Darvill R (2011) Peregrine falcons enable two species of colonial seabirds to breed successfully by excluding other aerial predators. Waterbirds 34:82–88
Hudgens BR (1997) Nest predation avoidance by the blue-billed malimbe Malimbus nitens (Ploceinae). Ibis 139:692–694
Ibáñez-Álamo JD, Magrath RD, Oteyza JC, Chalfoun AD, Haff TM, Schmidt KA, Thomson RI, Martin TE (2015) Nest predation research: recent findings and future perspectives. Journal of Ornithology 156:247–262
Jenni DA (1969) A study of ecology of 4 species of herons during breeding season at Lake Alice Alachua County, Florida. Ecological Monographs 39:245–270
Joanen T, McNease L (1980) Reproductive biology of the American alligator in Southwest Louisiana. In: Murphy JB, Collins JT (eds) Reproductive biology and diseases of captive reptiles, 1st edn. Society for the Study of Amphibians and Reptiles, Oxford, pp 153–159
Kelly JP, Pratt HM, Greene PL (1993) The distribution, reproductive success, and habitat characteristics of heron and egret breeding colonies in the San Francisco Bay area. Colonial Waterbirds 16:18–27
Kiers ET, Palmer TM, Ives AR, Bruno JR, Bronstein JL (2010) Mutualisms in a changing world: an evolutionary perspective. Ecology Letters 13:1459–1474
Kikvidze Z, Callaway RM (2009) Ecological facilitation may drive major evolutionary transitions. BioScience 59:399–404
Klause S (1984) Reproduction characteristics of the American alligator in North Carolina. North Carolina State University, MS Thesis
Kushlan JA, Kushlan MS (1980) Function of nest attendance in the American alligator. Herpetologica 36:27–32
Loveless CM (1959) A study of the vegetation in the Florida Everglades. Ecology 40:1–9
Martin TE (1993) Nest predation and nest sites – new perspectives on old patterns. Bioscience 43:523–532
Mazzotti FJ, Brandt LA (1994) Ecology of the American alligator in a seasonally fluctuating environment. Everglades: the ecosystem and its restoration. In: Mitsch WJ, Gosselink JG (eds) Wetlands, 2nd edn. USA: Wiley pp 485-505
Mitsch WJ, Gosselink JG (1993) Wetlands, 2nd ed. John Wiley, New York
Moe SR, Rutina L, Hytteborn H, du Toit JT (2014) Impala as controllers of elephant-driven change within a savanna ecosystem. In: Skarpe C, du Toit JT, Moe SR (eds) Elephants and savanna woodland ecosystems: a study from Chobe National Park. Wiley, Botswana, pp 154–171
Morosinotto C, Thompson RL, Hanninen M, Korpimaki E (2012) Higher nest predation risk in association with a top predator: Mesopredator attraction? Oecologia 170:507–515
Nell LA, Frederick PC (2016) Fallen nestlings and regurgitant as mechanisms of nutrient transfer from nesting wading birds to crocodilians. Wetlands 25:723–732
Nell LA, Frederick PC, Mazotti FJ, Vliet KA, Brandt LA (2016) Presence of breeding birds improves body condition for a crocodilian nest protector. PLoS One 11:e0149572
Nummi P, Hahtola A (2008) The beaver as an ecosystem engineer facilitates teal breeding. Ecography 31:519–524
Polak M (2014) Protective nesting association between the barred warbler Sylvia nisoria and the red-backed shrike Lanius collurio: an experiment using artificial and natural nests. Ecological Research 29:949–957
Post W (1998b) Reproduction of least bitterns in a managed wetland. Colonial Waterbirds 21:268–273
Post W (1998a) Advantages of coloniality in female boat-tailed grackles. Wilson Bulletin 110:489-496
Post W, Seals CA (1991) Bird density and productivity in an impounded cattail marsh. Journal of Field Ornithology 62:195–199
Post W, Seals CA (2000) Breeding biology of the common moorhen in an impounded cattail marsh. Journal of Field Ornithology 71:437–442
Prugh LR, Brashares JS (2012) Partitioning the effects of an ecosystem engineer: kangaroo rats control community structure via multiple pathways. Journal of Animal Ecology 81:667–678
Quinn JL, Prop J, Kokorev Y, Black JM (2003) Predator protection or similar habitat selection in red-breasted goose nesting associations: extremes along a continuum. Animal Behaviour 65:297–307
Quinn JL, Ueta M (2008) Protective nesting associations in birds. Ibis 150:146–167
Rice AN (2004) Diet and condition of American alligators (Alligator mississippiensis) in three central Florida lakes. University of Florida, MS Thesis
Robinson SK (1985) Coloniality in the yellow-rumped cacique as a defense against nest predators. Auk 102:506–519
Rodgers JA (1987) On the antipredator advantages of coloniality – a word of caution. Wilson Bulletin 99:269–271
Ruckdeschel C, Shoop CR (1987) Aspects of wood stork nesting ecology on Cumberland Island Georgia, USA. Oriole 52:21–27
Sergio F, Caro T, Brown D, Clucas B, Hunter J, Ketchum J, McHugh K, Hiraldo F (2008) Top predators as conservation tools: ecological rationale, assumptions and efficacy. Annual Review of Ecology, Evolution and Systematics 39:1–19
Shoop CR, Ruckdeschel CA (1990) Alligators as predators on terrestrial mammals. American Midland Naturalist 124:407–412
Silliman BR, Bertness MD, Altieri AH, Griffin JN, Bazterrica MC, Hidalgo FJ, Crain CM, Reyna MV (2011) Whole-community facilitation regulates biodiversity on Patagonian rocky shores. PLoS One 6:e24502
Stachowicz JJ (2001) Mutualism, facilitation, and the structure of ecological communities. BioScience 51:235–246
Tremblay J, Ellison LN (1979) Effects of human disturbance on breeding of black-crowned night herons. Auk 96:364–369
Tsai J, Reichert BE, Frederick PC, Meyer KD (2016) Breeding site longevity and site characteristics have intrinsic value for predicting persistence of colonies of an endangered bird. Wetlands 36:639
van der Zee EM, Angelini C, Govers LL, Christianen MJA, Altieri AH, van der Reijden KJ, Silliman BR, van de Koppel J, van der Geest M, van Gils JA, van der Veer HW, Piersma T, de Ruiter PC, Olff H, van der Heide T (2016) How habitat-modifying organisms structure the food web of two coastal ecosystems. Proceedings of the Royal Society B: Biological Sciences 283(1826):20152326
Acknowledgements
This work was supported by the United States Army Corps of Engineers (W912HZ-20010) and a graduate assistantship from the University of Florida’s School of Natural Resources and the Environment. This work was performed under permit from the Animal Research Committee at University of Florida #001-09ABE. We thank F. Mazzotti for alligator ecology guidance. Statistical support was provided by R. Fletcher and the University of Florida IFAS Statistics Department. We thank J. Fidorra, E. Fishel, L. Garner, B. Jeffery, E. Posthumous, M. Schlothan, J. Simon, G. Smith, L. Venne, N. Vitale and C. Winchester for their support in the field. We also thank four anonymous reviewers and A. Maklakov for their helpful comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Burtner, B.F., Frederick, P.C. Attraction of Nesting Wading Birds to Alligators (Alligator mississippiensis). Testing the ‘Nest Protector’ Hypothesis. Wetlands 37, 697–704 (2017). https://doi.org/10.1007/s13157-017-0900-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-017-0900-x