Abstract
Monsoonal estuaries, located along the coastline of the Indian subcontinent, differ from other estuaries by their time dependence on the salinity characteristics. Effective sustenance and retention of the mesozooplankton community in the estuarine habitats is often determined by their dominant behavioral patterns: diel vertical migration (DVM) and tidal vertical migration (TVM). The modes of these endogenous rhythms often vary among estuaries based on the river runoff and tidal characteristics. The present study is a pioneering attempt to depict the vertical migration pattern of zooplankton along a diel and tidal scale in a tropical, microtidal, monsoonal estuary. We observed that in spite of the prominent asymmetry in the magnitude of the river runoff between the seasons, most of the zooplankton groups exhibited strong DVM, with a clear increase in biomass and abundance in surface waters during night. The peak increase in biomass and abundance at night always synchronized with the slack periods in the tidal cycles, which differed from the general concepts of downward migration during ebb tide and upward migration during flood tide in estuarine systems. The weak currents during the slack period might have favored the effective vertical migration of the mesozooplankton community in this monsoonal estuarine system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mesozooplankton have pivotal roles in estuaries as an intermediate link in the plankton food web and a major player in nutrient recycling (Madhupratap 1987; Sterner et al. 1992). They occupy a key position in the plankton food web by transferring organic carbon from phytoplankton and bacteria to higher trophic levels (Azam et al. 1983). Estuarine zooplankton are enabled with several adaptive mechanisms to survive in their environment, where they have to cope with downstream drift (Speirs and Gurney 2001) and large fluctuations in salinity (Madhupratap 1987) induced by tidal incursion and high river runoff. This is particularly relevant in monsoonal estuaries, where large variations in salinity occur in short spatial and temporal scales. The monsoonal estuaries separate themselves from other estuaries in the world by the unsteady state of the salinity characteristics influenced by the Indian summer monsoon (June – September) (Vijith et al. 2009). In these estuaries, the balance between the salinity ingress and egress experience time-dependent changes controlled by the variability in the tidal incursion and seasonality in river influx. These estuaries are characterized by total runoff that is many times higher than its volume and thus points out the chance of total wash-over during monsoon. This dynamicity in the estuarine features thus signifies the need to study the fate and the behavior of the mesozooplankton community inhabiting this estuarine system.
Vertical migration is a common behavioral pattern among zooplankton in freshwater, estuarine, and marine environments (Lampert 1989; Hays 2003). In shallow, poorly stratified estuaries, the most common pattern of zooplankton migration is the diel vertical migration (DVM), where most of the individuals remain near the bottom during the day and migrate to the surface waters during the night (Hutchinson 1967; Fancett and Kimmerer 1985). The well-accepted benefits of this behavior are the reduction of light-dependent mortality by predator avoidance (Zaret and Suffern 1976; Bollens and Frost 1991) and protection of damage from both visible and ultraviolet rays (Hairston 1976, 1979; Ringelberg et al. 1984; Williamson et al. 2011). Though DVM is a well-known migration behavior of zooplankton in estuaries (Hutchinson 1967; Haney 1988), the actual mechanisms that structure and sustain the process in monsoonal estuaries are mostly unknown.
Zooplankton have limited ability for horizontal movement, and therefore their survival in the estuarine environment depends on their adaptive strategies against tidal flows and turbulence (Wooldridge and Erasmus 1980; Kimmerer and Mckinnon 1987). In many macrotidal estuaries, tidal vertical migration (TVM) is an effective mechanism adopted by the estuarine plankton for retaining their favorable estuarine habitats (Wooldridge 1976; Kimmerer et al. 1998, 2002). The efficiency of retention in the estuarine environments varies in organisms, which basically depends on the swimming capability of different zooplankton and the magnitude of physical factors in the system (Hill 1991; Kimmerer et al. 2002). TVM is an endogenous rhythm of organisms (Cronin and Forward 1979) and has been widely reported in Copepoda (Kimmerer and McKinnon 1987) Mysidacea (Kimmerer et al. 1998), Decapod larvae (Forward and Cronin 1980), and several other zooplankton taxa (Laprise and Dodson 1989; Bennett et al. 2002). Thus, a comprehensive study on the TVM of zooplankton is a prerequisite for the better understanding of the vertical migration pattern of the zooplankton community of monsoonal estuaries characterized by the time-dependent unsteady state of salinity and large runoff (Vijith et al. 2009).
In monsoonal estuaries, there is a general lack of data on DVM of mesozooplankton with respect to the tidal rhythm. In Cochin Backwaters Wetland (CBW), where salinity is taken as the major limiting factor governing the planktonic standing stock and abundance (George 1958; Nair and Tranter 1971; Menon et al. 1971; Madhupratap 1987; Madhu et al. 2007), our aim was to study in detail the patterns of vertical migration and to find out whether the wide variation in the magnitude of river runoff among seasons affects the migration behaviors (both DVM and TVM). This study will also help to describe the fate of the zooplankton community in monsoonal estuaries in relation to the high seasonal runoff associated with the Indian summer monsoon. This study, by exhibiting high-resolution data on tidal and diurnal rhythms of the estuarine mesozooplankton community, will be relevant for the better understanding of the plankton dynamics that are active in tropical monsoonal estuaries.
Materials and Methods
Study Area
The CBW, a monsoonal estuary (Vijith et al. 2009) located between Lat. 9° 30′–10° 10′ N and Long.76° 15′–76° 25′ E, is the second largest wetland in India (Revichandran et al. 2012). It is connected to the Arabian Sea through two barmouths; one at Cochin (450 m) and the other at Azheekkodu (250 m) (Fig. 1). It forms the largest estuary (area 231 km2) along the west coast of India and experiences a regular intrusion of seawater from the Arabian Sea by tidal incursion. Seasonal hydrography of CBW is mainly influenced by monsoonal rainfall, as the annual precipitation in the Cochin area is around 320 cm, of which more than 70 % occurs during the summer monsoon period (June – September) (Qasim 2003). The northern limb of CBW receives runoff from two rivers (Periyar and Chalakudy) and the southern limb from five rivers (Muvattupuzha, Pamba, Manimala, Meenachil, and Achancoil)—thus, altogether an annual freshwater influx of 22,000 × 106 m3 (Revichandran et al. 2012). The asymmetry in the balance between the freshwater influx and tidal incursion brings about tidal and seasonal variations in the estuarine characteristics of CBW.
Sampling and Methods
Five sampling locations were selected along the salinity gradients in the CBW, covering a distance of ~ 60 km (Fig. 1). Locations 1 and 3 represented the barmouths in Azheekkodu and Cochin, respectively, location 2 represented the mid-point between the two barmouths, and locations 4 and 5 were in the southern upstream part of the estuary. Sampling of mesozooplankton and associated environmental factors was carried out at 3-h intervals for 24 h over both spring (when sun, moon, and earth come in a line) and neap phase (sun and moon are at right angles with earth), covering two seasons: dry season - pre-monsoon (February – March, 2010) and wet season - summer monsoon (September, 2009).
The SBE 26 plus SEAGAUGE wave and tide recorders were deployed in all locations to collect tidal information every 10 min. A current meter (Aanderaa RCM-9), deployed near the main barmouth (location 3) was used to measure the surface currents. Surface water temperature was measured using a bucket thermometer. Water samples were collected from the surface (0.5 m) using Niskin samplers for salinity, pH, dissolved oxygen (DO), and chlorophyll a. Salinity was measured using a digital salinometer (DIGI – AUTO, 3G Tsurumi seiki, Japan, accuracy ± 0.001) and pH was measured using a pH meter (ELICO LI610, accuracy ± 0.01). Estimation of DO was carried out following Winkler’s method (Grasshoff 1983). Chlorophyll a was measured flourimetrically by filtering phytoplankton present in 500 ml water on GF/F filter papers (pore size 0.7 μm), and its extraction occurred in 10 ml 90 % acetone (Strickland and Parsons 1972).
Zooplankton samples were collected from 5 locations using a WP net (mesh size 200 μm, mouth area 0.28 m2). The net was towed horizontally just below the surface (speed ~1 knot) for 10 min. A digital flow meter (Hydro Bios, Model- 438110) was attached across the mouth of the net to estimate the amount of water filtered. A total of 180 zooplankton samples (90 in each season) were collected accordingly, and the biomass was measured following the displacement volume method after removing large detrital particles (Hagen 2000). The samples were then preserved in 4 % formaldehyde solution (Harris et al. 2000) and stored for further analyses. Later, various zooplankton groups were sorted, identified, and counted for their abundance, expressed in individuals/cubic meter (ind. m−3).
Data Analysis
In order to understand the spatial and temporal variations in zooplankton biomass and abundance on a diel scale, two-way analysis of variance without replication was used. The analysis was carried out separately for spring and neap tides of both seasons. Variations in biotic and abiotic parameters due to tidal forcing over spring and neap tides were analyzed, adopting Wilcoxon matched pair test using Graph Pad Prism (version 5.01). This paired test was done as a nonparametric test, not assuming Gaussian distributions, with two-tailed P-values and 95 % confidence interval. A t-test was also carried out for the statistical analysis of abiotic and biotic parameters between pre-monsoon and summer monsoon to analyze the seasonal variation. To check the similarity among the sampling periods during day and night, a non-metric multi-dimensional scaling (NMDS) plot was created based on the fourth root transformed data of the abundance of different zooplankton taxa during the study and was done separately for both seasons. The discriminating zooplankton taxa between day and night periods were identified using the similarity percentages routine (SIMPER) implemented in PRIMER (Clarke and Gorley 2006). For this purpose, we considered all those taxa whose average dissimilarity was higher than its standard deviation, at least by a factor of one. For both NMDS plot and SIMPER analysis, the abundance values of 6 am (dawn) and 6 pm (dusk) were not considered since they cannot be properly categorized either as day or night. Principal component analysis (PCA) was carried out using the statistical program PAST, version 2.02 (Hammer et al. 2001), to understand the relationship between the biotic and abiotic variables. The biotic variables chlorophyll a, zooplankton biomass, and zooplankton abundance and the abiotic variables temperature, salinity, pH, DO, and tidal height were used for this purpose. For tidal height, the absolute value was used for both high tide and low tide phases. The mean temporal abundance of the major plankton groups during diel cycle and seasons were plotted using the same statistical program. Considering the total abundance of a particular taxon within a diel scale as 100 %, the percentage contribution of each taxon was plotted to see how its percentage abundance varied with time.
Results
Hydrography
The surface temperature profile exhibited a diel variation, with higher values during the day than night (Fig. 2a). During the pre-monsoon, the temperature in the spring phase was slightly higher (av. 32 ± 1.2 °C) than the neap phase (av. 31.5 ± 1.1 °C). During the summer monsoon, the temperature exhibited relatively less variation over both spring (av. 30.1 ± 1.1 °C) and neap phases (av. 29.8 ± 0.9 °C), and the difference was statistically insignificant (P > 0.05), whereas the temperature distribution between two seasons was found to be significantly different (P < 0.05) (Table 1).
Surface salinity in CBW showed spatial and temporal variations (Fig. 2b). The seasonal picture was a prominently high saline condition during the pre-monsoon (av. 20.5 ± 8.1 and 19.5 ± 7.8 psu in spring and neap phases, respectively) and low saline condition during the summer monsoon (av. 3.8 ± 6.2 and 1 ± 1 psu in spring and neap phases, respectively), and the difference in salinity between the seasons was statistically significant (P < 0.05). During the pre-monsoon, the inlet locations (1 and 3) had evidently higher salinity (>25 psu in spring phase and >20 psu in neap phase) compared to the locations upstream of the estuary (locations 2, 4, and 5), which had medium salinity (≥10 psu). There was no significant variation in salinity between spring and neap phases during the pre-monsoon, but the variation was significant during the summer monsoon (Table 1).
During the summer monsoon, as a result of heavy freshwater influx, a marked decline in salinity was well evident in the CBW (Fig. 2b). This salinity decline was pronounced, even in the barmouth region, as the salinity in these locations was considerably lower (av. 5.4 ± 5.3 psu in Cochin barmouth and 4.8 ± 7.3 psu in Azheekkodu barmouth) as compared to the pre-monsoon period (av. 20.5 ± 8.1 and 19.5 ± 7.8 psu in spring and neap phases, respectively). All other locations in the CBW behaved as a freshwater-dominant region during the summer monsoon with salinity < 2 psu (Fig. 2b). Locations 4 and 5 in the upstream of the CBW were completely occupied with freshwater, where the tidal influence was less (Fig. 2b).
The spatial variation of pH was found to be negligible in the CBW (Fig. 2c), and it did not show any significant variation over tidal phases during either season (P > 0.05). The average values were relatively higher during the pre-monsoon (av. 7.25 ± 0.25 and 7.3 ± 0.31 during spring and neap, respectively) than the summer monsoon (av. 7.21 ± 0.36 and 7.06 ± 0.31 during spring and neap, respectively), and these seasonal differences were found to be significant (Table 1). DO values showed insignificant (P > 0.05) variation between the pre-monsoon (av. 5 ± 1.1 and 5.6 ± 1.3 mg l−1 during spring and neap, respectively) and the summer monsoon (av. 5.4 ± 0.7 and 5.6 ± 1, spring and neap, respectively). Along the diel scale, a noticeable change in the DO value was observed in most of the locations (Fig. 2d).
Tides and Currents
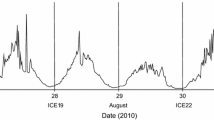
Mixed semidiurnal tides were predominant in the study area. But, during the spring phase of the pre-monsoon, semidiurnal tides were also found in a few locations (Figs. 3 and 4). The tidal amplitude progressively decreased, and the time lag in propagation of tide increased from the mouth to the head of the CBW. In the pre-monsoon, the tidal range in the barmouth was ~ 1 m in spring and ~ 0.8 m in neap, and the tidal activity was dominant throughout the study area (~0.6 m) (Fig. 3). During the summer monsoon, variation in tidal ranges were well marked in the barmouth region, with a prominently higher tidal height during the spring phase (~1 m) compared to the neap phase (~0.5 m) and this spring-neap variability was in agreement with the earlier observation in the CBW (Qasim and Gopinathan 1969). The current meter data from the barmouth showed a marked variation in the current speed during different tidal phases (Fig. 5). The highest current speed was during the peak ebb and flood tide periods, and the lowest was during the slack period between ebb and flood tide which was similar to the earlier observation in the CBW (Srinivas et al. 2003).
Phytoplankton Biomass (Chlorophyll a)
The concentration of chlorophyll a in the CBW was found to be >2 mg m−3 throughout the observation, with noticeable temporal and spatial variations (Fig. 2e). The highest chlorophyll a from the entire observations was recorded at location 2. A daytime increase in chlorophyll a was also evident in most of the locations (Fig. 2e). During the pre-monsoon, chlorophyll a was higher in the spring phase (av. 8.2 ± 6.2 mg m−3) compared to the neap phase (av. 5.9 ± 3.3 mg m−3), and these changes were significantly different (P < 0.05). It was vice versa during the monsoon period, with higher chlorophyll a in the neap phase (av. 7.4 ± 5.6 mg m−3) compared to spring phase (av. 6.6 ± 6.3 mg m−3), but the variation due to the tidal influence was insignificant (Table 1). Similarly, the seasonal influence in the chlorophyll a value was also found to be insignificant (P > 0.05).
Mesozooplankton Biomass and Abundance
The biomass and abundance values were generally high during the pre-monsoon (Biomass: av. 0.17 ± 0.19 and 0.22 ± 0.33 ml m−3; Abundance: av. 8625 ± 12,257 and 11,412 ± 19,465 ind. m−3 during spring and neap phase, respectively), with significant spatial variation (Table 2), and relatively high values were observed in the upstream region (Fig. 6). With the onset of the summer monsoon, there was a drastic drop in biomass and abundance throughout the study area, with a prominent decline in the upstream locations (Biomass: av. 0.06 ± 0.09 and 0.02 ± 0.05 ml m−3; Abundance av. 980 ± 1515 and 418 ± 990 ind. m−3 during spring and neap phase, respectively), and this seasonal variation in biomass and abundance values was found to be significant (P < 0.05). Mesozooplankton biomass and abundance always showed a clear indication of DVM in all locations, irrespective of tidal phases and seasons (Figs. 3 and 4). Though the occurrence of peak biomass and abundance accumulation in the surface waters varied spatially and seasonally, the highest values were observed consistently at night time during both seasons. Based on the abundance of different zooplankton taxa along the diel scale, the two dimensional plot of NMDS (stress 0.01) exhibited two distinct groups of the day and night time sampling periods during both seasons (Fig. 7). The zooplankton biomass and abundance accumulation in the surface waters were found to be either during the high water slack or low water slack during night (Figs. 3 and 4). In a few locations, a secondary peak in zooplankton biomass was also observed during night, corresponding to another slack period. The only contradiction to the pattern above was at the upstream most location (station 5) during the flood tide of the summer monsoonal spring phase (Figs. 4 and 5). During the premonsoon period, the biomass and abundance values did not show significant variation between two tidal phases (P > 0.05); during the summer monsoon, the variation was significant (Table 1). The results of the spatio-temporal analysis of biomass and abundance within the diel scale revealed a significant variation in both spatial and temporal scale at both tidal phases during the premonsoon (Table 2). During the summer monsoon, the temporal variation in abundance was significant during both tidal phases, whereas the spatial variation was significant only during the spring phase (Table 2).
The results of the PCA of the biotic and abiotic variables (excluding the diurnal factor) added insight towards the interrelation existing between them. During the summer monsoon, biomass and abundance values were found to be positively related to salinity and tidal height (Fig. 8). As the tidal height reaches the maximum values during the tidal slack period, the association of higher biomass and abundance with the higher tidal height validates the surface accumulation of zooplankton biomass and abundance during the slack period. However, during the pre-monsoon, these two biotic parameters did not show any close relation with any abiotic and biotic parameters (Fig. 8).
Mesozooplankton Distribution
The mesozooplankton community was composed of 20 taxonomic groups, of which 13 belonged to holoplankton and seven to meroplankton (Table 3). Among the holoplankton, Copepoda, Cladocera, Luciferidae, and Mysidacea were the major contributors to the total abundance. Based on abundance, Copepoda was the most dominant group during both the pre-monsoon (98 % in spring 90 % in neap) and summer monsoon periods (77 % in spring and 94 % in neap). During both seasons, a clear diel variation was noticed in their abundance, with higher abundance in surface waters during night time (Fig. 9). Luciferidae was present during both seasons, but its abundance peaked during the pre-monsoon period (0.3 and 0.1 %, spring and neap phase, respectively). On a diel scale, except for summer monsoon-neap, the abundance of Luciferidae was relatively higher during the night. Similarly, Mysidacea was also present during both seasons, with little seasonal preference (Table 3), and showed a clear diel pattern with higher abundance in surface waters during the night (Fig. 9). Cladocera were found only during the summer monsoon period in the lower estuary. Contrasting Cladocera, gelatinous zooplankton (Hydromedusae, Ctenophora, and Thaliacea) was present only during the pre-monsoon, and their abundance was clearly high during night. Several other holoplankton (Polychaeta, Isopoda and Amphipoda) also exhibited a clear diel pattern in abundance, with higher peaks in the surface waters during night (not shown in Figure).
Meroplankton generally showed a prominent diel pattern, with high abundance in the surface waters during night. They consisted of Decapod larvae, Gastropod larvae, and Fish larvae, of which Decapod larvae contributed to 1.5 % (during summer monsoonal spring) to 9.2 % (during pre-monsoonal neap) of the total zooplankton abundance. Both Decapod and Gastropod larvae showed a prominent DVM, whereas it was not clear in Fish larvae (Fig. 9). Though Fish larvae were recorded in all sampling locations, they were concentrated more towards the upstream locations, especially during the pre-monsoon period.
The result of the SIMPER analysis further helped to identify the discriminating taxa between the day and night samplings (Table 4). During pre-monsoon eleven taxa of total eighteen taxonomic groups and during monsoon ten out of total 15 taxa displayed prominent variation in their abundance between day and night period (Table 4).
Discussion
During the summer monsoon period, there is a high peak in freshwater influx into the monsoonal estuary (Vijith et al. 2009). Due to heavy rainfall and associated river runoff during the monsoon, a major part of the CBW was transformed into freshwater zones. Revichandran et al. (2012) have demonstrated that nearly 60 % of the total annual rainfall occurs during the summer monsoon period and that 60 to 70 % of the annual river runoff occurs during this period. This contention is significant when considering the recent study on the flushing rate of the CBW. Shivaprasad et al. (2012) have shown that the flushing time of the CBW is minimum during the summer monsoon period (3 days) compared to the pre-monsoon period (13–14 days). The monsoonal estuarine feature was clearly evident in the present study, also, as the salinity values in locations within the estuary were found to be < 2 psu during the wet summer monsoon. On the other hand, the high saline environment due to the low river influx and the resultant high marine water incursion during the dry pre-monsoon (av. 17.3 ± 10.4 psu in spring and av. 16.5 ± 9.9 psu in neap) supports the time-dependent unsteadiness of salinity of the monsoonal estuary. The seasonal variation in temperature might be due to its tropical nature, whereas in case of pH (Table 1), it might be due to the differences with respect to river influx and saline water intrusion (Saraladevi et al. 1983).
The mesozooplankton community in the CBW exhibits strong seasonal patterns, with prominently high biomass and abundance during the high-saline pre-monsoon period (Madhupratap 1987). During the summer monsoon, due to heavy rainfall and associated freshwater influx, the salinity in the CBW declines drastically (Haridas et al. 1973). During this time, the interaction between freshwater influx and tidal action creates significant variation in salinity distribution (Ramamirtham and Jayaraman 1963), especially in the downstream regions. This was the reason for the prominent difference in salinity values over the spring and neap phases during the summer monsoon (Table 1). The higher biomass and abundance values of mesozooplankton during the summer monsoon were contributed to by the barmouth locations (Fig. 4). Both biomass and abundance of mesozooplankton were more closely coupled with salinity than other variables (temperature, DO, pH, and chlorophyll a), signifying the control of salinity on biotic parameters during the summer monsoon (Fig. 8).
During the pre-monsoon period, low freshwater influx and high amount of saline water incursion from the Arabian Sea caused an overall increase in salinity throughout the CBW. As a result, temporal variations in salinity during both spring and neap tidal phases were minor and insignificant during the pre-monsoon period (Table 1). This minor change in salinity might be one factor responsible for the insignificant difference in zooplankton biomass and abundance between the tidal phases during this period. The relatively lower spatial variation in salinity distribution during this period might have a role in the inconspicuous relation between salinity and the biotic components (zooplankton biomass and abundance) (Fig. 8).
The interesting feature observed during the present study was the prominent night time increase of biomass and abundance in the surface waters during both the pre-monsoon and monsoon, in spite of the wide variation of the magnitude of the river influx and flushing rate between these periods. The distinct groups of the day and night sampling periods observed during both season clearly indicated the variability in their abundance along the diel scale (Fig. 7). The prominent dissimilarity in the day and night abundance of several zooplankton taxa evidenced through the SIMPER analysis further supports the diel variability of the mesozooplankton community. The evident DVM among the zooplankton community was similar to the observation in many temperate estuaries characterized with relatively less variation in seasonal salinity (Sweatt and Forward 1985; Cuker and Watson 2002).
The euphotic column in the CBW is very shallow and varies from a few centimeters to a few meters from the monsoon to pre-monsoon periods (Qasim 2003). Thus, the vertical migration might provide many advantages to zooplankton, of which the most important is the avoidance of mortality imposed by visually oriented predators, such as fishes (Bollens and Frost 1991; Loose and Dawidowicz 1994), and protection from ultraviolet rays (Williamson et al. 2011). These positive advantages might have taken important roles in the maintenance of their vertical migration behavior in the diurnal scale, irrespective of the vast changes in hydrographic conditions between the pre-monsoon and monsoon seasons.
The second important feature observed during the present study was the synchronization of peak zooplankton biomass accumulation in the surface waters, along with the tidal slack period during night during both seasons. It is also evident that the slack period varied temporally in the CBW, but irrespective of such changes, high zooplankton biomass had coincided with the tidal slack (Figs. 3, 4 and 8). During the premonsoon, apparently the biplot of the PCA showed no association of tidal height with zooplankton biomass and abundance. This might be due to the higher biomass and abundance in the southern upstream part (Fig. 6), where tidal influence was relatively less (Fig. 3). Excluding these locations, the output of the biplot (not shown in figure) was much similar to that observed during the monsoon period. As the ingress and egress current velocities are generally higher in the surface water during flood and ebb tide (Wooldridge and Erasmus 1980; Ueda et al. 2010) and decrease with depth mainly due to bottom friction (Hill 1991), the estuarine zooplankton might have avoided the surface waters during both flood and ebb tide and might have congregated in the surface waters during the tidal slack period, when the current velocity reaches minimum (Fig. 5). This is in contrast to the observations in macro tidal estuaries, where the zooplankton migrate to deeper layers during ebb tide and to surface waters during flood tide to escape from getting flushed out to the marine system (Laprise and Dodson 1989; Ueda et al. 2010). Regarding estuarine zooplankton, as it is difficult to recognize the flood and ebb phases separately—as in both cases, water flow is accompanied by the changes within the water column brought about by flow induced turbulence—the indigenous zooplankton in this micro tidal estuary (~1 m) might have adapted to retain their position by avoiding the higher velocity flow in surface waters associated with flood and ebb phases of the tidal cycle. This behavioral pattern of the estuarine zooplankton might provide ecological advantages against the challenge of getting washed out from their preferable estuarine environment. Though this was the general trend in vertical migration of zooplankton in the CBW, this feature was absent in the most upstream location (station 5) during the summer monsoon, which can be explained by the position-dependent vertical migration behavior of estuarine zooplankton. TVM varies according to the position of inhabitance of zooplankton in the estuary (Ueda et al. 2010), as the retention efficiency should be higher in organisms inhabiting the seaward side than those in the upstream locations of the estuary. Station 5 had the maximum distance from the inlet station and thus experienced the lowest tidal range (<0.4 m). Hence, the indigenous estuarine fauna was capable of overcoming the low tidal effect in this region and thus maintained higher population during tidal flow.
Since the tidal slack periods in the CBW usually exist for 2–3 h, we verified whether this time period is sufficient for zooplankton to carry out their vertical migration. In the CBW, the abundant zooplankton taxa that exhibited upward migration during night included Copepoda, Luciferidae, Mysidacae, and Decapod larvae (Fig. 9). Copepods have the smallest size among these groups, with less developed locomotory appendages compared to other groups. Since Copepods represented 77 to 98 % of the total zooplankton community in the CBW, they contributed significantly to the vertical migration rhythm observed in the system. Though the swimming speed of a Copepod varies during different life stages, an adult Copepod can migrate 20–30 m h−1 (Hattori 1989). With an approximately 20 m h−1 swimming speed, an adult Copepod can move 5 m distance in 15 min. Considering the shallowness of the CBW, it seems to be logical that the zooplankton can effectively utilize the time period during the tidal slack period to carry out effective vertical migration.
Conclusion
The present study is a pioneering attempt addressing the vertical migration pattern of the zooplankton community in a tropical monsoonal estuarine system. Though the Indian summer monsoon and associated river runoff play a crucial role in the seasonal variability in zooplankton biomass and abundance distribution, the vertical migration behavior (both DVM and TVM) among the zooplankton community remained similar in both dry (pre-monsoon) and wet (monsoon) periods. The indigenous mesozooplankton community exhibited a clear DVM pattern, with higher biomass and abundance in surface waters during night in both seasons. They followed a tidally timed DVM, with peak ascension to the surface waters during the slack period of the tidal cycle at night, and it was prominent irrespective of seasons. This result differs from the observations in macro tidal temperate estuaries, where zooplankton are mostly reported to migrate towards the lower layer on ebb tide and the upper layer on flood tide. The observed TVM helped them to avoid surface currents during the flood and ebb tide and also to retain their position in the estuarine habitat. Thus, the present study depicting the detailed vertical migration patterns of different zooplankton taxa in response to the hydrography and tidal rhythms of this estuarine system will be relevant for a better understanding of the adaptive strategies adopted by the monsoonal estuarine zooplankton community for their effective retention and survival in their preferable estuarine habitats.
References
Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F (1983) The ecological role of water-column microbes in the Sea. Marine Ecology Progress Series 10:257–263
Bennett WA, Kimmerer WJ, Burau JR (2002) Plasticity in vertical migration by native and exotic estuarine fishes in a dynamic low-salinity zone. Limnology and Oceanography 47:1496–1507
Bollens SM, Frost B (1991) Diel vertical migration in zooplankton: rapid individual response to predators. Journal of Plankton Research 1:1359–1365
Clarke KR, Gorley RN (2006) PRIMER v6: user manual/tutorial. PRIMER-E Ltd, Plymouth, p 91
Cronin TW, Forward RB (1979) Tidal vertical migration: an endogenous rhythm in estuarine crab larvae. Science 205:1020–1022
Cuker BE, Watson MA (2002) Diel vertical migration of zooplankton in contrasting habitats of the Chesapeake Bay. Estuaries 25:296–307
Fancett MS, Kimmerer WJ (1985) Vertical migration of the demersal copepod Pseudodiaptomus as a means of predator avoidance. Journal of Experimental Marine Biology and Ecology 88:31–43
Forward RB, Cronin TW (1980) Tidal rhythms of activity and phototaxis of an estuarine crab larva. The Biological Bulletin 158:295–303
George MJ (1958) Observations on the plankton of the Cochin backwaters. Indian Journal of Fisheries 5:375–401
Grasshoff K (1983) Determination of oxygen. In: Grasshoff K, Ehrhardt M, Kremling K (eds) Methods of sea water analysis. Verlag Chemie, Weinheim, pp 61–72
Hagen W (2000) Biovolume and biomass determinations. In: Harris R, Wiebe P, Lenz J, Skjoldal HR, Huntley ME (eds) ICES zooplankton methodology manual. Academic, London, pp 87–147
Hairston NG (1976) Photoprotection by carotenoid pigments in the copepod Diaptomus nevadensis. Proceedings of the National Academy of Sciences 73:971–974
Hairston NG (1979) Adaptive significance of color polymorphism in 2 species of Diaptomus (Copepoda). Limnology and Oceanography 24:15–37
Hammer Ø, Harper DAT, Ryan PD (2001) PAST: paleontological statistics software package for education and data analysis
Haney JF (1988) Diel patterns of zooplankton behavior. Bulletin of Marine Science 43:583–603
Haridas P, Madhupratap M, Rao TSS (1973) Salinity, temperature, oxygen & zooplankton biomass of the backwaters from Cochin to Alleppey. Indian Journal of Marine Sciences 2:94–102
Harris R, Wiebe P, Lenz J, Skjoldal HR, Huntley ME (eds) (2000) ICES zooplankton methodology manual. Academic, London
Hattori H (1989) Bimodal vertical distribution and diel migration of the copepods Metridia pacifica, M. okhotensis and Pleuromamma scutullata in the western North Pacific Ocean. Marine Biology 103:39–50
Hays GC (2003) A review of the adaptive significance and ecosystem consequences of zooplankton diel vertical migrations. Hydrobiologia 503:163–170
Hill AE (1991) Vertical migration in tidal currents. Marine Ecology Progress Series 75:39–54
Hutchinson GE (1967) A Treatise on limnology: Vol. 2. Introduction to lake biology and the limnoplankton. Wiley, New York
Kimmerer WJ, McKinnon AD (1987) Zooplankton in a marine bay. II. Vertical migration to maintain horizontal distributions. Marine Ecology Progress Series 41:53–60
Kimmerer WJ, Burau JR, Bennett WA (1998) Tidally oriented vertical migration and position maintenance of zooplankton in a temperate estuary. Limnology and Oceanography 43:1697–1709
Kimmerer WJ, Burau JR, Bennett WA (2002) Persistence of tidally oriented vertical migration by zooplankton in a temperate estuary. Estuaries 25:359–371
Lampert W (1989) The adaptive significance of diel vertical migration of zooplankton. Functional Ecology 3:21–27
Laprise R, Dodson JJ (1989) Ontogeny and importance of tidal vertical migrations in the retention of larval smelt Osmerus mordax in a well mixed estuary. Marine Ecology Progress Series 55:101–111
Loose CJ, Dawidowicz P (1994) Trade-offs in diel vertical migration by zooplankton: the costs of predator avoidance. Ecology 75:2255–2263
Madhu NV, Jyothibabu R, Balachandran KK, Honey UK, Martin GD, Vijay JG, Shiyas CA, Gupta GVM, Achuthankutty CT (2007) Monsoonal impact on planktonic standing stock and abundance in a tropical estuary (Cochin Backwaters— India). Estuarine Coastal and Shelf Science 73:54–64
Madhupratap M (1987) Status and strategy of zooplankton of tropical Indian estuaries: a review. Bulletin of the Plankton Society of Japan 34:65–81
Menon NR, Venugopal P, Goswami SC (1971) Total biomass and faunistic composition of the zooplankton in the Cochin backwater. Journal of the Marine Biological Association of India 13:220–225
Nair KKC, Tranter DJ (1971) Zooplankton distribution along salinity gradient in the Cochin Backwater before and after the monsoon. Journal of the Marine Biological Association of India 13:203–210
Qasim SZ (2003) Indian estuaries. Allied Publication, Mumbai
Qasim SZ, Gopinathan CK (1969) Tidal cycle and the environmental features of Cochin backwater (a tropical estuary). Proceedings of the Indian Academy of Sciences 69:336–348
Ramamirtham CP, Jayaraman R (1963) Some aspects of the hydrographical conditions of the backwaters around Willington island (Cochin). Journal of the Marine Biological Association of India 5:170–177
Revichandran C, Srinivas K, Muraleedharan KR, Rafeeq M, Amaravayal S, Vijayakumar K, Jayalakshmi KV (2012) Environmental set-up and tidal propagation in a tropical estuary with dual connection to the sea (SW coast of India). Environmental Earth Sciences 66:1031–1042. doi:10.1007/s12665-011-1309-0
Ringelberg J, Keyser AL, Flik BJG (1984) The mortality effect of ultraviolet radiation in a translucent and in a red morph of Acanthodiaptomus denticornis (Crustacea, Copepoda) and its possible ecological relevance. Hydrobiologia 112:217–222
Saraladevi K, Venugopal P, Remani KN, Zacharias D, Unnithan RV (1983) Nutrients in some estuaries of Kerala. Mahasagar 16:161–173
Shivaprasad A, Vinita J, Revichandran C, Manoj NT, Srinivas K, Reny PD, Ashwini R, Muraleedharan KR (2012) Influence of saltwater barrage on tides, salinity, and Chlorophyll a in Cochin Estuary, India. Journal of Coastal Research. doi:10.2112/JCOASTRES-D-12-00067
Speirs DC, Gurney WSC (2001) Population persistence in rivers and estuaries. Ecology 82:1219–1237
Srinivas K, Revichandran C, Thottam TJ, Maheswaran PA, Asharaf TTM, Murukesh N (2003) Currents in the Cochin estuarine system (southwest coast of India) during March 2000. Indian Journal of Marine Sciences 32:123–132
Sterner RW, Elser JJ, Hessen DO (1992) Stoichiometric relationships among producers, consumers and nutrient cycling in pelagic ecosystems. Biogeochemistry 17:49–67
Strickland JDH, Parsons TR (1972) In: a practical handbook of seawater analysis. Bulletin of Fisheries Research Board, Canada
Sweatt AJ, Forward RB (1985) Diel vertical migration and photoresponses of the chaetognath Sagitta hispida Conant. The Biological Bulletin 168:18–31
Ueda H, Kuwatani M, Suzuki KW (2010) Tidal vertical migration of two estuarine copepods: naupliar migration and position-dependent migration. Journal of Plankton Research 32:1557–1572
Vijith V, Sundar D, Shetye SR (2009) Time-dependence of salinity in monsoonal estuaries. Estuarine Coastal and Shelf Science 85:601–608
Williamson CE, Fischer JM, Bollens SM, Overholt EP, Breckenridge JK (2011) Toward a more comprehensive theory of zooplankton diel vertical migration: Integrating ultraviolet radiation and water transparency into the biotic paradigm. Limnology and Oceanography 56:1603–1623
Wooldridge T (1976) The zooplankton of msikaba estuary. Zoologica Africana 11:23–44
Wooldridge T, Erasmus T (1980) Utilization of tidal currents by estuarine zooplankton. Estuarine and Coastal Marine Science 11:107–114
Zaret TM, Suffern JS (1976) Vertical migration in zooplankton as a predator avoidance mechanism. Limnology and Oceanography 21:804–813
Acknowledgments
The authors thank Director, CSIR-National Institute of Oceanography, India for facilities and encouragement. We thank all NIO staff who helped in carrying out the simultaneous time series measurements in Cochin backwaters. The authors thank ICMAM-PD Chennai for the financial support. This work forms a part of the Supra Institutional Project (SIP) 1302 of NIO and Grant-in-Aid project ‘Ecosystem modeling of Cochin backwaters’ funded by ICMAM – PD Chennai. The first author is thankful to CSIR for the award of Senior Research Fellowship. This is NIO contribution number 5725.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vineetha, G., Jyothibabu, R., Madhu, N.V. et al. Tidal Influence on the Diel Vertical Migration Pattern of Zooplankton in a Tropical Monsoonal Estuary. Wetlands 35, 597–610 (2015). https://doi.org/10.1007/s13157-015-0650-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-015-0650-6