Abstract
Denitrification in tidal freshwater river channels and their adjoining freshwater wetlands greatly affects nitrogen export from river networks, yet the relative importance of these two habitats to nitrogen export has not been examined. Knowledge of how these habitats contribute to denitrification of the river nitrogen load is critical for improving models of nitrogen transport. Denitrification rates were measured in sediments from the channel, bank, and floodplain at upstream and downstream sites of two forested tidal freshwater zones (TFZs) in North Carolina, the New River and Newport River, using membrane inlet mass spectrometry to measure N2 production. Denitrification rates did not usually differ statistically between the channel, bank, and floodplain, although denitrification was highest on the floodplain at the upstream site in the Newport River. When these rates were extrapolated across the entire area of the TFZ, the channel contributed more to the N2 flux than the riparian zone. These results indicate that denitrification rates are comparable between the channel and riparian zone in forested TFZs, and that the importance of the channel versus the riparian zone depends on channel and floodplain morphology.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen loading to the biosphere has approximately doubled in recent decades due to human activities, which has caused increased nitrogen loading from rivers to estuaries (Nixon 1995, Vitousek et al. 1997, Howarth et al. 2002). Since nitrogen usually limits estuarine primary production (Howarth 1988), excess nitrogen loading can cause eutrophication and subsequently fish kills, benthic infauna mortality, and harmful algal blooms (Nixon 1995, Paerl et al. 1998, Howarth et al. 2002, Smayda 1990, Rabalais and Turner 2001). Rivers and their floodplains play a critical role in the delivery of nitrogen from watersheds to estuaries by removing nitrogen through burial of nitrogen via sediment accretion and two anaerobic microbial processes which permanently remove nitrogen from rivers: denitrification, the conversion of nitrate to N2, and anaerobic ammonium oxidation (the conversion of nitrite and ammonium to N2). Denitrification is thought to dominate N2 production in river and estuarine environments (Burgin and Hamilton 2007, Rich et al. 2008, Nicholls and Trimmer 2009), and removes a substantial portion of the nitrogen load (Whalen et al. 2008, Mulholland et al. 2009).
Denitrification occurs in both a river's channel and floodplain wetlands. Floodplains can provide a larger area over which denitrification can occur compared with the river channel, and can be more organic rich due to higher productivity on floodplains, and the scouring of fine sediments in channels (Johnston et al. 1997, Ross et al. 2004), although denitrification rates in floodplains may be lower due to oxic conditions, and lower nitrate concentration when hydrologic exchange with the channel is low (Baldwin and Mitchell 2000, Baker and Vervier 2004, Forshay and Stanley 2005, Racchetti et al. 2011). While inundation of river floodplains occurs seasonally in most rivers, inundation occurs on a daily basis in the tidal freshwater zone (TFZ) of rivers (Day et al. 2007). The high frequency of tidal inundation of freshwater riparian wetlands, combined with their continual hydrologic exchange with the channel, may enhance their capacity to remove nitrogen via denitrification (Ensign et al. 2013b). For example, Ensign et al. (2008) found that denitrification in the riparian zone of a small coastal watershed accounted for an average of 38 % of the total N2 flux from the watershed. In addition, denitrification in tidal freshwater wetlands may be enhanced by higher organic matter (OM) than non-tidal wetlands (Odum 1988, Hill and Cardaci 2004), the lack of sulfide which can inhibit denitrification in saline wetlands (Rysgaard et al. 1999, Joye and Hollibaugh 1995), and enhanced coupling between nitrification (the conversion of ammonium to nitrate) and denitrification due to fluctuating redox conditions (Reddy and Patrick 1976, Reddy and Patrick 1984, Groffman and Tiedje 1988, Courtwright and Findlay 2011, Noe et al. 2013). The position of the TFZ immediately upstream from the estuary where coastal eutrophication is a concern due to typically N-limited conditions, and intense urban and agricultural development (Howarth 1988, Paerl 2006), combined with the biogeochemical factors which may enhance denitrification in the TFZ relative to other aquatic systems, makes it a particularly important ecosystem to the overall nitrogen budget of estuaries.
Because denitrification in the TFZ can have a major influence on estuarine nitrogen budgets, it is important to develop accurate models of TFZ denitrification. Most river nitrogen cycling models do not include explicit channel-floodplain linkages (Boyer et al. 2006, Groffman et al. 2009), which are critical to the TFZ due to the daily riparian zone inundation. Reduction of a river's nitrogen load via denitrification in the TFZ is a function of spatial and temporal variability in denitrification rate (mass area−1 time−1) combined with the area of channel and floodplain that are inundated and the duration of inundation and lag time to reducing conditions on the floodplain. The product of these two factors yields the total mass of nitrogen removed from downstream flow through the TFZ of a river when integrated over time. The high temporal variability in water level in tidal freshwater floodplains combined with the spatial variation in floodplain topography results in large spatio-temporal variation in inundated area within the tidal freshwater reach. When extrapolating denitrification rates across the tidal freshwater floodplain, accounting for this variation in inundated area is as important as accounting for temporal variation in the rate of denitrification (Ensign et al. 2013b).
One important gap in modeling denitrification in the TFZ is determining the relative contributions of denitrification in the channel versus the floodplain. Previous studies in the upper Mississippi River and in tidal freshwater marshes have suggested that the rate of denitrification is lower in the channel than the riparian zone (Richardson et al. 2004, Greene 2005, Hopfensperger et al. 2009). Such lateral differences in denitrification might be expected due to higher organic matter content on the floodplain (Johnston et al. 1997, Ross et al. 2004), but studies which have concurrently measured rates of denitrification in channel sediments and the adjacent riparian zone are rare, and are nonexistent in the case of tidal freshwater forested wetlands. Knowledge of the lateral differences in the denitrification rate in the TFZ is particularly important for development of landscape-scale models of nitrogen flux which incorporate the spatial and temporal dynamics of tidal inundation.
To explore lateral differences in denitrification, this study compared denitrification rates between the channel, bank, and floodplain in two forested TFZs. The riparian zone was separated into the bank and floodplain because TFZ rivers are often fringed by levees, and consequently banks can consist of coarser, more organic-poor sediments than floodplains (Anderson and Lockaby 2007). Denitrification measurements from the channel and riparian zone were combined with estimates of inundated floodplain area over a hypothetical tidal wave to compare the relative importance of the channel and riparian zone to the N2 flux due to denitrification in the TFZ. A second objective was to determine if spatial patterns in denitrification varied between upstream and downstream sites in the TFZ, or between tidal freshwater rivers with different nitrate concentrations. Controlling factors of denitrification (OM and nitrate) were measured to explain spatial patterns in denitrification. In addition, sediment oxygen demand (SOD) was measured because it is an indicator of denitrification.
Materials and Methods
Site Selection
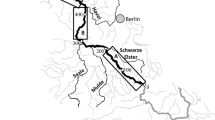
The forested TFZs of two coastal rivers in Eastern North Carolina were selected for denitrification measurements: the New River and the Newport River (Fig. 1). Although denitrification rates have been studied extensively in tidal freshwater emergent marshes (Greene 2005, Neubauer et al. 2005, Gribsholt et al. 2006, Hopfensperger et al. 2009), they have received less attention in forested TFZs (except see Verhoeven et al. 2001, Ensign et al. 2008) which comprise the majority of the tidal freshwater wetlands in the southeastern U.S. (Field et al. 1991). The New and Newport Rivers have watershed areas of 243 km2 and 310 km2, and TFZs 12 km and 8 km long, respectively (Ensign et al. 2008, Ensign et al. 2013b). An upstream site, and a downstream site were selected in the New River at 34° 50.944'N, 77° 31.157'W and 34° 47.655'N, 77° 28.104'W, and in the Newport River at 34° 47.274'N, 76° 53.097'W and 34° 46.845'N, 76° 52.338'W. These sites fell within the range of the TFZ reported in previous studies (Ensign et al. 2012, Ensign et al. 2013b). The upstream site in the Newport River had a tidal range of approximately 0.5 m (Ensign et al. 2013a). The tidal range at the upstream site in the New River was between approximately 0.3-0.6 m during the fall and summer sampling (USGS gauge 02093000, New River at Gum Branch), although the tidal signal was not apparent during the winter and spring. At each site a bank and floodplain site were selected approximately 1.5 m and 15 m from the low tide channel, respectively. In the New River, steep banks flanked the upstream site and no floodplain was present.
Denitrification Measurements
Sediment cores 12 cm in length, 6.4 cm in diameter, were collected from each river in triplicate from the channel, bank, and floodplain at the upstream and downstream sites. Sampling was carried out on 1 Sept 2010, 11 Jan 2011, 23 March 2011, and 7 June 2011 in the New River, and on 7 Sept 2010, 25 Jan 2011, 14 March 2011, and 13 June 2011 in the Newport River (fall, winter, spring, and summer in each river, respectively). Cores were collected from the channel using a check valve corer which kept the overlying water column intact (Gardner et al. 2009). A water quality sonde (Yellow Springs Instruments) was used to measure the salinity and temperature at each site, and carboys of surface water were collected from the river channel at the upstream and downstream sites.
The cores and carboys of water were returned to the Institute of Marine Sciences, Morehead City, NC, and rates of denitrification were measured using membrane inlet mass spectrometry (MIMS, Kana et al. 1994). The cores were incubated using a continuous flow-through system in an environmental chamber set at the average of the ambient water temperatures between the upstream and downstream sites (Miller-Way and Twilley 1996). The cores were submerged in site water and capped with gas tight tops equipped with sampling ports, yielding an overlying water volume of ~ 400 ml. A peristaltic pump pulled water through the inlet port, and out through a tube 2.5 cm above the sediment surface. The inflowing water was aerated to prevent oxygen depletion. The pumped flow rate was set at 1 ml minute−1, which is in the range of flow rates typically used during MIMS (McCarthy et al. 2007). A pre-incubation period of 24 h allowed equilibration of dissolved gases with the PVC and tubing, and allowed concentration gradients in the surficial sediments to reach steady state (Eyre et al. 2002). Inflow and outflow samples were collected by filling 5 ml glass test tubes until overflowing, and capping the tubes so that no bubbles were present. The rate of denitrification and SOD were calculated as the flux of N2 and O2, respectively, where the flux is equal to the change in concentration between the inflow and outflow samples, multiplied by the flow rate and divided by the core area (Miller-Way and Twilley 1996).
Signals of N2, argon (Ar), and O2 were measured using a Balzers Prisma QME 200 quadropole mass spectrometer (Pfeiffer Vacuum, Nashua, NH, USA). The ratio of N2:Ar and O2:Ar was used to calculate the N2 and O2 concentrations, respectively, using Ar as a conservative tracer (Kana et al. 1998, Laursen and Seitzinger 2002). Standard gas saturation tables were used to obtain the Ar concentration (Lide 2004), and the N2:Ar and O2:Ar ratios were multiplied by the Ar concentration to obtain the concentration of N2 and O2, respectively. MIMS signals were calibrated using standards from a temperature controlled, air-equilibrated water bath.
Concentration of NOx (NO3 − + NO2 −) and ammonium (NH4 +) were measured at the inflow and outflow of each core during incubation. Samples were filtered through Whatman GF/F filters (25 mm diameter, 0.7 μm nominal pore size), and the filtrate was analyzed on a Lachat Quick-Chem 8000 nutrient autoanalyzer (Lachat Instruments, Milwaukee, WI). Fluxes of NOx in μmol m−2 h−1 were calculated as the change in NOx concentration, multiplied by the flow rate and divided by the core area (Miller-Way and Twilley 1996). The NOx flux was used to estimate the percentage of denitrification which was direct (denitrification of nitrate from the water column) or coupled to nitrification. Denitrification was assumed to be direct up to the point where it was supported by a flux of nitrate into the sediments. The percentage coupled denitrification was calculated by the following equation:
In this equation where NOx was positive (out of the sediments), % denitrification was assumed to be completely coupled, and if NOx was negative (into the sediments) and greater than or equal to denitrification, denitrification was assumed to be completely direct.
Upon completion of the experiment, the top centimeter of sediment and organic material was collected, dried at 105 °C for 3 hours, combusted at 525 °C for 3 hours, and the quantity of OM was calculated as the percent change in weight between the dry sample and combusted sample (Schulte and Hopkins 1996).
Statistical Analysis
Statistical analyses were carried out separately for each river due to the uneven sampling design (e.g., lack of a floodplain site in the upper New River). Linear regression was used to test for a relationship between denitrification and SOD, and denitrification and OM using R (R Development Core Team 2011). Spatial variation in denitrification was tested for with 3-way ANOVA (SPSS 15.0, SPSS Inc. 2006) using three factors: 1) lateral position (channel, bank, and floodplain), 2) upstream/downstream position, and 3) season. ANOVA models were simplified by excluding non-significant interactions. A type IV sum of squares was used for the New River and hypotheses not automatically tested for were examined using contrast coding (SAS 9.0, SAS Institute Inc. 2002; Miliken and Johnson 2009). Spatial variability in SOD was examined with the same statistical method as denitrification. Denitrification in both rivers and SOD in the Newport River were square root transformed to meet the assumption of normality. Least Squared Difference (LSD) pairwise tests with a Sidak correction were used for post-hoc testing. Normality of the residuals was assessed using a normal-Q-Q plot, and by performing a Shapiro-Wilks test, and the assumption of homoscedasticity was tested using Levene's test on the median (R, R Development Core Team 2011). Statistical analyses were performed on OM after averaging OM across seasons, since the magnitudes and spatial patterns of OM were generally similar between seasons. OM data did not fit the assumptions of 2-way ANOVA, so nonparametric tests were performed separately for upstream and downstream sites (Kruskal-Wallis or Mann–Whitney; R, R Development Core Team 2011). An alpha of 0.05 was chosen for all tests.
Estimation of Total N2 Flux
The total N2 fluxes from the channel and riparian zone were calculated as the product of denitrification rate and inundated area. The channel bed area was estimated using imagery provided by Google EarthTM (Google Inc. 2011) using the ruler tool to measure channel length in segments of approximately 500 m, and multiplying the length of each segment by the channel width, measured as the distance between visible vegetated banks. The shape of the channel bed was not considered in this analysis; the channel bed was assumed to be a flat, horizontal surface. Although the assumption of a flat channel bed may have led to an underestimate of channel area, there was not enough information available to calculate wetted perimeter, so this assumption was more appropriate for this study. Denitrification rate in the riparian zone was calculated as the average of the bank and floodplain rates, and upstream and downstream rates were averaged together for both the channel and riparian zone. The inundation area of the riparian zone was estimated using hypsometric curves from Ensign et al. (2013b), and assuming a hypothetical sinusoidal tidal wave varying around 0 m elevation in NAVD88, and tidal amplitudes based off of measurements in Ensign et al. (2013b). While the NAVD88 vertical datum may differ from local mean sea level in this region by approximately 0.1 m, correction for this difference was not incorporated into our calculations due to the lack of precise information at the inland locations studied. Ensign et al. (2013b) developed hypsometric curves for the floodplains within a 50 m buffer from the river channel using a combination of water level measurements and a topographic model created using Light Image Detection and Ranging data (LIDAR). The buffer was based on observations that floodplain inundation can extend at least that far.
Results
Denitrification
In the New River the seasonal average of denitrification ranged from 35–248 μmol N m−2 hr−1, and was highest in the fall (Fig. 2). Denitrification was lower in the winter than all seasons, and was also significantly higher in the fall than the spring (ANOVA, P < 0.001, LSD: P < 0.001). No other interactions or main effects were significant for denitrification in the New River.
In the Newport River the seasonal average of denitrification ranged from 36–161 μmol N m−2 hr−1 (Fig. 3). Denitrification was significantly higher during the fall and summer than the winter and spring (ANOVA: P < 0.001, LSD: P < 0.001). There were significant main effects of lateral position (P < 0.001) and upstream/downstream position (P = 0.028), and a significant 2-way interaction between these two factors (P = 0.048). Post hoc tests showed that the average annual denitrification was higher in the upstream floodplain than the downstream floodplain (LSD: P = 0.004), and that at the upstream site the average annual denitrification was higher on the floodplain than the bank (LSD: P =0.037) and the channel (LSD: P < 0.001).
The average annual denitrification rate was significantly higher in the New River than the Newport River during the fall and spring (Mann–Whitney-Tests: P < 0.001). The average percent coupled denitrification ranged from 76.5 % to 87.2 % in the Newport River, and from 14.7 % to 50.0 % in the New River (Fig. 4). The average percent coupled denitrification was significantly higher in the Newport River than the New River (Mann–Whitney: P < 0.001).
Site Characteristics
In the New River the average annual OM upstream (3 %) was significantly lower than downstream (21 %) (Fig. 5, Mann–Whitney Test: P < 0.001). At the downstream site OM was highest in the floodplain, lower in the bank, and lowest in the channel (Kruskal-Wallis: P < 0.001, Pairwise Mann–Whitney Tests: P < 0.01). OM was not statistically different between the channel and bank at the upstream site in the New River (Mann–Whitney Test: P = 0.48). In the Newport River the average annual OM was significantly higher upstream than downstream (Mann–Whitney Test: P = 0.0011). At the upstream site OM was lower in the channel than both the floodplain and the bank (Kruskal-Wallis: P < 0.001, Pairwise Mann–Whitney Tests: P < 0.001). At the downstream site OM was highest on the floodplain, lower in the bank, and lowest in the channel (Kruskal-Wallis: P < 0.001, Pairwise Mann–Whitney Tests: P < 0.05).
The average annual NOx concentration in the New River was significantly higher upstream than downstream (Table 1, T-Test: P = 0.020). In the Newport River, the NOx concentration ranged from 3 μM to 20 μM, and was higher downstream during the fall and summer. Salinity in the two rivers remained below 0.5 PSU during most seasons, but occasionally rose above 0.5 PSU (Table 1).
In the New River SOD was significantly different between seasons (Fig. 6, ANOVA: P < 0.001) but not between sites (ANOVA: P > 0.05). SOD was significantly lower during the winter than all other seasons (LSD: P < 0.001), higher during the fall than both the spring (LSD: P < 0.001) and summer (LSD: P = 0.006), but SOD was not statistically different between the spring and summer (LSD: P = 0.67). In the Newport River, the SOD differed significantly between all seasons (Fig. 7, ANOVA: P < 0.001, LSD: P < 0.001). There was a significant interaction between upstream/downstream position and lateral position (ANOVA: P = 0.017). Pairwise comparisons showed that the average annual SOD in the channel at the downstream site was greater than the channel at the upstream site (LSD: P =0.037).
Relationship between Denitrification and Controlling Factors
Denitrification was significantly correlated with OM during the fall in the New River, and during the spring and winter in the Newport River (Pearson correlation coefficient: P < 0.05, Table 2). There was a strong correlation between SOD and denitrification during the fall, spring, and summer in the New River (Pearson correlation coefficient: P <0.05). Denitrification was correlated to SOD in the Newport River during the winter, spring, and summer, but the correlation was generally weaker than the New River (Pearson correlation coefficient: P < 0.05, Table 2). Both denitrification and SOD tended to be higher during seasons where the ambient water temperature was warmer (Table 1).
Modeled N2 Flux
N2 flux in the channel and riparian zone was calculated as the product of the inundated area and the denitrification rate. The channel area was 31 ha in the New River, 35 ha in the Newport River, 63 ha in the White Oak River, and 827 ha in the Northeast Cape Fear River. In each of the rivers, the channel generally had a larger inundation area than the lower and upper riparian zones combined, although the riparian zone was greater than the channel at high tide in the White Oak River and New River (Fig. 8). The total N2 flux from the TFZ ranged from 2.5 Kg N per tidal cycle to 19.2 kg N per tidal cycle in the New River, and from 1.9 Kg N per tidal cycle to 10.1 Kg N per tidal cycle in the Newport River (New River: Table 3, Newport River: Table 4). In the New River, the riparian zone and the channel represented on average 17 % and 83 % of the total N2 flux, respectively. In the Newport River, the riparian zone and the channel represented on average 26 % and 74 % of the total N2 flux, respectively.
Discussion
Spatial Variation of Denitrification
Comparison of denitrification in the riparian zone and the channel provided insights into mechanisms controlling spatial variability of denitrification in forested tidal freshwater wetlands. Previous comparisons of denitrification between the river channel and floodplains in the TFZ have been in emergent marshes, and have found lower denitrification rates in the channel than the riparian zone. Greene (2005) found denitrification rates in Jug Bay to be approximately 225 μmol N m−2 h−1 in the high marsh, and 100 μmol N m−2 h−1 in the marsh creek. Denitrification rates of 49 ± 28 and 63 ± 34 μmol N m−2 h−1 were found during the spring and summer in the Potomac River, while average rates of 147 ± 24 μmol N m−2 h−1 were found in the adjacent Dyke Marsh (Hopfensperger et al. 2009). Similarly, in this study the annual average of denitrification was higher on the floodplain (128 ± 24 μmol N m−2 h−1) than the bank (101 ± 20 μmol N m−2 h−1) and the channel (72 ± 11 μmol N m−2 h−1) at the upstream site of the Newport River. In contrast, denitrification rates were comparable between the channel, bank, and floodplain in the New River and at the downstream site of the Newport River. The denitrification rates in this study were similar to rates reported in other studies of TFZs (Neubauer et al. 2005, Greene 2005, Ensign et al. 2008, Hopfensperger et al. 2009), but were higher than those reported in Verhoeven et al. (2001), and lower than those reported in Gribsholt et al. (2006).
OM quantity showed considerable variation between the channel, bank, and floodplain. The lateral differences in OM were likely caused by deposition of organic rich sediments on the floodplain as flow velocity declined, and increased productivity in the riparian zone (Johnston et al. 1997, Ross et al. 2004). Other studies in TFZs have reported increasing organic matter with elevation and higher total organic carbon accumulation rates in the riparian zone than the channel (Khan and Brush 1994, Hopfensperger et al. 2009). Compared to this study, Johnston et al. (2001) found a greater difference in %OM between the channel and organic rich backwaters in freshwater riverine wetlands (Johnston et al. 2001), while a similar difference in %OM occurred in the upper Mississippi river (Richardson et al. 2004). A lack of forested floodplain flanking the upstream site in the New River may have resulted in extremely low OM at the upstream site.
The positive correlations between denitrification and %OM during some seasons could indicate a link between organic matter supply and denitrification, and higher denitrification rates at the upstream site of the Newport River could have been associated with the high %OM relative to other sites. On the other hand, correlations between denitrification and %OM were relatively weak and only occurred during a few seasons. Also, despite higher %OM on the floodplain than the channel, denitrification did not usually significantly differ between the channel and floodplain. In these cases, the difference in %OM may have been too small to cause a significant difference in denitrification. Still, denitrification rates were high at the upstream site in the New River despite very low %OM (near 1 %). This suggests that denitrification was limited by organic matter quality rather than quantity (Hill and Cardaci 2004). OM quality could have been similar between sites despite significant differences in %OM. If only a small percentage of OM was labile, it may have taken a very high %OM, such occurred at the upstream site in the Newport River, to cause significantly higher denitrification rates.
Alternatively, limitation of denitrification by nitrate could explain why denitrification was similar between some sites despite significant differences in %OM. A statistically higher denitrification rate in the New River than the Newport River coinciding with higher nitrate concentrations in the New River may indicate nitrate limitation. In contrast, denitrification was comparable between the upstream and downstream sites despite differences in the nitrate concentration of up to 19 μM in the Newport River and up to 45 μM in the New River. Still, Greene (2005) found a Km (half saturation constant) of 93 μM in tidal freshwater marsh sediments with denitrification rates relatively similar to this study. If the New and Newport Rivers followed a similar saturation response to their study, the differences in nitrate concentration could have been too small to cause a significant difference in denitrification.
SOD was usually a strong predictor for denitrification in the New River, and in the Newport River the correlation ranged from weak during the winter and spring, to strong in the summer. Although organic matter quality was not measured, the correlations between SOD and denitrification could reflect a link between SOD and OM quality (Mulholland et al. 2008). Since SOD was generally comparable between the channel, bank and floodplain, and between upstream and downstream sites, it may suggest that OM quality, and consequently denitrification was also comparable between sites. Under nitrate limiting conditions, relationships between denitrification and SOD can also reflect differences in nitrate supply due to a link between SOD and nitrification or the oxygen penetration depth, (Rysgaard et al. 1994, Seitzinger and Giblin 1996, Seitzinger et al. 2006, Piehler and Smyth 2011). Without experimental nitrate additions or organic matter quality measurements it is difficult to confirm the limiting factor for denitrification in these rivers and the mechanisms behind the SOD-denitrification relationships.
Increased denitrification at the floodplain of the upstream site in the Newport River shows that patterns of spatial variability of denitrification can differ between upstream and downstream sites in the TFZ, or between TFZ rivers. Increased spatial variability in the Newport River compared to the New River could simply reflect the large quantity of OM on the floodplain at the upstream site of the Newport River. Alternatively, increased spatial variability in the Newport River as compared to the New River could reflect differences in the frequency of tidal inundation of the floodplains between these rivers: water level fluctuations are wind driven in the New River, resulting in irregular patterns of floodplain inundation (Ensign et al. 2013b). In the Newport River, the microbial community on the floodplain could be primed for denitrification due to more regular inundation patterns, resulting in increased floodplain denitrification.
Denitrification has been suggested to be higher on floodplains than channel sediments at the onset of inundation due to increased nitrate availability from nitrification (Reddy and Patrick 1976, Reddy and Patrick 1984, Groffman and Tiedje 1988), but the experimental setup used to measure denitrification in flow-through cores is not compatible with the variable inundation that would be needed to simulate tidal flooding due to requiring steady state N2 production. Therefore, if denitrification was nitrate limited in these rivers, the denitrification measurements from the riparian zone could have been underestimated. On the other hand, nitrate could become depleted on the floodplain over the course of an inundation cycle, but the flow-through design continuously supplies cores with new water, which may have led to an overestimation of denitrification for the floodplain. The approach used in this study also fails to account for differences in water temperature between floodplain and channel environments. Floodplains may have higher water temperatures due to a greater surface area of water exposed to solar radiation, which could enhance denitrification in floodplains compared to the channel.
Analysis of System-wide N Removal
The total N removal via denitrification in the channel and riparian zones in these TFZ rivers were estimated based on two factors: the denitrification rate, and the surface area of inundation. Although this calculation was not meant as a robust model of N removal since it was based on a hypothetical tidal wave instead of observed water level variation, it was useful for comparing the relative importance of the floodplain and channel denitrification between rivers. In both the New River and the Newport River, the channel represented a greater proportion of the net N2 flux than both the upper and lower river riparian zones combined, indicating that the higher riparian zone denitrification rates in the Newport River were insufficient to compensate for the smaller area of riparian zone inundation. This suggests that at base flow conditions, the channel dominates the N2 flux in both rivers. The relationship between channel area and floodplain inundation was similar in the White Oak River where floodplain inundation only exceeded the channel area at high tide. In contrast, the Northeast Cape Fear River is a much larger river whose channel area greatly exceeded the inundated floodplain area. In summary, while the riparian TFZ may have greater rates of denitrification than the channel in some cases, we predict that under baseflow conditions the riparian TFZ will produce a much lower denitrification flux than the river channel. Based on the limited survey of rivers performed here, we anticipate that the larger the river the greater the dominance of river channel N2 flux than riparian flux.
More thorough predictions of the relative importance of the riparian zone versus the channel would require more precise hydrologic, geomorphic, and biogeochemical data. Hydrologic data such as annual flow-duration curves and inundation data would be required to incorporate the long-term influence of storm events on riparian inundation. More extensive morphologic data on floodplain elevation and riparian flow velocities would be needed to fine-tune the spatial extent of inundation analysis. Finally, more biogeochemical data are needed on the delay between inundation of the floodplain and occurrence of reducing conditions for denitrification to occur. A previous study found a delay of 4.6 h between inundation and reducing conditions suitable for denitrification (Ensign et al. 2008); incorporation of that delay into our predictions of N2 flux would greatly reduce the floodplain denitrification flux predictions made here.
In rivers such as the Potomac River or Jug Bay (Greene 2005, Hopfensperger et al. 2009), where the differences in denitrification between the riparian zone and channel are larger than this study, the N2 flux from the riparian zone may be enhanced. The average for the Newport River over four seasons was 6 kg N per tidal cycle while the New River average was 11 kg N per tidal cycle. The difference in N2 flux was mainly due to higher denitrification rates in the channel of the New River. For rivers with a broader range of channel areas, differences in channel morphology would likely have been a more important factor. Also, floodplain topography may play a larger role in some rivers, especially during high river flow when a large portion of the floodplain can be permanently inundated (such as in Ensign et al. 2013b). The influence of topography and morphology on nitrogen cycling and denitrification flux have been examined at a variety of scales, from the sub-meter scale (Courtwright and Findlay 2011, Wolf et al. 2011) to entire tidal creek basins (Seldomridge and Prestegaard 2012). The current study demonstrates how integration of this physical heterogeneity at the landscape-scale can help elucidate the relative importance of physical versus biogeochemical processes that regulate denitrification flux.
It is possible that denitrification is one process which contributes to the decline in nitrate concentration of between 16-34 % from the upstream to the downstream section of the New River TFZ. Algal uptake, and long term burial of nitrogen was not quantified in this study, but could also play a role. The New River is relatively nitrate rich and thereby has the potential to act as a major nitrate source to the New River Estuary, so it is important to quantify sinks like denitrification in the TFZ. The decline in nitrate concentration throughout the TFZ suggests that predicting nitrate loading to estuaries from a single gauge at an upstream site does not take into account longitudinal changes in nitrate concentration due to processes such as denitrification that occur throughout the TFZ. These results support previous studies which have noted that gaps in monitoring of coastal regions can reduce accuracy of nutrient budgets (Destouni et al. 2008).
Conclusions
Advancing the knowledge of spatial variability in denitrification in the forested TFZ is important for determining the ability of the TFZ to act as a sink for nitrogen, and the relative importance of the riparian zone and channel sediments as a sink for N2. Previous studies have shown that the riparian zone in the TFZ is a reactive site for nitrogen removal, but this has not previously been confirmed in the less organic-rich river channel sediments. This study suggests that in general the denitrification rates between the channel and riparian zone area are comparable in the TFZ. Denitrification may be higher on the floodplain than the channel at some sites such as the upstream site of the Newport River. The presence of different patterns of spatial variability between upstream and downstream sites or between tidal freshwater rivers suggests that models may benefit from incorporating multiple denitrification measurements at kilometer scales along the TFZ, and from determining patterns of lateral variation in denitrification unique to tidal freshwater rivers. Under baseflow conditions, the channel may be a more important N2 sink in TFZ rivers than the floodplain. These results significantly improve our understanding of spatial variability of denitrification in the TFZ, and are useful for constraining N budgets in the TFZ.
References
Anderson CJ, Lockaby BG (2007) Soils and biogeochemistry of tidal freshwater forested wetlands. In: Conner WH, Doyle TW, Krauss KW (eds) Ecology of tidal freshwater forested wetlands of the southeastern United States. Springer, Netherlands, pp 65–88
Baldwin DS, Mitchell AM (2000) The effects of drying and re-flooding on the sediment and soil nutrient dynamics of lowland river-floodplain systems: a synthesis. Regulated Rivers: Research & Management 16:457–467. doi:10.1002/1099-1646(200009/10)16:5<457::AID-RRR597>3.0.CO;2-B
Baker MA, Vervier P (2004) Hydrological variability, organic matter supply and denitrification in the Garonne River ecosystem. Freshwater Biology 49:181–190. doi:10.1046/j.1365-2426.2003.01175.x
Boyer EW, Alexander RB, Parton WJ, Li C, Butterbach-Bahl K, Donner SD, Skaggs RW, Del Grosso SJ (2006) Modeling denitrification in terrestrial and aquatic ecosystems at regional scales. Ecological Applications 16:2123–2142. doi:10.1890/1051-0761(2006)016[2123:MDITAA]2.0.CO;2
Burgin AJ, Hamilton SK (2007) Have we overemphasized the role of denitrification in aquatic ecosystems? A review of nitrate removal pathways. Frontiers in Ecology and the Environment 5:89–96. doi:10.1890/1540-9295(2007)5[89:HWOTRO]2.0.CO;2
Courtwright J, Findlay SEG (2011) Effects of Microtopography on Hydrology, Physicochemistry, and Vegetation in a Tidal Swamp of the Hudson River. Wetlands 31:239–249. doi:10.1007/s13157-011-0156-9
Day RH, Williams TW, Swarzenski CM (2007) Hydrology of tidal freshwater forested wetlands of the southeastern United States. In: Conner WH, Doyle TW, Krauss KW (eds) Ecology of tidal freshwater forested wetlands of the southeastern United States. Springer, Netherlands, pp 29–63
Destouni G, Hannerz F, Prieto C, Jarsjö J, Shibuo Y (2008) Small unmonitored near-coastal catchment areas yielding large mass loading to the sea. Global Biogeochem Cycles 22. doi:10.1029/2008GB003287
Ensign SH, Piehler MF, Doyle MW (2008) Riparian zone denitrification affects nitrogen flux through a tidal freshwater river. Biogeochemistry 91:133–150. doi:10.1007/s10533-008-9265-9
Ensign SH, Doyle MW, Piehler MF (2012) Tidal geomorphology affects phytoplankton at the transition from forested streams to tidal rivers. Freshwater Biology 57:2141–2155. doi:10.1111/j.1365-2427.2012.02856.x
Ensign SH, Doyle MW, Piehler MF (2013a) The effect of tide on the hydrology and morphology of a freshwater tidal river. Earth Surface Processes and Landforms 38:655–660. doi:10.1002/esp.3392
Ensign SH, Siporin K, Piehler M, Doyle M, Leonard L (2013b) Hydrologic versus biogeochemical controls of denitrification in tidal freshwater wetlands. Estuaries and Coasts 36:519–532. doi:10.1007/s12237-012-9491-1
Eyre BD, Rysgaard S, Dalsgaard T, Christensen PB (2002) Comparison of isotope pairing and N2:Ar methods for measuring sediment-denitrification-assumptions, modifications, and implications. Estuaries 25:1077–1087. doi:10.1007/BF02692205
Field DW, Reyer A, Genovese P, Shearer B (1991) Coastal wetlands of the United States-an accounting of a valuable national resource. Strategic Assessment Branch, Ocean Assessments Division, Office of Oceanography and Marine Assessment, National Ocean Service, National Oceanic and Atmospheric Administration, Rockville, Maryland
Forshay KJ, Stanley EH (2005) Rapid nitrate loss and denitrification in a temperate river floodplain. Biogeochemistry 75:43–64. doi:10.1007/s10533-004-6016-4
Gardner WS, McCarthy MJ, Carini SA, Souza AC, Lijun H, McNeal KS, Puckett MK, Pennington J (2009) Collection of intact sediment cores with overlying water to study nitrogen- and oxygen-dynamics in regions with seasonal hypoxia. Continental Shelf Research 29:2207–2213. doi:10.1016/j.csr.2009.08.012
Greene SE (2005) Nutrient removal by tidal fresh and oligohaline marshes in a Chesapeake Bay tributary. M.S. Thesis, University of Maryland, Solomons
Gribsholt B, Struyf E, Tramper A, Andersson MGI, Brion N, De Brabandere L, Van Damme S, Meire P, Middelburg JJ, Dehairs F, Boschker HTS (2006) Ammonium transformation in a nitrogen-rich tidal freshwater marsh. Biogeochemistry 80:289–298. doi:10.1007/s10533-006-9024-8
Groffman PM, Tiedje JM (1988) Denitrification hysteresis during wetting and drying cycles in soil. Soil Science Society of America Journal 52:1626–1629. doi:10.2136/sssaj1988.03615995005200060022x
Groffman PM, Butterbach-Bahl K, Fulweiler RW, Gold AJ, Morse JL, Stander EK, Tague C, Tonitto C, Vidon P (2009) Challenges to incorporating spatially and temporally explicit phenomena (hotspots and hot moments) in denitrification models. Biogeochemistry 93:49–77. doi:10.1007/s10533-008-9277-5
Hill AR, Cardaci M (2004) Denitrification and organic carbon availability in riparian wetland soils and subsurface sediments. Soil Science Society of America Journal 68:320–325. doi:10.2136/sssaj2004.0320
Hopfensperger KN, Kaushal SS, Findlay SEG, Cornwell JC (2009) Influence of plant communities on denitrification in a tidal freshwater marsh of the Potomac River, United States. Journal of Environmental Quality 38:618–626. doi:10.2134/jeq2008.0220
Howarth RW (1988) Nutrient limitation of net primary production in marine ecosystems. Annual Review of Ecology and Systematics 19:89–110. doi:10.1146/annurev.es.19.110188.000513
Howarth RW, Boyer EW, Pabich WJ, Galloway JN (2002) Nitrogen use in the United States from 1961–2000 and potential future trends. Ambio 31:88–96. doi:10.1579/0044-7447-31.2.88
Johnston CA, Schubauer-Berigan JP, Bridgham SD (1997) The potential role of riverine wetlands as buffer zones. In: Haycock NE, Burt TP, Goulding KWT, Pinay G (eds) Buffer zones: their processes and potential in water protection. Quest Environmental, United Kingdom
Johnston CA, Bridgham SD, Schubauer-Berigan JP (2001) Nutrient dynamics in relation to geomorphology of riverine wetlands. Soil Science Society of America 65:557–577
Joye SB, Hollibaugh JT (1995) Influence of sulfide inhibition of nitrification on nitrogen regeneration in sediments. Science 270:623–625. doi:10.1126/science.270.5236.623
Kana TM, Darkangelo C, Hunt MD, Oldham JB, Bennett GE, Cornwell JC (1994) Membrane inlet mass-spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples. Analytical Chemistry 66:4166–4170. doi:10.1021/ac00095a009
Kana TM, Sullivan MB, Cornwell JC, Groszkowski KM (1998) Denitrification in estuarine sediments determined by membrane inlet mass spectrometry. Limnology and Oceanography 43:334–339. doi:10.4319/lo.1998.43.2.0334
Khan H, Brush GS (1994) Nutrient and metal accumulation in a freshwater tidal marsh. Estuaries 17:345–360. doi:10.2307/1352668
Laursen AE, Seitzinger SP (2002) The role of denitrification in nitrogen removal and carbon mineralization in Mid-Atlantic Bight sediments. Continental Shelf Research 22:1397–1416. doi:10.1016/S0278-4343(02)00008-0
Lide DR (2004) Handbook of chemistry and physics. CRC Press, Florida
McCarthy MJ, Lavrentyev PJ, Yang LY, Zhang L, Chen YW, Qin BQ, Gardner WS (2007) Nitrogen dynamics and microbial food web structure during a summer cyanobacterial bloom in a subtropical, shallow, well-mixed, eutrophic lake (Lake Taihu, China). Hydrobiologia 581:195–207. doi:10.1007/s10750-006-0496-2
Miliken GA, Johnson DE (2009) Analysis of Messy Data Volume 1 Designed Experiments, 2nd edn. CRC Press, Florida
Miller-Way T, Twilley RR (1996) Theory and operation of continuous flow systems for the study of benthic-pelagic coupling. Marine Ecology-Progress Series 140:257–269. doi:10.3354/meps140257
Mulholland PJ, Hall RO, Sobota DJ, Dodds WK, Findlay SEG, Grimm NB, Hamilton SK, McDowell WH, O’Brien JM, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Gregory SV, Johnson SL, Meyer JL, Peterson BJ, Poole GC, Valett HM, Webster JR, Arango CP, Beaulieu JJ, Bernot MJ, Burgin AJ, Crenshaw CL, Helton AM, Johnson LT, Niederlehner BR, Potter JD, Sheibley RW, Thomas SM (2009) Nitrate removal in stream ecosystems measured by 15 N addition experiments: denitrification. Limnology and Oceanography 54:666–680. doi:10.4319/lo.2009.54.3.0666
Mulholland PJ, Helton AM, Poole GC, Hall RO, Hamilton SK, Peterson BJ, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Dodds WK, Findlay SEG, Gregory SV, Grimm NB, Johnson SL, McDowell WH, Meyer JL, Valett HM, Webster JR, Arango CP, Beaulieu JJ, Bernot MJ, Burgin AJ, Crenshaw CL, Johnson LT, Niederlehner BR, O'Brien JM, Potter JD, Sheibley RW, Sobota DJ, Thomas SM (2008) Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 452:202–U46. doi:10.1038/nature06686
Neubauer SC, Anderson IC, Neikirk BB (2005) Nitrogen cycling and ecosystem exchanges in a Virginia tidal freshwater marsh. Estuaries and Coasts 28:909–922. doi:10.1007/BF02696019
Nicholls JC, Trimmer M (2009) Widespread occurrence of the anammox reaction in estuarine sediments. Aquatic Microbial Ecology 55:105–113. doi:10.3354/ame01285
Nixon SW (1995) Coastal marine eutrophication - a definition, social causes, and future concerns. Ophelia 41:199–219
Noe GB, Krauss KW, Lockaby BG, Conner WH, Hupp CR (2013) The effect of increasing salinity and forest mortality on soil nitrogen and phosphorus mineralization in tidal freshwater forested wetlands. Biogeochemistry 114:225–244. doi:10.1007/s10533-012-9805-1
Odum WE (1988) Comparative ecology of tidal freshwater and salt marshes. Annual Review of Ecology and Systematics 19:147–176. doi:10.1146/annurev.ecolsys.19.1.147
Paerl HW (2006) Assessing and managing nutrient-enhanced eutrophication in estuarine and coastal waters: interactive effects of human and climatic perturbations. Ecological Engineering 26:40–54
Paerl HW, Pinckney JL, Fear JM, Peierls BL (1998) Ecosystem responses to internal and watershed organic matter loading: consequences for hypoxia in the eutrophying Neuse River Estuary, North Carolina, USA. Marine Ecology Progress Series 166:17–25. doi:10.3354/meps166017
Piehler MF, Smyth AR (2011) Habitat-specific distinctions in estuarine denitrification affect both ecosystem function and services. Ecosphere 2:art12. doi:10.1890/ES10-00082.1
Rabalais NN, Turner RE (eds) (2001) Coastal Hypoxia: Consequences for living resources and ecosystems. Coastal and Estuarine Studies 58. American Geophysical Union, Washington, D.C. doi: 10.1029/CE058
Racchetti E, Bartoli M, Soana E, Longhi D, Christian RR, Pinardi M, Viaroli P (2011) Influence of hydrological connectivity of riverine wetlands on nitrogen removal via denitrification. Biogeochemistry 103:335–354. doi:10.1007/s10533-010-9477-7
Reddy KR, Patrick WH Jr (1976) Effect of frequent changes in aerobic and anaerobic conditions on redox potential and nitrogen loss in a flooded soil. Soil Biology and Biochemistry 8:491–495. doi:10.1016/0038-0717(76)90091-2
Reddy KR, Patrick WH (1984) Nitrogen transformations and loss in flooded soils and sediments. Critical Reviews in Environmental Control 13:273–309. doi:10.1080/10643388409381709
Rich JJ, Dale OR, Song B, Ward BB (2008) Anaerobic ammonium oxidation (anammox) in Chesapeake Bay sediments. Microbial Ecology 55:311–320. doi:10.1007/s00248-007-9277-3
Richardson WB, Strauss EA, Bartsch LA, Monroe EM, Cavanaugh JC, Vingum L, Soballe DM (2004) Denitrification in the upper Mississippi River: rates, controls, and contribution to nitrate flux. Canadian Journal of Fisheries and Aquatic Sciences 61:1102–1112. doi:10.1139/f04-062
Ross KM, Hupp CR, Howard AD (2004) Sedimentation in floodplains of selected tributaries of the Chesapeake Bay. In: Bennett SJ, Simon A (eds) Riparian vegetation and fluvial geomorphology. American Geophysical Union, Washington, DC, pp 187–208
Rysgaard S, Risgaard-Petersen N, Sloth NP, Jensen K, Nielsen LP (1994) Oxygen regulation of nitrification and denitrification in sediments. Limnology and Oceanography 39:1643–1652. doi:10.4319/lo.1994.39.7.1643
Rysgaard S, Thastum P, Dalsgaard T, Christensen PB, Sloth NP (1999) Effects of salinity on NH4 + adsorption capacity, nitrification, and denitrification in Danish estuarine sediments. Estuaries 22:21–30. doi:10.2307/1352923
Schulte EE, Hopkins BG (1996) Estimation of soil organic matter by weight loss-on-ignition. In: Magdoff FR, Tabatabai MA, Hanlon EA Jr (eds) Soil organic matter: analysis and interpretation. Soil Science Society of America, Wisconsin, pp 21–31
Seitzinger SP, Giblin AE (1996) Estimating denitrification in North Atlantic continental shelf sediments. Biogeochemistry 35:235–260. doi:10.1007/BF02179829
Seitzinger S, Harrison JA, Bohlke JK, Bouwman AF, Lowrance R, Peterson B, Tobias C, Van Drecht G (2006) Denitrification across landscapes and waterscapes: a synthesis. Ecological Applications 16:2064–2090. doi:10.1890/1051-0761
Seldomridge ED, Prestegaard KL (2012) Use of geomorphic, hydrologic, and nitrogen mass balance data to model ecosystem nitrate retention in tidal freshwater wetlands. Biogeosciences 9:2661–2672. doi:10.5194/bg-9-2661-2012
Smayda TJ (1990) Novel and nuisance phytoplankton blooms in the sea: evidence for a global epidemic. In: Granéli E, Sundström BG, Edler L, Anderson DM (eds) Toxic marine phytoplankton. Elsevier Science, New York, pp 29–40
Verhoeven JTA, Whigham DF, van Logtestijn R, O'Neill J (2001) A comparative study of nitrogen and phosphorus cycling in tidal and non-tidal riverine wetlands. Wetlands 21:210–222. doi:10.1672/0277-5212(2001)021[0210:ACSONA]2.0.CO;2
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman GD (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecological Applications 7:737–750. doi:10.2307/2269431
Whalen SC, Alperin MJ, Nie Y, Fischer EN (2008) Denitrification in the mainstem Neuse River and tributaries, USA. Fundamental and Applied Limnology 171:249–261. doi:10.1127/1863-9135/2008/0171-0249
Wolf KL, Ahn C, Noe GB (2011) Microtopography enhances nitrogen cycling and removal in created mitigation wetlands. Ecological Engineering 37:1398–1406. doi:10.1016/j.ecoleng.2011.03.013
Acknowledgments
We thank Stacy Davis, Claude Lewis, Teri O'Meara, Joe Purifoy, Kaylyn Siporin, Ashley Smyth, and Suzanne Thompson for field and laboratory assistance. Thanks to Dr. Hans Paerl for providing access to analytical equipment, and to Hermann Godwin and Betty Allen for providing access to the Newport and New Rivers from their property. We are grateful for financial support from NSF EAR 0815627 and the Defense Coastal/Estuarine Research Program funded by Strategic Environmental Research and Development Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Von Korff, B.H., Piehler, M.F. & Ensign, S.H. Comparison of Denitrification Between River Channels and Their Adjoining Tidal Freshwater Wetlands. Wetlands 34, 1047–1060 (2014). https://doi.org/10.1007/s13157-014-0545-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-014-0545-y