Abstract
The influence of inundation depth on phytomass, shoot density, photosynthesis, elongation, and nutrients of Typha domingensis in Stormwater Treatment Area-1East (STA-1East) was examined and results were evaluated relative to phosphorus (P) treatment from 2006 to 2011. Biomass and nutrient concentrations of T. domingensis were not related to inundation depth. Necromass and belowground/leaf biomass ratio increased with increasing inundation depth from 30 to 80 cm. Shoot density, photosynthesis, and elongation decreased with inundation depth. The contents of necromass nutrients and phytomass nitrogen increased with inundation depth. Annual total P (TP) concentration increase (−) or decrease (+) from inflow to outflow was −21 %, −3 %, +8 %, −54 %, +41 %, and +29 % in 2006–2011, respectively. Decreases in soluble reactive P (SRP), total dissolved P (TDP), dissolved organic P (DOP), and/or particulate P (PP) from inflow to outflow were low or did not occur annually. Prolonged inundation damaged T. domingensis community and thus adversely impacted the capacity of the system to treat P. Inundation depths in the STAs can be categorized into optimal, subtle stress, and lethal stress zones. This study stressed the importance in maintaining vegetation within an optimal zone of inundation for a sustainable system.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Typha species (cattail) have been widely used to reduce contaminant and nutrient concentrations in surface-flow constructed treatment wetlands (CTWs; Vymazal 2007; Chen 2011). Typha domingensis (southern cattail), a tropical species of the Typhaceae family, grows in a wide range (i.e., −5–122 cm) of water depths (Grace 1989). This species survives at up to 1.5 m of inundation in the San Juan River Reservoir in Cuba (Fraga and Kvet 1993) because it has high pressurized gas flow to supply oxygen to the rhizosphere (Chabbi et al. 2000; Sorrell and Hawes 2010). However, T. domingensis has high shoot density and flowering incidence only under shallow inundation conditions (Grace 1989). A recent study indicated that increasing depth from 40 to 137 cm for 6 weeks decreased growth, biomass, photosynthesis, and belowground non-structural carbohydrate storage of T. domingensis (Chen et al. 2010, 2013). After T. domingensis returned to a depth of 40 cm during a 4-week recovery, previous damage to roots and loss in belowground biomass of plants previously subjected to in excess of 90-cm inundation did not recover. Increasing inundation also reduces the anchorage capacity of T. domingensis by causing the plant to decrease biomass allocation to rhizomes and roots and increase allocation to shoots (Grace 1989; Chen et al. 2010; Miao and Zou 2012).
Hydrologic regimes are a major driving factor in CTWs and affect the physical, chemical, and biological processes of phosphorus (P) cycling (Kadlec and Wallace 2009; Chen 2011). Phosphorus loosely bound to sediments is released during extended hydroperiods, due to the reduction of iron oxides and sulfate (Lamers et al. 1998; Aldous et al. 2005). The solubility of phosphates, particularly in Fe enriched soil, increases under an extended hydroperiod, which causes some adsorbed P to be released (Reddy et al. 1999). At shallow (i.e., 5–10 cm) inundation conditions, T. latifolia can assimilate released soluble reactive P (SRP) from sediments and thus increase its spread and dominance in wetlands (Boers and Zedler 2008). Deep inundation or soil reduction conditions results in stress to roots of wetland plants, such as T. domingensis, limiting the capability for P uptake (DeLaune et al. 1999; Chen et al. 2013). The released SRP can also be taken up rapidly by microbial communities, thus stimulating litter decomposition and consequently contributing to additional P release (Qualls and Richardson 2000; DeBusk and Reddy 2005). In addition, water depth influences P removal in CTWs by affecting both biotic communities and hydraulic loading (Chimney and Newman 2006; Kadlec and Wallace 2009). The volumetric P uptake rate consistently decreased with increasing inundation depths in two wetlands in Listowel, Ontario and Hillsboro, Oregon (see Kadlec and Wallace 2009). A study on several Florida lakes demonstrates that at low hydraulic loading rates, the lakes exhibit similar P removal rates to emergent dominated wetlands, but at high hydraulic loading rates P removal performance declines (Knight et al. 2003).
Macrophytes are an essential component for P removal in surface-flow CTWs because of their direct nutrient uptake and structure that foster various P removal processes including particulate setting (Kadlec and Wallace 2009; Chen 2011). In P-enriched T. domingensis dominated zones of Water Conservation Area 2A of the Everglades, microbial biomass P acts as short-term storage and accounts for 13 % of annually sequestered P (Qualls and Richardson 1995; Richardson 2008). T. domingensis P uptake accounts for approximately 32 % of the radioactive 32P added to the system, and the remaining 55 % of the added 32P is found in the soil layer after 1 week (Davis 1982). Some of the P absorbed by macrophytes during the growing season is released during turnover and senescence through litter decomposition (Qualls and Richardson 2000). Other macrophyte P is stored as recalcitrant organic matter through litter burial and peat accretion and provides long-term P sequestration (Craft and Richardson 1993; Richardson 2008). The sediment-litter compartment contains the largest amount of P, and P accretion can reach nearly 1 g P m−2 year−1, accounting for 80–90 % of total annual P storage in WCA-2A (Richardson 2008). Therefore, macrophyte communities are intimately involved in P removal processes through direct nutrient uptake and subsequent biogenic accumulation or autogenic burial (Chen 2011).
The Everglades Stormwater Treatment Areas (STAs) are the largest CTWs in the United States, encompassing approximately 180 km2. The STAs are operated by the South Florida Water Management District (SFWMD) to treat P enriched runoff from agricultural and other sources as part of the Everglades restoration effort (Sklar et al. 2005). The STAs are highly influenced by fluctuating hydrologic conditions (Ivanoff et al. 2012). High flows during storm events can result in inflows that cause excessive inundation for T. domingensis dominated treatment cells of the STAs (Pietro et al. 2011). Uneven topography within treatment cells can also lead to deep water areas even when target stages are maintained. Understanding the influence of long-term hydrologic conditions on T. domingensis and their roles in P retention and release processes in the STAs is essential in developing strategies to improve the water quality of STA discharges.
The objective of this study was to determine the influence of inundation depth on phytomass (biomass and necromass), shoot density, photosynthesis, and elongation of T. domingensis in an STA and to evaluate these results relative to P treatment performance in order to understand the relationship among hydrology, vegetation, and P treatment in these systems. This field investigation was a follow-up to previous studies (Chen et al. 2010, 2013) and was performed to identify the effects of inundation depth on cattail communities in relation to P treatment performance and to provide further insights to STA management.
Methods
Study Site
STA-1East is a surface-flow CTW that has a treatment area of 2078 ha, located west of West Palm Beach, Florida (Fig. 1). This CTW is divided into eight treatment cells separated by interior levees. Water flows through the system via various structures, such as water-control gates and pumps. Currently STA Operation Plans include guidance to maintain a target water depth of 38 cm between flow events to create conditions favorable for optimal and sustainable plant communities (SFWMD 2007). The target plant community in each treatment cell is either emergent aquatic vegetation (EAV) or submerged aquatic vegetation (SAV) although most cells have a mixture of both in varying extent. This study was conducted in Cell 7, a 169-ha, T. domingensis-dominated treatment cell. Cell 7 had a P loading rate varying from 0.01 to 5.33 g m−2 year−1 and a hydraulic loading rate from 0.3 to 6.4 cm day−1 from 2006 to 2011. Soil in Cell 7 is Okeelanta muck. Soil bulk density, total carbon, total nitrogen (N), and total P (TP) of the surface layer (0–10 cm) was 0.56 g cm−3, 241 g kg−1, 16.4 g kg−1, and 320 mg kg−1, respectively. The topography in Cell 7 is highly variable, resulting in localized portions of the cell experiencing different depths of inundation even when the target stage is achieved.
Effect of Inundation Depth on T. domingensis Community
To examine the effects of inundation depth on phytomass, shoot density, photosynthesis, and shoot elongation of T. domingensis, 17 2 m × 4 m plots were established in May 2009: 14 plots in Cell 7 and three plots in Cell 6 (reference site), an adjacent treatment cell that was dominated by SAV with patches of T. domingensis stands (Fig. 1). All 17 plots were established in monospecific T. domingensis stands with the same soil type and similar surface water quality. The Cell 7 plots experienced deepwater conditions while the three plots within Cell 6 experienced lower water levels. Field observations and sampling were conducted during May–November 2009. Water depth in the plots was monitored biweekly using a graduated PVC pole. The number of shoots of rooted plants was counted in each of the plots in June, August, and October 2009. Shoot density was estimated as the number of plant shoots per square meter. In plots with floating T. domingensis mats in Cell 7, neither the floating mats of T. domingensis nor newly recruited seedlings growing on the mats were considered in shoot density calculations. Photosynthesis was measured on three fully-expanded, representative leaves from three different plants in each of the plots between 10:00 am and 12:00 pm in June, August, and October 2009, using a LI-6400 Portable Gas Exchange System (LI-COR, Lincoln, Nebraska, USA). In addition, five newly emerged T. domingensis leaves on five different mature, rooted plants were selected and labeled in each of the plots. The length of those labeled leaves was measured at 5-day intervals in June, August, and October 2009, respectively. Shoot elongation was calculated as the length increment of newly emerged leaves in a 5-day period and was expressed as cm/day. Phytomass in a 0.25 m2 quadrat randomly placed within the plot was collected with a woodcutting hand saw cutting along the quadrat and hand pulling out all plant materials, and transported to a laboratory and stored in a refrigerator in November 2009. The phytomass was separated into leaf, shoot base, root, rhizome, and necromass. Biomass and necromass were separated with hand sorting based on visual cues. The plant materials were individually dried at 70 °C to a constant weight and weighed for biomass and necromass. Plant tissue samples were analyzed for total N and TP.
Surface Water P Concentrations
The water stage and flow at each of the water-control inflow and outflow structures (S373A-B and S374A-C, respectively) in Cell 7 (Fig. 1) were measured daily. Daily water depth in Cell 7 was calculated as the difference between daily inflow and outflow surface water stage and the average ground elevation of the cell. Surface water samples were collected weekly at S373A-B and S374A-C for TP, SRP, and total dissolved P (TDP). Surface water TP, SRP, and TDP were analyzed at the Chemistry Laboratory of the SFWMD using SM4500PF method (American Public Health Association 1998). Dissolved organic P (DOP) was calculated by subtracting SRP from TDP. Particulate P (PP) was defined as particulate-bound P including both organic and inorganic forms and was calculated by subtracting TDP from TP. The percent change (%Δ) in P concentration by P species was calculated as the difference between the inflow and outflow P species concentrations (flow-weighed means) as the follows:
Statistical Data Analysis
Statistical analyses were performed using the SAS GLM procedure (SAS v9.3; SAS Institute Inc., Cary, NC, USA). The effect of water depth and sampling date on shoot density, photosynthesis, and shoot elongation of T. domingensis in plots was analyzed with a two-way analysis of variance (ANOVA). Pearson’s regression was performed to examine the effect of inundation depth on biomass, belowground and leaf biomass ratio, necromass, necromass N and P, and phytomass N and P of T. domingensis in plots. Statistical significance was determined at α = 0.05. The normality and equality of variances were tested prior to all of the analyses and no data transformation was performed.
Results
Monthly Flow and Daily Water Depth
Monthly inflow to Cell 7 of STA-1E varied seasonally from zero to 15 × 106 m3 (Fig. 2a). Inflow and outflow were high in the wet season (May to October) and were low in the dry season (November to April) prior to May 2010. Due to the declining cell condition and poor P removal performance, both inflow and outflow were maintained at low levels from May 2010 to July 2011. The average daily water depths of Cell 7 in 2006–2011 were 56, 61, 74, 57, 57, and 61 cm, respectively that were substantially greater than the target depth of 38 cm between flow events (Fig. 2b). From June 2007 to October 2009 the average cell water depth was 69 cm. Five extreme high-water episodes with >100-cm depth also occurred during this 28-month period. In contrast, there was a 48-day period of less than 38-cm depth from October to December 2009, due to water level management activities.
Effect of Inundation Depth on T. domingensis
Leaf, belowground, and total biomass were not significantly related to inundation depth in the plots (p > 0.05, Fig. 3a). Total biomass was 2,992 ± 331 g m−2, consisting of 43 % leaf and 57 % belowground biomass. Necromass and belowground biomass/leaf biomass ratio significantly increased with increasing inundation depths (p < 0.05, Fig. 3b and c).
Shoot density and photosynthesis were affected by inundation depth (p < 0.01) but did not significantly differ among sampling dates. Shoot density and photosynthesis significantly decreased with increasing inundation depth from 30 to 80 cm (p < 0.01, Fig. 4a and b).
Shoot elongation was affected by inundation depth (p < 0.01) and differed among the sampling dates (p < 0.01), but the interaction of sampling date and inundation depth on elongation was not significant. Shoot elongation decreased with increasing inundation depth from 5 to 90 cm (Fig. 5). Shoot elongation in June and August (6.8 cm/day) was higher than in October (4.8 cm/day).
Effect of Inundation Depth on the Nutrient Uptake of T. domingensis
The concentrations and contents of N and P in shoot, shoot base, root and rhizome of T. domingensis were not significantly related to inundation depth (p > 0.05). While concentrations of necromass N and P were not significantly related to inundation depth (p > 0.05), the contents of necromass N and P and phytomass N significantly increased with increasing inundation depth from 30 to 80 cm (Fig. 6a, b, and c). Phytomass P content did not significantly differ with increasing inundation depth from 30 to 80 cm. In addition, necromass N concentration was higher compared with other tissues (except for roots). Necromass P concentration was lowest compared with other tissues, and necromass N/P molar ratio and N and P contents were highest compared with other tissues (Table 1).
Changes in Inflow and Outflow P Concentrations
Mean weekly TP concentrations of the inflow and outflow from July 2006 to July 2011 were 0.123 ± 0.004 (mean ± SE) and 0.112 ± 0.004 mg P L−1, respectively. While much lower outflow concentrations compared to inflow are expected for STAs, outflow TP observed in Cell 7 between January 2009 and May 2010 was generally higher than inflow TP (Fig. 7a). Lower outflow TP than inflow TP was observed between June 2010 and July 2011. The annual change in TP concentration ranged from an increase of 54 % to a decrease of 41 % (Table 2).
Inflow SRP had an average concentration of 0.047 ± 0.004 mg P L−1 and accounted for 38 % of inflow TP. Outflow SRP, with an average concentration of 0.024 ± 0.003 mg P L−1, was generally lower than inflow SRP from 2006 to 2011, with few exceptions (Fig. 7b). The annual decrease in SRP concentration was approximately 30 % in 2006–2007. In 2008–2009 an increase in SRP concentration ranged from zero to 13 %. The decrease in SRP concentration was the highest, at 38 % in 2011 (Table 2).
Inflow TDP had an average concentration of 0.064 ± 0.004 mg P L−1 and accounted for 52 % of inflow TP. Outflow TDP, with an average concentration of 0.045 ± 0.002 mg P L−1, did not substantially differ from inflow TDP (Fig. 7c), with a decrease of ≤10 % in 2006–2009 and 25–41 % in 2010–2011 (Table 2).
Inflow DOP had an average concentration of 0.019 ± 0.0004 mg P L−1 and accounted for 15 % of inflow TP. Mean outflow DOP concentration was 0.023 ± 0.001 mg P L−1 from 2006 to 2011. Outflow DOP concentrations were generally higher than inflow DOP concentrations prior to June 2010 (Fig. 8a). The annual decrease of DOP was ≤10 % or did not occur in 2006–2011 (Table 2). Compared with other P species, this treatment cell was the least effective in reducing DOP concentrations.
Inflow PP had an average concentration of 0.058 ± 0.001 mg P L−1 and accounted for 48 % of inflow TP. Mean outflow DOP concentration was 0.068 ± 0.003 mg P L−1 from 2006 to 2011. The concentration of outflow PP was not substantially less than inflow PP from 2006 to 2009 (Fig. 8b). Annual decreases of PP concentration was <5 % or did not occur in 2006–2009 and were 23–26 % in 2010–2011 (Table 2).
Discussion
Flow and Water Depth
Cell 7 was either operationally off line or online with restricted flow after June 2010 due to poor P removal performance that was attributed to vegetation decline (Ivanoff et al. 2012). Therefore, this cell had low inflow and outflow hydraulic loading in 2010–2011 (Fig. 2a). Ground elevation in Cell 7 is much lower than adjoining areas and, as a consequence, prevailing deepwater conditions (Fig. 2b) resulted in unsuccessful vegetation establishment and gradual decline in vegetation coverage over the years of operation (Pietro et al. 2011). The 48-day shallow inundation from October to December 2009 (Fig. 2b) was a result of water-level drawdown (Germain and Pietro 2010).
Impacts of Inundation Depth on Ecophysiology of T. domingensis
Although total biomass did not differ with increasing inundation depths (Fig. 3a), similar to the result of recent mesocosm study (Miao and Zou 2012), increased belowground and leaf biomass ratio (Fig. 3b) indicates that T. domingensis has a positive response strategy to survive deep inundation stress. Increased belowground biomass allocation helps flooded plants anchor to sediment. Lack of significant regression between inundation depth and total biomass to some degree implies the plasticity of T. domingensis to varying inundation depths. However, the response of the biomass allocation of T. domingensis in the field is inconsistent with previous mesocosm studies (Chen et al. 2010; Miao and Zou 2012). With greater space and light availability, T. domingensis may allocate more resources for rhizomes and roots to survive stressed conditions. Although these adaptive strategies help T. domingensis survive deep inundation in wetlands (Grace 1989; Fraga and Kvet 1993), photosynthesis and the incidence of flowering decrease with increasing inundation depths (Fig. 4b; Grace 1989; Chen et al. 2010). Decreased shoot density and elongation caused by increasing inundation depths (Figs. 4a and 5) suggest that deepwater conditions adversely affect reproduction and propagation of T. domingensis communities in the STAs. Studies by Chen et al. (2010, 2013) indicates that a 6-week-long period of inundation from 91 to 137 cm decreased relative growth rate, the number of both new shoots and live leaves, chlorophyll fluorescence, and belowground non-structural carbohydrate storage of T. domingensis, compared with 40-cm inundation.
Phosphorus Reduction in Relation to T. domingensis Decline
The poor performance in P treatment in Cell 7 (Table 2 and Figs. 7 and 8) indicates that wetland conditions did not favor processes for P assimilation and storage from 2006 to 2011. In particular, persistent deep inundation had adverse effects on the health of T. domingensis community (Figs. 4 and 5; Chen et al. 2010) and presumably the processes of P cycling in wetlands (Reddy and DeLaune 2008). T. domingensis community decline, including long-term coverage decline (Pietro et al. 2011), is likely one of the primary reasons for such a low P removal performance. Floating T. domingensis mats have been observed in Cell 7, which has been subjected to extended periods of deepwater conditions (Germain and Pietro 2010; Pietro et al. 2011). The T. domingensis floating mats consisting of dying and dead plant materials adversely affect treatment performance . First, when floating T. domingensis mats occur, nutrient uptake by plant roots are likely compromised or lost. Second, due to exposure to air, dying and dead plant materials on the mat are rapidly decomposed by microbial communities and solar radiation (Tate 1980; D’Angelo and Reddy 1999), resulting in nutrients being released to the water column and thus increasing nutrient availability for uptake and/or transport. Increased necromass and associated nutrients (Fig. 6) have a great potential to contribute to increased internal nutrient availability. Consistently higher PP and DOP concentrations in the outflow than inflow in 2009 (Fig. 8a and b) indicate that the decomposing plant fragments and particles from the interior wetland contributed to increased PP and DOP concentrations of the outflow as reported in other studies (Qualls and Richardson 2000; DeBusk and Reddy 2005). Therefore, the impacted and damaged T. domingensis community may account for low P treatment performance in this treatment cell.
Following the water-level drawdown during October – December 2009, efforts to improve vegetation were initiated. Schoenoplectus californicus (giant bulrush) was planted and established in the eastern open-water area of the wetland (Ivanoff et al. 2012), and a consequent establishment of SAV was observed in some of the impacted areas. Improved P treatment in 2010–2011 (Table 2) is likely a result of vegetation condition improvements with low hydraulic and TP loading rates, 0.3 cm/day and 0.01 g P/m2/year, respectively, to Cell 7 (Fig. 2a; Ivanoff et al. 2012).
Implications for Management of STAs
Multiple interactive factors including hydrology, nutrient loading, and vegetation influence P removal in CTWs (Kadlec and Wallace 2009; Chen 2011). In particular, the level of interaction of hydroperiod and the macrophyte community on P treatment processes is not well quantified (Chen 2011). A parallel relationship between vegetation density and the P removal rate constant was observed during the first 3 years of operation of the Orlando Easterly Wetland for tertiary effluent polishing but this relationship was not found in the following 3 years of operation (Kadlec and Knight 1996). Little information is available on the quantitative relationship between vegetation density and P treatment, although the importance of maintaining a critical hydroperiod (depth, duration, frequency, and timing of inundation) at which selected vegetation thrives is recognized (Kadlec and Wallace 2009; Chen et al. 2010).
Because CTWs are often maintained in flooded conditions for nutrient removal purposes (Wetzel 2001) as long as water sources are available, this study focused on the effect of inundation depth and did not consider the effect of the duration and frequency of inundation. To help understand the complex relationships among hydroperiod, vegetation, and P removal in STAs, inundation depth zones in EAV cells can be categorized as follows: optimal zone (i.e., 20–40 cm), subtle stress zone (i.e., 40–60 cm), and lethal stress zone (i.e., >60 cm) in a hypothetical conceptual model outlining the effect of long-term inundation on T. domingensis community and P removal in surface-flow CTWs (Fig. 9). Inundation depths of <20 cm are not considered here because maintaining significantly shallow depths is not economically feasible for treating flows to STAs. In the optimal zone, where wetlands have high vegetation health index in terms of shoot density or productivity (Fig. 4a; Grace 1989), plant associated treatment is robust. With increasing inundation depth in the subtle stress zone, shoot density or productivity gradually decreases (Fig. 4a; Grace 1989) and T. domingensis floating mats may occur (Chimney et al. 2000). Following the occurrence of T. domingensis floating mats, P treatment likely declines rapidly because of PP and DOP releases through decomposition of increased necromass (Fig. 6a and b; Qualls and Richardson 2000; DeBusk and Reddy 2005). In the lethal stress zone, where T. domingensis is severely damaged or doesn’t survive, long-term deep inundation may drive STAs to become an open-water habitat with low P removal (Wetland Solutions Inc. 2004) and consequently STAs likely become a nutrient source, instead of a nutrient sink. Therefore, it is important to maintain an optimal range of inundation depths for sustainable operation of STAs. The operation of the STAs is dictated by both rainfall events and water management practices that control runoff from drainage basins to the system. Therefore, long-term inundation depths in the subtle stress zone are a warning sign that sustainable treatment performance may not be maintained in an STA. When STAs have inundation depths in the lethal stress zone, there should be an immediate relief to lower water levels to help recover T. domingensis communities and maintain their P-removal capability.
A hypothetical conceptual model outlining the effect of long-term inundation on T. domingensis community and P removal in surface-flow constructed treatment wetlands. Zones I (i.e., 20–40 cm), II (i.e., 40–60 cm), and III (i.e., >60 cm) represent optimal, subtle-stress, and lethal-stress ranges of water depth in the wetlands, respectively. Water depths of <20 cm are not considered because keeping shallow water depths may not economically feasible for treating stormwater. Solid and dashed curves represent a response of vegetation health index and net phosphorus removal to water depth in the wetlands, respectively
Conclusions
The study was conducted to explore the relationship between long-term inundation and P removal in surface-flow CTWs and identified that vegetation plays a key role in understanding this complex relationship. As a primary variable in CTWs, hydrologic regime drives both system metabolism, including production and decomposition, and nutrient cycling through vegetation community dynamics. The role of vegetation builds a bridge between hydrologic regime and P removal and is closely associated with P removal, particularly through providing carbon for microbes and oxygen in the rhizosphere of plants in wetlands (Wetzel 2001). Therefore, the interactive effects of hydrologic regime and vegetation likely determine P removal efficiency and whether CTWs likely become a nutrient source or sink (Chen 2011). The conceptual model (Fig. 9) sheds light on relationship among hydrologic regime, vegetation, and P removal, but this study did not quantify a direct linkage between hydrologic regime and P removal in STAs. There is need for further studies to understand how inundation impacts P removal in CTWs.
References
Aldous A, McCormick P, Ferguson C, Graham S, Craft C (2005) Hydrologic regime controls soil phosphorus fluxes in restoration and undisturbed wetlands. Restoration Ecology 13:341–47
American Public Health Association (1998) Standard methods for the examination of water and wastewater, 20th edn. American Water Works Association and Water and Environment Federation, Washington, DC
Boers AM, Zedler JB (2008) Stabilized water levels and Typha invasiveness. Wetlands 28:676–685
Chabbi A, McKee KL, Mendelssohn IA (2000) Fate of oxygen losses from Typha domingensis (Typhaceae) and Cladium jamaicense (Cyperaceae) and consequences for root metabolism. American Journal of Botany 87:1081–1090
Chen H (2011) Surface-flow constructed treatment wetlands for pollutant removal: applications and perspectives. Wetlands 31:805–814
Chen H, Zamorano MF, Ivanoff D (2010) Effect of flooding depth on growth, biomass, photosynthesis, and chlorophyll fluorescence of Typha domingensis. Wetlands 30:957–965
Chen H, Zamorano MF, Ivanoff D (2013) Effects of deep flooding on nutrients and non-structural carbohydrates of mature Typha domingensis and its post-flooding recovery. Ecological Engineering 53:267–274
Chimney MJ, Newman J (2006) Everglades nutrient removal project test cell research: optimizing Stormwater Treatment Area performance—the importance of hydrologic conditions in maximizing nutrient retention by the STAs. Technical Publication ERA #438. South Florida Water Management District, West Palm Beach
Chimney MJ, Nungesser M, Newman J, Pietro K, Germain G, Lynch T, Goforth G, Moustafa MZ (2000) Chapter 6 Stormwater Treatment Areas. 2000 Everglades consolidation report. South Florida Water Management District, West Palm Beach
Craft CB, Richardson CJ (1993) Peat accretion and phosphorus accumulation along an eutrophication gradient in the northern Everglades. Biogeochemistry 22:133–156
D’Angelo EM, Reddy KR (1999) Regulators of heterotrophic microbial potentials in wetland soils. Soil Biology and Biochemistry 31:815–830
Davis SM (1982) Patterns of radiophosphorus accumulation in the Everglades after its introduction into surface water. Tech. Pub. 82-2. South Florida Water Management District, West Palm Beach
DeBusk WF, Reddy KR (2005) Litter decomposition and nutrient dynamics in a phosphorus enriched Everglades marsh. Biogeochemistry 75:217–240
DeLaune RD, Jugsujinda A, Reddy KR (1999) Effect of root oxygen stress on phosphorus uptake by cattail. Journal of Plant Nutrition 22:459–466
Fraga JMP, Kvet J (1993) Production dynamics of Typha domingensis (Pers.) Kunth populations in Cuba. Journal of Aquatic Plant Management 31:240–243
Germain G, Pietro K (2010) Chapter 5 performance and optimization of the Everglades Stormwater Treatment Areas. In 2011 South Florida Environmental Report, West Palm Beach, FL, USA
Grace JB (1989) Effect of water depth on Typha latifolia and Typha domingensis. American Journal of Botany 76:762–768
Ivanoff D, Chen H, Gerry L (2012) Chapter 5 performance and optimization of the Everglades Stormwater Treatment Areas. In 2012 South Florida Environmental Report, West Palm Beach, FL, USA
Kadlec RH, Knight RL (1996) Treatment wetlands. CRC Press, Boca Raton
Kadlec RH, Wallace SD (2009) Treatment wetlands, 2nd edn. CRC Press, Boca Raton
Knight RL, Gu B, Clarke RA, Newman JM (2003) Long-term phosphorus removal in Florida aquatic systems dominated by submerged aquatic vegetation. Ecological Engineering 20:45–63
Lamers LPM, Tomassen MHBM, Roelofs JGM (1998) Sulfate-induced eutrophication and phytotoxicity in freshwater wetlands. Environmental Science and Technology 32:199–205
Miao SL, Zou CB (2012) Effects of inundation on growth and nutrient allocation of six major macrophytes in the Florida Everglades. Ecological Engineering 42:10–18
Pietro K, Germain G, Bearzotti R, Iricanin N (2011) Chapter 5 performance and optimization of the Everglades Stormwater Treatment Areas. In: 2011 South Florida Environmental Report, South Florida Water Management District, West Palm Beach, FL, USA
Qualls RG, Richardson CJ (1995) Forms of soil phosphorus along a nutrient enrichment gradient in the northern Everglades. Soil Science 160:183–198
Qualls RG, Richardson CJ (2000) Phosphorus enrichment affects litter decomposition, immobilization, and soil microbial phosphorus in wetland mesocosms. Soil Science Society of America Journal 64:799–808
Reddy KR, DeLaune RD (2008) Biogeochemistry of wetlands. CRC Press, Taylor and Francis Group, Boca Raton
Reddy KR, Kadlec RH, Flaig E, Gale PM (1999) Phosphorus retention in streams and wetlands: a review. Critical Reviews in Environmental Science and Technology 29:83–146
Richardson CJ (2008) Long-term phosphorus assimilative capacity (PAC) in the Everglades. In: Richardson CJ (ed) The Everglades experiments. Springer, NY, pp 567–578
Sklar FH, Chimney MJ, Newman S, McCormick P, Gawlik D, Miao S, McVoy C, Said W, Newman J, Coronado C, Cozier G, Korvela M, Rutchey K (2005) The ecological-societal underpinnings of the Everglades restoration. Frontiers in Ecology and the Environment 3:161–169
Sorrell BK, Hawes I (2010) Convective gas flow development and the maximum depths achieved by helophyte vegetation in lakes. Annals of Botany 105:165–174
South Florida Water Management District (SFWMD) (2007) STA-1East operation plan. West Palm Beach, FL, USA
Tate RL III (1980) Microbial oxidation of histosols. Advances in Microbial Ecology 4:169–210
Vymazal J (2007) Removal of nutrients in various types of constructed wetlands. Science of the Total Environment 380:48–65
Wetland Solutions, Inc (2004) Constraints on hydraulic loading rates to Stormwater Treatment Areas. Gainesville, FL, USA
Wetzel RG (2001) Fundamental processes within natural and constructed wetland ecosystems: short-term versus long-term objectives. Water Science and Technology 44:1–8
Acknowledgments
We thank M. Zamorano and M. Korvela for assistance in the field and S. Gray, D. Ivanoff, T. Piccone, L. Schwartz, and D. Unsell for comments on an early version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, H., Vaughan, K. Influence of Inundation Depth on Typha domingensis and its Implication for Phosphorus Removal in the Everglades Stormwater Treatment Area. Wetlands 34, 325–334 (2014). https://doi.org/10.1007/s13157-013-0500-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-013-0500-3


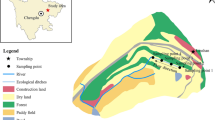

 ) and three outflow structures (
) and three outflow structures ( ) in Cell 7 of STA-1East, respectively
) in Cell 7 of STA-1East, respectively






