Abstract
Freezing-thawing in mid-high latitudes is an important factor controlling nutrient dynamics. We transplanted peatland columns (TQ) and freshwater marsh columns (SJ) in different latitudes into south seasonal frozen regions to determine the responses of greenhouse gas emissions from different wetlands to the freezing-thawing under climate warming. The decrease in CO2 and CH4 emissions during freezing stage were interrupted by a short emission peak. While N2O uptake rate reduced with decreasing temperature. In the thawing stage, all the three greenhouse gases exhibited emission peaks. CO2 were 159.83 mg m−2 h−1 (TQ) and 86.83 mg m−2 h−1 (SJ); CH4 were 1.32 mg m−2 h−1 (TQ) and 4.07 mg m−2 h−1 (SJ); N2O were 72.14 ug m−2 h−1 (TQ) and 22.15 ug m−2 h−1 (SJ). Meanwhile, N2O transferred from sink into source. With temperature increase, the emission rate of CO2 increased fast, while CH4 and N2O decreased. CO2 emission during freezing-thawing periods was significantly correlated with soil temperature and CH4 emission. Soil active organic carbon also played important roles in greenhouse gases emissions. Our study suggested that more greenhouse gases may release from wetlands into atmosphere in the context of global warming, and the potential release of CO2 and N2O during freezing-thawing periods was much higher in peatlands of permafrost zone.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Freezing-thawing is the fluctuations of soil temperature across the 0 °C isotherm (Hershfield 1979). It is the water transformation between liquid water and solid ice (Miller 1980). As one climate-driven pedoturbation, freezing-thawing regularly happens in mid-high latitude and high altitude regions (Grogan et al. 2004). It is the important meteorological event in boreal regions. Freezing-thawing can change the processes of soil nutrient dynamics, organic matter decomposition and affect carbon and nitrogen balance (Kidd et al. 2004). Recently, many studies focused on the effects of freezing-thawing on the key biogeochemical cycles of carbon and nitrogen, especially in the sensitive mid-high latitude regions (e.g., Grogan et al. 2004; Wang et al. 2011). And the greenhouse gas emission during seasonal freezing-thawing periods plays an important role in the atmosphere chemical change. For example, Some studies found that CO2 emissions during freezing-thawing periods make up about 3–50 % of that of the whole year (Lafluer et al. 2001; Wickland et al. 2001); CH4 emission rate in mires during thawing stage was about 2–3 times than that in summer, and is about 11 % of the whole year (Hargreaves et al. 2001); N2O emission peaks during freezing-thawing periods were detected in agricultural, fallow and forest land, and the N2O emissions during the entire winter period contributed to about 50 % of the annual N2O emissions (Teepe et al. 2000; Wang et al. 2009). Meanwhile, Song et al. (2006) also found the high emission peaks of CO2, CH4 and N2O during freezing-thawing periods in a freshwater marsh of Sanjiang Plain. Other ecosystems such as forest, farmland, temperate peatland, tundra and alpine meadow also exhibited emission peaks of greenhouse gas during freezing-thawing periods (Priemé and Christensen 2001; Bubier et al. 2002; Mikan et al. 2002; Kato et al. 2005). Therefore, the emission of greenhouse gas during freezing-thawing periods can’t be ignored. However, their source and mechanism are far from certain (Song et al. 2006; Wang et al. 2009). And the existing studies of greenhouse gas emissions are mainly via indoor controlled experiments, but few studies consider the effect of global warming. In addition, the lack of consensus on freezing-thawing manipulations and variation in methodologies resulted in the inconsistent results (Henry 2007). Therefore, the conclusions from indoor controlled experiments are yet to be verified.
Wetlands store large amounts of organic carbon, and play an important role in the global carbon balance. In Northeast China, the area of wetlands is about 657.4 × 104 hm2, and accounts for 48.3 % of total wetland area in China (Liu 2005). Freshwater marshes and peatlands are the two main wetland types in this region. Freshwater marshes are widely distributed in the Sanjiang plains, and peatlands are common in the Da and Xiao Xing’anling mountains. They are sensitive to climate change. Seasonal freezing-thawing is an important event in Northern China, and it impacts the evolution process of the ecosystem, controls the soil development, and affects the chemistry process of mass and energy cycles in the global ecosystems (Song et al. 2006). At present, global warming and human activity are changing the wetland ecosystem structure and function. They can affect greenhouse gas emissions during freezing-thawing periods by changing physical properties, freezing time, freezing depth and thawing process (Henry 2007). However, there was no study of emissions of CO2, CH4 and N2O in the wetlands of China during freezing-thawing periods in the context of climate warming. Therefore, we transplanted the peatland columns in permafrost zone (TQ) and freshwater marsh columns in seasonal frozen regions (SJ) into a southern seasonal frozen ground to simulate the plots under climate warming. The objectives of this study are to reveal the characters of greenhouse gas emissions from different wetlands during freezing-thawing periods in the context of climate warming, and to discuss the associated mechanisms impacts by seasonal freezing-thawing.
Materials and Methods
Study Areas and Sampling
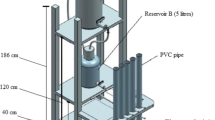
In order to study the effects of warmer climate on the greenhouse gas emission during freezing-thawing periods, we chose the two important kinds of wetlands in Northeast China: a peatland in Da Xing’anling Mountains, and a freshwater marsh in Sanjiang Plain. Their characteristics are shown in Table 1. In October 2010, using a polypropylene tube (diameter 10 cm; height 60 cm), we took seven soil columns (diameter 10 cm; soil height 30 cm) from peatland and freshwater marsh respectively, and transplanted them into the seasonal frozen ground at the Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences (43°48′N, 125°14′E). The climate is continental monsoon, with an average altitude of 211-m, mean annual temperature of 5.6 °C, and annual precipitation ranging from 522 mm to 615 mm. The soil columns from different latitudes make two treatments. Every treatment has four replicates. The bottoms of the tubes were sealed with caps and buried in the ground in the late October 2010. The distance between the soil surface and the upper of the tube is 30 cm. Meanwhile, the thermometers, whose sensitivity is ±0.3 °C, were set at depths of 0, 10, 20, 30, 40, and 60 cm. The PT1000 data logger measures the outputs of thermometer every 10 min, and the format of data is the same as the recording of meteorological data. Because the soil in the polypropylene tube is only 30 cm thick and the tubes are 60 cm long, the thermometers were set at 60 cm. The soil columns were covered with a foam box to avoid precipitation and reduce evaporation (Fig. 1). Additionally, we analyzed the active organic carbon fractions in the other soil columns as the control.
Sampling and Analyzing
After the soil equilibrated for about one month, we observed CO2, CH4 and N2O emissions in the soil columns once per week during winter and twice per week during freezing-thawing periods from late November 2010 to April 2011. Soil and air temperature were recorded simultaneously. When collecting gas samples, the upper caps with tree-way valves were screwed tightly into the permanently polypropylene tubes, and sealed with vaseline and plastic film to avoid leaks. 50 ml nylon syringes were connected with the three-way valves to collect gas at 10 min interval over 30 min. The samples were measured by a modified gas chromatography (GC Agilent 7820A) within 12 h. CH4 and CO2 were separated with a 2 m stainless-steel column with inner diameter of 2 mm column (80/100 mesh). FID works at 200 °C using highly pure nitrogen as a carrier gas at a flow rate of 30 ml min−1. N2O was separated using a 1 m stainless-steel column with inner diameter 2 mm Porapak Q (80/100 mesh) and measured using the ECD, which was set at 330 °C using highly pure nitrogen as a carrier gas at a flow rate of 35 ml min−1. The column temperatures were maintained at 55 °C for separation (Song et al. 2006). The GC was standardized using a CO2, CH4 and N2O standard gas after approximately every eight samples.
The flux was calculated according to the following equation (Song et al. 2006):
Where \( \frac{ dc}{ dt} \) is the slope of the curve of gas concentration variation with time; M is the mole mass of gas, P is the atmospheric pressure in sampling site, T is the absolute temperature during sampling, and H is the relative height of column above the water surface. V 0 , T 0 , P 0 are gas mole volume, air absolute temperate and atmospheric pressure under standard conditions. When the linear regression with coefficient R 2 > 0.90 occurred, the data are available.
Sample Analyses
Soil organic carbon (SOC) (using potassium dichromate-external heating method) and total nitrogen (TN) (using Kjeldahl digests method) were determined following the laboratory methods described by Zhang (2000). Soil light fraction organic carbon (LFOC) was determined using the density fractionation method (Gregorich and Ellert 1993). Soil particle organic carbon (POC) was measured by particle size (Cambardella and Elliott 1992). Soil microbial biomass carbon (MBC) was determined by a fumigation-extraction method (Vance et al. 1987). Soil dissolved organic carbon (DOC) was determined by the method of Jones and Willett (2006).
Statistical Analysis
One-way analysis of variance (ANOVA) was performed to assess the differences in active organic carbon fractions before and after freezing-thawing periods and gas emission in two kinds of wetlands (LSD). Correlation analysis was used to examine the relationships among CO2, CH4 and soil temperature. SPSS (13.0) software package for windows performed all statistical analysis. The Origin (7.5) software drew figures.
Results and Discussion
Seasonal Variations of Freezing-Thawing Characteristics
Northeast Institute of Geography and Agroecology, Chinese Academy of Sciences belongs to the seasonal frozen ground. From the Fig. 2a, we know that the freezing-thawing curve was separated by freezing and thawing in two stages. The first stage is freezing period. It is about from the beginning of November 2010 that the soil columns began to freeze. With decreasing temperature, the freezing process proceeded from the upper part of the column to the bottom part. And until 9 January 2011, the soil columns were fully frozen. But the lowest temperature in the 10 cm soil layer was not more than −6 °C (Fig. 2b). The second stage is thawing period. It is about from the beginning of March 2011 that the soil columns began to thaw. With increasing temperature, the thawing process proceeded from both the upper and bottom parts of soil column to the middle. And by 5 April 2011, soil columns were fully thawed. Meanwhile, the 10 cm soil temperature continues to increase, but it increased slowly near 0 °C, and then increased quickly.
Characteristics of CO2 and CH4 Emissions During Freezing-Thawing Periods
During freezing period, CO2 and CH4 emission rates in TQ and SJ gradually reduced with decreasing temperature but the decrease in CO2 and CH4 emissions were interrupted by short emission peaks before the soil fully froze. The short peaks of CO2 were 232.99 mg m−2 h−1 (TQ) and 46.32 mg m−2 h−1 (SJ). The CH4 were 0.89 mg m−2 h−1 (TQ) and 1.66 mg m−2 h−1 (SJ). This result was consistent with the findings of CO2 peak in agriculture (Teepe et al. 2001) and CH4 burst in tundra (Mastepanov et al. 2008). This emission peak was possibly caused by water soluble CO2 and CH4 being forced out of the growing ice structure (Teepe et al. 2001; Mastepanov et al. 2008). Even though the soil columns were fully frozen during winter, there were still gas emissions: about 0.90 mg m−2 h−1(TQ) and 0.35 mg m−2 h−1 (SJ) for CO2, and 0.084 mg m−2 h−1 (TQ) and 0.012 mg m−2 h−1 (SJ) for CH4 (Fig. 3). It suggested that microbes which produce CO2 and CH4 were activated even in winter, and the gas can escape through frost-induced cracks (Teepe et al. 2001; Sharma 2006).
During the thawing period, there was obvious emission peaks in CO2 and CH4. The peaks of CO2 were 159.83 mg m−2 h−1 (TQ) and 86.83 mg m−2 h−1 (SJ). While there were two peaks in CH4 emissions, the highest values were 1.32 mg m−2 h−1 (TQ) and 4.07 mg m−2 h−1 (SJ). It is possible that the thawing process proceeded from upper and bottom to middle, and resulted in the two peaks. When the soil columns were fully thawed, the CO2 emission rate increased rapidly with soil temperature. The significant positive correlation between CO2 emission and soil temperature (Fig. 4b) suggested that temperature as an indirect factor controlled CO2 emission by adjusting microbial activities. However, CH4 emission rate gradually decreased after the peak, and exhibited absorption in the late thawing stage. That was because there was not external water supply during freezing-thawing periods, and soil water might be the important factor to limit CH4 emission (Song et al. 2003). Meanwhile, methanotrophs are concentrated in the upper soil layer (Schimel 1995). CH4 from the underlying soil layer will be oxidized by methanotrophs during the diffusion, which resulted in a massive CO2 release and CH4 uptake in the late thawing stage (Fig. 3a).
The emission peaks of CO2 and CH4 emission during the thawing stage were consistent with other studies (Priemé and Christensen 2001; Teepe et al. 2001; Song et al. 2006; Mastepanov et al. 2008). The causes of high emission peaks during thawing periods might be as follows. Firstly, with temperature increase, the activity of dormant microorganisms might be recovered and even elevated due to the repeatedly freezing-thawing cycles (Wagner et al. 2005; Sharma et al. 2006). Secondly, freezing-thawing resulted in much more soil active organic carbon release, and the released active organic carbon can supply enough substrate for microbial activity (Feng et al. 2007). From Table 2, we know that the accumulated LFOC, POC, DOC and MBC in fall decreased after the long freezing-thawing cycles. It suggested that these active organic carbon fractions might play important roles in CO2 and CH4 emission peaks during thawing stages. It is possible that the micromolecule like DOC and MBC will be utilized by microbe directly, but the macromolecule like LFOC and POC as the indirect carbon source. Lastly, with soil columns thawing, the diffusion barriers have been eliminated, and then the accumulated gas in the soil below the frozen soil surface immediately emitted (Herrmann and Witter 2002).
From the mean flux and cumulative CO2 and CH4 emissions during freezing-thawing periods (Table 3, Fig. 3), we know that at the same freezing-thawing periods, the emission of CO2 in SJ was significantly lower than that in TQ, which was contrary to CH4 emission. But all of them are the sources of CO2 and CH4. The different emissions of CO2 and CH4 in freshwater marsh and peatland were mainly due to the differences in soil microbial activities, active organic matter and soil physical properties. Compared to the in situ observations of CO2 and CH4 emission during snow covered season (Miao et al. 2012), our carbon emission rates were much higher. And the CO2 emission in TQ was higher than that in SJ, which was different from the in situ observations. It suggested that the potential release of CO2 during freezing-thawing periods was much higher in peatlands of permafrost zone than that in freshwater marsh of seasonal frozen regions. However, the lower temperature in Da Xing’anling Mountains restricted the carbon emission (Wang et al. 2010). Therefore, under global warming, more CO2 and CH4 will be released during freezing-thawing periods and their roles cannot be ignored.
Characteristics of N2O Emission During Freezing-Thawing Periods
From Fig. 5, we know that during freezing period, all the soil columns were the sink of N2O and the uptake rate decreased from 24.68 ug m−2 h−1 (TQ) and 11.98 ug m−2 h−1 (SJ) into 0.24 ug m−2 h−1 (TQ) and 0.15 ug m−2 h−1 (SJ). In our study, there is no emission peak of N2O during freezing period, which was different from the findings of Teepe et al. (2001). But the uptake capacity of N2O decreased with soil column freezing. It suggested that microbes in freezing soil were still active. And the anaerobic environment due to the ice barrier supported favorable conditions for denitrification. But the ice layers prevent the escape of soluble N2O into the liquid water film and may result in supersaturated soil solutions (Teepe et al. 2001). However, part of the trapped N2O may still escape through cracks in the ice layer into the air filled pore space (Teepe et al. 2001). Therefore, the capacity of soils for N2O uptake became smaller. However, when the soil thawed, all the soil columns transferred from N2O sink to source. The emission rate gradually increased with temperature increase, and the peak were 72.14 ug m−2 h−1 (TQ) and 22.15 ug m−2 h−1 (SJ). Our emission peak of N2O during thawing stage was consistent with other studies (Teepe et al. 2001; Koponen and Martikainen 2004; Ludwig et al. 2006; Song et al. 2006). When the soil columns were fully thawed, the emission rate began to decrease but they were always the N2O source. Usually, N2O production in soil is mainly from nitrification and denitrification by microbial activity (Bouwman 1994). Whether ecosystems acted as source or sink of N2O depended on the balance of N2O production and consumption. Our high N2O emissions during soil thawing may be the burst emission of accumulated N2O in the soil below the frozen soil surface due to the disappeared diffusion barriers (Burton and Beauchamp 1994). Meanwhile, denitrification is the main cause of N2O production during thawing periods. Ludwig et al. (2004) found that immediately after the beginning of the thawing, denitrification contributed by 83 % to the N2O production. That was because the enzyme reductase activity and active organic matter released by broken aggregates or from microorganisms killed by freezing-thawing cycles play an important role in controlling the N2O emissions during freezing-thawing periods (Wang et al. 2005).
From the mean flux and cumulative N2O emissions during freezing-thawing periods (Table 3, Fig. 5), the emission of N2O in TQ was significantly higher than that in SJ, which was contrary to the in situ observation of N2O emission during snow covered season (Miao et al. 2012). It suggested that under global warming, the potential emission capacity of N2O during thawing periods was much higher in the peatlands of permafrost zone than that in the freshwater marsh of seasonal frozen regions. Therefore, available nitrogen will be activated and more N2O will be emitted from wetlands during freezing-thawing periods, which intensify the greenhouse effect combined with CO2 and CH4.
Conclusion
In our study, we found that the emission rates of CO2 and CH4 during freezing stage decreased with soil freezing but the short peaks appeared before soil columns were fully frozen. However, all the soil columns were a N2O sink during freezing stage and the capacity of the sink reduced with temperature decrease. During the thawing stage, all the three greenhouse gases exhibited obvious peaks and N2O transferred from sink into source. Meanwhile, there was significant correlation between CO2, CH4 and 10 cm soil temperature. Soil active organic carbon also plays an important role in greenhouse gases emissions during freezing-thawing periods. The high CO2 and N2O emissions in the peatland of the permafrost zone suggested that their potential releases during freezing-thawing periods were much higher than that in the freshwater marsh of seasonal frozen regions. Therefore, under global warming, greater amounts of CO2, CH4 and N2O will release into atmosphere, and intensify the greenhouse effect.
References
Bouwman AF (1994) Direct emissions of nitrous oxide from agriculture soil. Report No. 773994004, National Institute of Public Health and Environmental Protection, Bilthoven, Netherlands
Bubier J, Crill P, Mosedale A (2002) Net ecosystem CO2 exchange measured by autochambers during the snow-covered season at a temperate peatland. Hydrological Processes 16:3667–3682
Burton DL, Beauchamp EG (1994) Profile nitrous oxide and carbon dioxide in a soil subject to freezing. Soil Science Society of America Journal 58:115–122
Cambardella CA, Elliott ET (1992) Particulate soil organic-matter changes across a grassland cultivation sequence. Soil Science Society of America Journal 56:777–783
Feng XJ, Nielsen LL, Simpson MJ (2007) Responses of soil organic matter and microorganisms to freeze-thaw cycles. Soil Biology and Biochemistry 39:2027–2037
Gregorich EG, Ellert BH (1993) Light fraction and macroorganic matter in mineral soils. In: Carter MR (ed) Soil sampling methods and analysis. Canadian Society of Soil Science, Lewis
Grogan P, Michelsen A, Ambus P et al (2004) Freeze–thaw regime effects on carbon and nitrogen dynamics in sub-arctic heath tundra mesocosms. Soil Biology and Biochemistry 36:641–654
Hargreaves KJ, Fowler D, Pitcairn CER, Aurela M (2001) Annual methane emission from Finnish mires estimated from eddy covariance campaign measurements. Theoretical and Applied Climatology 70:203–213
Henry HAL (2007) Soil freeze-thaw cycle experiments: trends, methodological weaknesses and suggested improvements. Soil Biology and Biochemistry 39:977–986
Herrmann A, Witter E (2002) Sources of C and N contributing to the flush in mineralization upon freeze-thaw cycles in soils. Soil Biology and Biochemistry 34:1495–1505
Hershfield DM (1979) Freeze-thaw cycles, potholes, and the winter of 1977–78. Journal of Applied Meteorology 18:1003–1007
Jones DL, Willett VB (2006) Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biology and Biochemistry 38:991–999
Kato T, Hirota M, Tang Y et al (2005) Strong temperature dependence and no moss photosynthesis in winter CO2 flux for a Kobresia meadow on the Qinghai–Tibetan plateau. Soil Biology and Biochemistry 37:1966–1969
Kidd RA, Bartsch A, Wagner W (2004) Development and validation of a diurnal difference indicator for freeze-thaw monitoring in the Siberia П project. Proceedings ENVISAT Symposium, Salzburg
Koponen HT, Martikainen PJ (2004) Soil water content and freezing temperature affect freeze-thaw related N2O production in organic soil. Nutrient Cycling in Agroecosystems 69:213–219
Lafluer PM, Roulet NT, Admiral SW (2001) Annual cycle of CO2 exchange at a bog peatland. Journal of Geophysical Research 106:3071–3081
Liu XT (2005) Wetlands in northeastern China. Science Press, Beijing (in Chinese)
Ludwig B, Wolf I, Teepe R (2004) Contribution of nitrification and denitrification to the emission of N2O in a freeze-thaw event in an agricultural soil. Journal of Plant Nutrition and Soil Science 167:678–684
Ludwig B, Teepe R, Gerenyu VL, Flessa H (2006) CO2 and CH4 emissions from gleyic soils in the Russian tundra and a German forest during freeze-thaw periods-a microcosm study. Soil Biology and Biochemistry 38:3516–3519
Mastepanov M, Sigsgaard C, Dlugokencky EJ et al (2008) Large tundra methane burst during onset of freezing. Nature Letters 456:628–631
Miao YQ, Song CC, Wang XW et al (2012) Greenhouse gas emissions from different wetlands during the snow-covered season in Northeast China. Atmospheric Environment 62:328–335
Mikan CJ, Schimel JP, Doyle AP (2002) Temperature controls of microbial respiration in arctic tundra soils above and below freezing. Soil Biology and Biochemistry 34:1785–1795
Miller RD (1980) Freezing phenomena in soils. In: Hillel D (ed) Applications of soil physics. Academic, San Diego, pp 254–299
Priemé A, Christensen S (2001) Natural perturbations, drying–wetting and freezing–thawing cycles, and the emission of nitrous oxide, carbon dioxide and methane from farmed organic soils. Soil Biology and Biochemistry 33:2083–2091
Schimel JP (1995) Plant transport and methane production as controls on methane flux from arctic wet meadow tundra. Biogeochemistry 28:183–200
Sharma S, Szele Z, Schilling R, Munch JC, Schloter M (2006) Influence of freeze-thaw stress on the structure and function of microbial communities and denitrifying populations in soil. Applied and Environmental Microbiology 72:2148–2154
Song CC, Yan BX, Wang YS et al (2003) Fluxes of carbon dioxide and methane from swamp and impact factors in Sanjiang Plain, China. Chinese Science Bulletin 48:2749–2753
Song CC, Wang YS, Wang YY, Zhao ZC (2006) Emission of CO2, CH4 and N2O from freshwater marsh during freeze-thaw period in Northeast of China. Atmospheric Environment 40:6879–6885
Teepe R, Brumme R, Beese F (2000) Nitrous oxide emissions from frozen soils under agricultural, fallow and forest land. Soil Biology and Biochemistry 32:1807–1810
Teepe R, Brumme R, Beese F (2001) Nitrous oxide emissions from soil during freezing and thawing periods. Soil Biology and Biochemistry 33:1269–1275
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biology and Biochemistry 19:703–707
Wagner D, Lipski A, Embacher A, Gattinger A (2005) Methane fluxes in permafrost habitats of the Lena Delta: effects of microbial community structure and organic matter quality. Environmental Microbiology 7:1582–1592
Wang LF, Cai ZC, Yang LF et al (2005) Effect of disturbance and glucose addition on nitrous oxide and carbon dioxide emissions from a paddy soil. Soil and Tillage Research 82:185–194
Wang F, Gao SB, Zhang KQ, Yang P, Bai LJ, Huang ZP (2009) Progress on N2O emission from soil in the freeze-thaw process. Ecology and Environmental Sciences 18:1933–1937 (in Chinese)
Wang XW, Li XZ, Hu YM et al (2010) Potential carbon mineralization of permafrost peatlands in Great Hing’an Mountains, China. Wetlands 30:747–756
Wang JY, Song CC, Wang XW, Wang LL (2011) Progress in the study of effect of freeze-thaw processes on the organic carbon pool and microorganisms in soils. Journal of Glaciology and Geocryology 33:442–452 (in Chinese)
Wickland KP, Striegl RG, Mast MA et al (2001) Carbon gas exchange at a southern Rocky Mountain wetland, 1996 1998. Global Biogeochemical Cycles 15:321–335
Zhang ZY (2000) Development and utilization of peat resources. Chinese Jilin Science and Technology Press, Changchun (in Chinese)
Acknowledgments
We gratefully acknowledge the “Strategic Priority Research Program—Climate Change: Carbon Budget and Related Issue” of the Chinese Academy of Sciences (Nos. XDA05050508 and XDA05020501), National Natural Science Foundation of China (Nos. 40930527, 41125001), CAS/SAFEA International Partnership Program for Creative Research Teams, and National Basic Research Program (973) of China (No.2009CB421103) for financial support. We thank Dr. Xianwei Wang and Rong Mao for soil sample collection.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, J., Song, C., Miao, Y. et al. Greenhouse Gas Emissions from Southward Transplanted Wetlands During Freezing-Thawing Periods in Northeast China. Wetlands 33, 1075–1081 (2013). https://doi.org/10.1007/s13157-013-0463-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-013-0463-4