Abstract
Rice fields provide important habitats for many endemic and endangered species originally dependent on wetlands as habitat. However, the value of rice fields has rarely been evaluated from a multi-scale perspective. We examined abundance of two frogs, the montane brown frog Rana ornativentris and the forest green tree frog Rhacophorus arboreus, that use rice fields as breeding sites, and explored local and landscape-level factors determining their abundance. To determine appropriate spatial scales influencing abundance, we generated different sized buffer circles around a focal rice field, calculated landscape composition in each buffer, and determined the regression model that best explained frog abundance using Akaike’s Information criterion (AIC). The montane brown frog and the forest green tree frog exhibited the lowest AIC at buffer sizes of 300 and 1,000 m, respectively. Both species exhibited a higher abundance at intermediate water depths (7–10 cm). At the landscape-level, the montane brown frog showed highest abundance at intermediate forest cover (50%–60%). Forest green tree frogs showed a monotonic increase with forest cover. Because each species responded somewhat differently to spatial scale and landscape composition, context and species dependent outcomes of local restoration practices are required for particular rice fields to achieve cost-effective results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Agricultural landscapes experienced a dramatic change in the second half of the 20th century by intensification of farmland management aiming to increase productivity and efficiency (Blaxter and Robertson 1995; Krebs et al. 1999). This led to a severe decline in biodiversity worldwide (e.g., Benton et al. 2003; Tscharntke et al. 2005). Because many species of indigenous plants and animals now depend on human-dominated landscapes, preservation and restoration of biodiversity in agricultural landscapes is a pressing conservation issue (e.g., Benton et al. 2003; Bennett et al. 2006).
Rice fields are considered to be important substitutes for natural wetlands and many organisms are known to depend on rice fields as foraging and breeding sites (Elphick 2000; Lawler 2001; Donald 2004). Rice fields occupy about 11% of the world’s arable lands (Falso and Ruiz 1997), and approximately 90% of them are distributed in Asian countries (FAO Statistics Division 2008). In Japan, rice cultivation has a history of more than a thousand years, and rice fields occupy a substantial portion of habitats for many indigenous and endangered species inhabiting wetlands (Washitani 2001; Kobori and Primack 2003). However, agricultural intensification and abandonment of rice fields since the 1970s deteriorated habitat quality for many species including insects, amphibians, and birds, some of which are now facing extinction (Washitani 2001; Kobori and Primack 2003). To seek effective restoration and conservation practices, research has been conducted to explore factors affecting distribution and abundance of organisms in rice fields (Fujioka and Lane 1997; Mukai et al. 2005; Fujimoto et al. 2008). As a result, water management of rice fields, such as flooding in winter, and enhancing connection between rice fields and streams are considered important restoration practices. In addition to these small-scale factors, landscape structure is also likely to play an important role in habitat quality. However, few studies have examined the influence of surrounding environments on organisms in rice fields (but see Amano et al. 2008). Because the rural landscape in Japan comprises a fine-scale mosaic of rice fields, forests, and human settlements (Kobori and Primack 2003), a research approach that incorporates different spatial scales is essential. Frogs that utilize rice fields as breeding sites may be an example requiring such an approach, because they are characterized by ontogenetic habitat shifts from aquatic to terrestrial ecosystems. It is well recognized that terrestrial land use has a large influence on the presence or density of frog species in ponds or marshes (Marsh and Trenham 2001; Guerry and Hunter 2002; Cushman 2006), including agricultural landscapes (Zanini et al. 2008; Babbitt et al. 2009). Moreover, several studies have shown that different species of pond or marsh breeding frogs respond to landscapes at different spatial scales (Houlahan and Findlay 2003; Gagne and Fahrig 2007; Eigenbrod et al. 2008). Thus, identifying multi-scale limiting factors for the target species will help predict the outcome of a particular restoration practice in a spatial context. However, no studies have been conducted from multi-scale perspectives on frogs that use rice fields as breeding sites.
Here we focused on the montane brown frog Rana ornativentris and the forest green tree frog Rhacophorus arboreus to explore local and landscape factors influencing the abundance of each frog species. Both species are declining in Japan due to habitat alteration and agricultural intensification (Ise and Mitsuhashi 2006; Yoshida et al. 2006), and have been red-listed by local governments (Association of Wildlife Research & Envision 2007). Both of these species breed, spend their larval periods in rice fields and use terrestrial habitats following metamorphosis. As they lay a single egg mass in one breeding season (montane brown frog: Maeda and Ueda 2010, forest green tree frog: Kasuya et al. 1996), the number of egg masses are a good measure for the relative abundance of each species breeding in a particular rice field. Adult montane brown frogs live on the forest floor in the non-breeding season (Osawa and Katsuno 2001), while adult forest green tree frogs spend their arboreal life in forests (Kusano 1998). The migration distances of the adults from the breeding sites to forests are known to differ, with the montane brown frog exhibiting a 500 m (Osawa and Katsuno 2001) and the forest green tree frog approximately 120 m (Kusano 1998) migration distance. Adults of both species exhibit site fidelity in forests across years in non-breeding season (Kusano 1998; Osawa and Katsuno 2001).
The objective of our study was to identify factors influencing the abundance of egg masses in a rice field from two spatial scales. We hypothesized that the forest green tree frog requires a larger proportion of forest cover at the landscape level, but the spatial extent to which the abundance of the montane brown frog responds is larger than that of the tree frog due to the longer migration distance of the adult montane brown frog. Regarding local factors, we hypothesized that water depth in the breeding season as well as ditch size adjacent to forests are important, because water depth may affect local oviposition-site suitability (e.g., Watson et al. 2003; Ficetola et al. 2006) and large ditches could prevent movement between forests and rice fields (Lane and Fujioka 1997).
Methods
Study Area
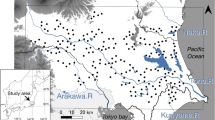
Field surveys were conducted in 58 rice fields located on Sado Island (38N,138E; altitude: 0–300 m), Niigata prefecture, Japan (Fig. 1). The surveys covered an area of approximately 131 km2, and the landscape consisted mostly of rice fields (30%) and forests (60%), ranging from forest-dominated hilly areas to rice-dominated plains along an east to west geographical gradient. The forest mainly consisted of native deciduous hardwood trees (such as Quercus serrata, Carpinus tschonoskii, Acer spp.) mixed with patchy plantations of Japanese cedar (Cryptomeria japonica) introduced from the mainland of Japan. Nearly all natural wetlands have been converted into rice fields. We selected rice fields that had varying degrees of forest cover in surrounding landscapes (0%–97% within a 500 m-radius). Seasonal management practices of rice fields were similar across the study area, i.e., flooding, rice plantation, and harvesting took place in April, early May, and late September, respectively. We did not have quantitative data on pesticide application, but the use of agricultural chemicals was likely not correlated with local and landscape factors used in our analyses (personal communication, local government). This suggests that the effect of pesticide use, when present, was not confounded with these factors.
Seven native amphibian species have been recorded on Sado Island (Sekiya 2006), but the montane brown frog, the forest green tree frog, and another species of tree frog (Hyla japonica) use rice fields as major breeding sites, while others use mainly farm ponds. We did not include H. japonica in our surveys because it was difficult to locate its egg masses in the field.
Breeding sites for the montane brown frog on Sado Island are predominantly in rice fields (Sekiya 2006) because there are few natural wetlands. The forest green tree frog, however, is also found in small ponds and temporary water bodies in and near the forests, but rice fields are likely to be major habitats because the total area available for breeding appears to be much more abundant in rice fields than in small ponds and temporal water bodies that are distributed sparsely on this island.
Sampling
Egg masses of the montane brown frog and the forest green tree frog were counted in late March and in mid-May 2008, respectively, after the peak of each breeding season. The montane brown frog lays jelly-like egg masses in the shallow water of rice fields (e.g., Osawa and Katsuno 2001), and the forest green tree frog lays frothy egg masses along rice-field margins on Sado Island (personal observation). We counted the number of egg masses, which were identified by eye, while walking along the paths surrounding rice fields. When egg masses of the montane brown frog were located too far from the paths to identify or count, binoculars were used to confirm their numbers. Because egg masses were large (>10 cm diameter) and water in the rice fields was clear with minimal floating material, we are confident that almost all egg masses were counted. We conducted field surveys when the majority of egg masses were fresh to minimize estimation error.
We measured the following environmental variables at the local (rice field) level: water depth in the rice field, the number of ditches adjacent to the rice field, the width and depth of each ditch, landuse type (paved road, river, paddy, forest) across the ditch from the rice field, and the perimeter length of the rice field. We recorded paddy water depth at six different points along the periphery of each rice field and used the average as the water depth value. Water depths were recorded in March and May when egg surveys were conducted. Other variables such as vegetation and water chemistry were not measured because there was almost no vegetation in rice fields in the season surveyed, and conductivity and pH are known to have little influence on the abundance of egg masses within the range of those found in most rice fields (Yoshida et al. 2006).
In late-March 2009 and 2010, we surveyed egg masses of the montane brown frog in 28 and 19 rice fields, respectively, that had been included in a 2008 survey using the same methodologies. We did not survey egg masses of the forest green tree frog because this species is known to show fairly stable yearly variations in egg mass abundance (Toda 2004).
GIS Analysis
Landscape variables and the areas of the rice fields were evaluated using a geographic information system (GIS; Arc GIS ESRI). We extracted the percent forest cover (hardwood forest and cedar plantation), percent rice-field cover, distance to the nearest forest, and paved road length as landscape variables, because forest is a major terrestrial habitat for adults of our target species, and paved road length is suggested as a landscape variable affecting abundances of some amphibians (Carr and Fahrig 2001). The mean and the range of environmental variables measured are listed in Table 1.
We employed the patch-landscape approach for extracting landscape factors by generating circular buffers centered around the focal paddy field (McGarigal and Cushman 2002; Holland et al. 2004; Bennett et al. 2006). To identify the appropriate spatial scale of the landscape factors affecting the abundance of a target species, we generated different sized circular buffers from 100 m to 1,500 m at 100 m intervals, and extracted landscape variables for 15 spatial scales. Although anurans may have an average maximum movement distance of about 2,000 m or more (Smith and Green 2005), we set 1,500 m as a maximum spatial scale to avoid a very large overlap of buffer circles between sites. Even within this range, occasional large overlaps between sites occurred, but this seemed not to be a serious problem, as abundance of egg masses (dependent variable) exhibited no clear spatial autocorrelations (see Results). Percent forest cover and rice-field cover were calculated using a digital vegetation map downloaded from J-IBIS (Japan Integrated Biodiversity Information System; http://www.biodic.go.jp/J-IBIS.html; Ministry of the Environment, Japan). The areas of the paddy fields were calculated using an aerial photograph of the study region taken in the winter of 2006.
Statistical Analysis
We assessed the spatial autocorrelation of the egg mass distribution for each frog species by generating a correlogram of Moran’s I. We then examined the factors affecting egg mass abundance in rice fields using a generalized linear model (GLM) with a negative binomial error distribution and log-link function. The independent variables (Table 1) were categorized as local and landscape level variables according to differences in spatial scales. Five variables were used to represent the local level: rice-field size, water depth, (water depth)2, number of ditches adjacent to the rice field, and the size of ditches lying between the focal rice field and the adjacent forest. Rice-field size represents the space available for frogs to lay egg masses. We used the area of the rice field for the montane brown frog and the perimeter length of the rice field for the forest green tree frog, because the brown frog lays egg masses inside the rice field whereas the tree frog does so alongside the paddy shore. We calculated the size of the ditches adjacent to forests because this likely affects the migration of frogs between rice fields and forests. Ditch size was defined as the first principal component (PCA) of the width and depth of the ditch, which explained 90.6% of the variance.
We used three landscape variables for the analyses: percent of total forest cover, (forest cover)2, and percent hardwood forest within a total forest cover. We included the quadratic terms for water depth and total forest cover, as these variables might exhibit a peak at intermediate values. We did not use distance to the nearest forest and paved road length for statistical analyses because they were highly correlated with percent forest cover, with correlation coefficients being larger than 0.5, except in the case of the smallest buffer size.
An information theoretic approach based on Akaike’s Information Criterion (AIC; Burnham and Anderson 2002) was used to select the generalized linear model that best explained the abundance of egg masses. This approach is superior to traditional stepwise procedures because it accounts for uncertainties concerning model structure and parameter estimation when there are several competing models with respect to their performance on the observed dataset (Whittingham et al. 2006). Here we used a hierarchical approach using AIC. First, to find the appropriate spatial scale determining the abundance of each frog species, we constructed models for all possible subsets of independent variables at each buffer size, and computed their AICs. We then compared the lowest AICs obtained from different buffer sizes, and determined the appropriate spatial scale based on the grand-lowest AIC across all buffer sizes. Next, to identify the environmental factors affecting the abundance, we calculated \( \Delta {\hbox{AIC }}(\Delta {\hbox{AIC}} = {\hbox{AIC}} - {\hbox{AI}}{{\hbox{C}}_{lowest}}) \) for all models at the appropriate spatial scale. As models having ΔAIC < 2 are considered to have similar performance, and hence no single best model can be chosen in such a case (Burnham and Anderson 2002), we listed all these models in the results (see Table 2). When the estimated regression coefficient of a variable was more than twice the standard error, we considered the variable influential because the confidence interval of the regression coefficient did not include zero (Burnham and Anderson 2002).
All statistical procedures were conducted using the statistical software R-2.6.2 (R Development Core Team 2005).
Results
The mean number (and ranges) of egg masses in rice fields were 26.9 (0–193) and 5.2 (0–42) for the montane brown frog and the forest green tree frog, respectively. Correlograms for the number of egg masses indicated that neither species exhibited a trend in Moran’s I with increasing distance, suggesting no strong spatial autocorrelations for either species (Fig. 2). Therefore, we did not consider spatial autocorrelation in subsequent analyses.
The two species of frogs showed a difference in their spatial scales determining their abundance of egg masses, i.e., 300 m for the montane brown frog and 1,000 m for the forest green tree frog, as revealed by the lowest AIC at these buffer sizes (Fig. 3). At these spatial scales, forest cover was an important landscape variable determining the abundance of egg masses in both frog species, because the regression coefficients were more than twice their standard errors, and these variables were almost always included in competing models (ΔAIC < 2; Appendix). According to the best model, the abundance of egg masses peaked at approximately 60% forest cover for the montane brown frog, while it showed a monotonic increase for the forest green tree frog (Fig. 4). This difference was due to whether or not the quadratic term of forest cover was included in the best model (Appendix). Percent hardwood forest within a total forest cover was not influential to either species because the estimated coefficient was equal to or less than its standard error (Appendix).
Regarding local factors, water depth was a common important variable for both species (Appendix). The number of egg masses exhibited high values at intermediate water depths of approximately 7–10 cm in both species (Fig. 4), as the quadratic term of water depth was included for both species (Appendix). Ditch size and rice-field size were also important local factors for the montane brown frog and forest green tree frog, respectively (Appendix). Larger ditches adjacent to rice fields had a negative effect on the montane brown frog, and larger rice-fields had a positive effect on the forest green tree frog.
There were highly significant correlations between the number of egg masses of the montane brown frog in 2008 and 2009 (r = 0.717, n = 28, p < 0.001), and in 2009 and 2010 (r = 0.587, n = 19, p = 0.007).
Discussion
We found that the montane brown frog showed maximum abundance in a landscape with approximately 60% forest cover in the best model, while maximum forest green tree frog abundance was observed in a landscape with more than 90% forest cover. As the entire landscape consisted mostly of forests and rice fields, the montane brown frog appeared to prefer landscapes with an intermediate mixture of forests and rice fields. This is consistent with the report that while adult montane brown frogs inhabit both forests and grasslands, adult forest green tree frogs are mainly arboreal (Maeda and Matsui 1989). Similar findings for European and American amphibians have shown that forest-dependent species were more abundant in ponds with more surrounding forest cover (Guerry and Hunter 2002; Houlahan and Findlay 2003; Porej et al. 2003; Van Buskirk 2005; Gagne and Fahrig 2007).
The spatial scale affecting the abundance of the montane brown frog was smaller than that of the forest green tree frog (300 vs. 1,000 m). This was opposite to the trend expected from the migration distance of adults from the breeding sites to forest habitats, i.e., the montane brown frog is known to exhibit a longer maximum distance than the forest green tree frog (500 vs. 120 m, respectively; Kusano 1998; Osawa and Katsuno 2001). Thus, the effective spatial scale for the montane brown frog estimated by our model (300 m) was mostly consistent with the movement distance reported in an earlier study (500 m; Osawa and Katsuno 2001), but for the forest green tree frog (1,000 m) it was much larger than the movement distance reported earlier (120 m; Kusano 1998). Some amphibian species exhibit an effective spatial scale similar to their movement distance (Houlahan and Findlay 2003), whereas others have shown a larger effective spatial scale than their movement distance (Herrman et al. 2005). This suggests that ecological processes operating at the landscape level differ among species. Several researchers have suggested that landscape features affect amphibian abundance in two ways: adult habitat use and metapopulation processes (e.g., Van Buskirk 2005; Ficetola et al. 2008). The concordance of the effective spatial scale and movement distance reported in the montane brown frog suggests the process of adult habitat use, i.e., most individuals exhibit site-fidelity in the sense that they utilize a particular range including breeding sites and nearby forests throughout their lives. In contrast, the discrepancy between the effective spatial scale and movement distance in the forest green tree frog may reflect a long-distance movement that occasionally occurs. Smith and Green (2005) speculated by reviewing literature that such migrants may connect populations separated by tens of kilometers.
With respect to local factors, the abundance of egg masses for both species exhibited a peak at approximately 7–10 cm water depths in the rice fields. This is consistent with the finding that the montane brown frog prefers to lay eggs in relatively shallow water (Maeda and Matsui 1989), which is also true for other frog species (e.g., Watson et al. 2003; Ficetola et al. 2006; Wang et al. 2008). Frogs may show preferences for intermediate water depths as a tradeoff, i.e., water that is too shallow poses a high risk of drying up, while deep water exhibits low temperatures and oxygen concentrations, that may inhibit rapid development (Watson et al. 2003; Ficetola et al. 2006) or survival (Wang et al. 2008). Another local factor that influenced egg abundance was the size of the ditch between the rice field and forest, but this effect was only detected for the montane brown frog. This is plausible because the forest green tree frog possesses digital disks on its feet while the montane brown frog does not, making it difficult for the montane brown frog to climb up the wall of deep ditches once it dropped off. It appears that large ditches may act as barriers preventing the montane brown frog from immigrating to the rice fields.
Our statistical model was constructed using single-year data. Some frog species show large annual variations in the number egg masses laid at a given locality (Loman and Anderson 2007). However, we found positive correlations in the number of egg masses laid by the montane brown frog in a given rice field over three consecutive years. This suggests that the qualitative importance of landscape variables on the number of egg masses of the montane brown frog does not change greatly, at least within a few years. We did not have multiple-year data for the forest green tree frog in our field, but Toda (2004) reported that the annual variation in the number of egg masses of this species was within the range of 250–300 over seven years. Thus, we believe that our use of single-year data did not lead to severely biased estimation of suitable habitats for the forest green tree frog.
Our study provides important implications for rice-field restoration in rural landscapes. In Japan, reserving water in winter and non-tillage practices are undertaken to enhance or restore biodiversity (Maeda and Yoshida 2009; Mineta et al. 2009). It should be noted however that rice fields are one of the components of rural landscapes called “Satoyama.” Mosaic structure in a Satoyama landscape is thought to enhance biodiversity by providing composite habitats to organisms such as amphibians and aquatic insects that undergo ontogenetic habitat changes (Washitani 2001; Kobori and Primack 2003). Thus, the outcomes of restoration practices in particular rice fields are likely to differ greatly depending on the surrounding landscape structure, but such a context-dependent view has been totally lacking in previous studies. We found that the montane brown frog preferred a landscape with a mixture of rice fields and forests at a small spatial scale, while the forest green tree frog required large-scale forest surroundings. These results suggest that reserving shallow water in winter could greatly enhance the abundance of the montane brown frog, which breeds in early spring, if performed in a landscape with a moderate mixture of forests and rice fields. Likewise, reviving cultivation in abandoned rice fields surrounded by extensive forests could enhance forest green tree frog abundance. Habitat restoration for the montane brown frog may be particularly important because it would increase the likelihood of persistence of many other organisms that depend on small scale heterogeneity in Satoyama landscapes. This hypothesis should be tested in future studies that target a variety of organisms.
References
Amano T, Kusumoto Y, Tokuoka Y, Yamada S, Kim E, Yamamoto S (2008) Spatial and temporal variations in the use of rice-paddy dominated landscapes by birds. Biological Conservation 141:1704–1716
Association of Wildlife Research & Envision (2007) Search system of Japanese Red Data. http://www.jpnrdb.com/category.html
Babbitt KJ, Baber MJ, Childers DL, Hocking D (2009) Influence of agricultural upland habitat type on larval anuran assemblages in seasonally inundated wetlands. Wetlands 29:294–301
Bennett AF, Radford JQ, Haslem A (2006) Properties of land mosaics: Implications for nature conservation in agricultural environments. Biological Conservation 133:250–264
Benton TG, Vickery JA, Wilson JD (2003) Farmland biodiversity: is habitat heterogeneity the key? Trends in Ecology & Evolution 18:182–188
Blaxter K, Robertson N (1995) From dearth to plenty: the Second Agricultural Revolution, Cambridge University Press
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information-theoretic approach. Springer, New York
Carr LW, Fahrig L (2001) Effects of road traffic on two amphibian species of differing vagility. Conservation Biology 15:1071–1078
Cushman SA (2006) Effects of habitat loss and fragmentation on amphibians: a review and prospectus. Biological Conservation 128:231–240
Donald PF (2004) Biodiversity impacts of some agricultural commodity production systems. Conservation Biology 18:17–37
Eigenbrod F, Hecnar SJ, Fahrig L (2008) The relative effects of road traffic and forest cover on anuran populations. Biological Conservation 141:35–46
Elphick C (2000) Functional equivalency between rice fields and seminatural wetland habitats. Conservation Biology 14:181–191
Falso M, Ruiz X (1997) Rice farming and waterbirds: integrated management in an artificial landscape. In: Pain D, Pienckowski MW (eds) Farming and birds in Europe. Academic, London, pp 210–235
FAO Statistics Division (2008) FAO, Rome.
Ficetola GF, Valota M, de Bernardi F (2006) Temporal variability of spawning site selection in the frog Rana dalmatina: consequences for habitat management. Animal Diversity and Conservation 29:157–163
Ficetola GF, Padoa-Schioppa E, de Bernardi F (2008) Influence of landscape elements in riparian buffers on the conservation of semiaquatic amphibians. Conservation Biology 23:114–123
Fujimoto Y, Ouchi Y, Hakuba T, Chiba H, Iwata M (2008) Influence of modern irrigation, drainage system and water management on spawning migration of mud loach, Migurnus anguillicaudatus. Environmental Biology of Fishes 81:185–194
Fujioka M, Lane S (1997) The impact of changing irrigation practices in rice fields on frog populations of the Kanto Plain, central Japan. Ecological Research 12:101–108
Gagne SA, Fahrig L (2007) Effect of landscape context on anuran communities in breeding ponds in the National Capital Region, Canada. Landscape Ecology 22:205–215
Guerry AD, Hunter ML Jr (2002) Amphibian distributions in a landscape of forests and agriculture: an examination of landscape composition and configuration. Conservation Biology 16:745–754
Herrman HL, Babbitt KJ, Baber MJ, Congalton RG (2005) Effects of landscape characteristics on amphibian distribution in a forest-dominated landscape. Biological Conservation 123:139–149
Holland JD, Bert DG, Fahrig L (2004) Determining the spatial scale of species’ response to habitat. Bioscience 54:227–233
Houlahan JE, Findlay CS (2003) The effects of adjacent land use on wetland amphibian species richness and community composition. Canadian Journal of Fisheries and Aquatic Sciences 60:1078–1094
Ise H, Mitsuhashi H (2006) Habitat evaluation of the tree frog and its application for conservation planning in Japan. Ecology and Civil Engineering 8:221–232
Kasuya E, Hirota M, Shigehara H (1996) Reproductive behavior of the Japanese treefrog, Rhacophorus arboreus (Anura: Rhacophoridae). Researches on Population Ecology 38:1–10
Kobori H, Primack RB (2003) Participatory conservation approaches for Satoyama, the traditional forest and agricultural landscape of Japan. Ambio 32:307–311
Krebs JR, Wilson JD, Bradbury RB (1999) The second silent spring ? Nature 400:611–612
Kusano T (1998) A radio-tracking of post-breeding dispersal of treefrog, Rhacophorus arboreus (Amphibia: Rhacophoridae). Japanese Journal of Herpetology 17:98–106
Lane S, Fujioka M (1997) The impact of changes in irrigation practices on the distribution of foraging egrets and herons (Ardeidae) in the rice fields of central Japan. Biological Conservation 83:221–230
Lawler SP (2001) Rice fields as temporary wetlands: a review. Israel Journal of Zoology 47:513–528
Loman J, Anderson G (2007) Monitoring brown frogs Rana arvalis and Rana temporaria in 120 south Swedish ponds 1989–2005. Mixed trends in different habitats. Biological Conservation 135:46–56
Maeda N, Matsui M (1989) Frogs and toads of Japan. Bun-ichi Sogo Shuppan, Tokyo. (In Japanese with English Abstract)
Maeda N, Ueda U (2010) The handbook of frogs and toad sounds of Japan: with SNG Soundreader & U-SPEAK. Bun-ichi Sogo Shuppan, Tokyo. (In Japanese)
Maeda T, Yoshida H (2009) Responses of birds in rice fields to winter flooding. Japanese Journal of Ornithology 58:55–64 (In Japanese with English Abstract)
Marsh DM, Trenham PC (2001) Metapopulation dynamics and amphibian conservation. Conservation Biology 15:40–49
McGarigal K, Cushman SA (2002) Comparative evaluation of experimental approaches to the study of habitat fragmentation effects. Ecological Application 12:335–345
Mineta T, Koizumi N, Ishida K (2009) Contribution of winter flooding in multifunctionality of paddy fields. Journal of Rural Planning Association 27:335–340 (In Japanese with English Abstract)
Mukai Y, Baba N, Ishii M (2005) The water system of traditional rice paddies as an important habitat of the giant water bug, Lethocerus deyrollei (Heteroptera: Belostomatidae). Journal of Insect Conservation 9:121–129
Osawa S, Katsuno T (2001) Dispersal of brown frogs Rana japonica and R. ornativentris in the forests of Tama Hills. Current Herpetology 20:1–10
Porej D, Micacchion M, Hetherington TE (2003) Core terrestrial habitat for conservation of local populations of salamanders and wood frogs in agricultural landscapes. Biological Conservation 120:399–409
R Development Core Team (2005). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org.
Sekiya K (2006) Amphibians on Sado Island. Bulletin of the Herpetological Society of Japan 2006:83–84
Smith MA, Green DM (2005) Dispersal and the metapopulation paradigm in amphibian ecology and conservation: are all amphibian populations metapopulations? Ecography 28:110–128
Toda M (2004) Population decline of the tree frog Rhacophorus arboreus in Kanazawa Castle Park. Bulletin of the Herpetological Society of Japan 2004:60–61
Tscharntke T, Klein AM, Kruess A, Steffan-Dewenter I, Thies C (2005) Landscape perspectives on agricultural intensification and biodiversity—ecosystem service management. Ecology Letters 8:857–874
Van Buskirk J (2005) Local and landscape influence on amphibian occurrence and abundance. Ecology 86:1936–1947
Wang Y, Zhengjun W, Ping LU, Zhang F, Yiming LI (2008) Breeding ecology and oviposition site selection of black-spotted pond frog (Rana nigromaculata) in Ningbo, China. Frontiers of Biology in China 3:530–535
Washitani I (2001) Traditional sustainable ecosystem ‘Satoyama’ and biodiversity crisis in Japan: conservation ecological perspective. Global Environmental Research 5:119–133
Watson JW, McAllister KL, Pierce DJ (2003) Home range, movements, and habitat selection of Oregon spotted frogs (Rana pretiosa). Journal of Herpetology 37:292–300
Whittingham MJ, Stephens PA, Bradbury RB, Freckleton RP (2006) Why do we still use stepwise modelling in ecology and behaviour? The Journal of Animal Ecology 75:1182–1189
Yoshida M, Yabu S, Yamada H (2006) Studies on the restoration technique of breeding environment of Rana ornativentris Werner. Journal of the Japanese Society of Revegetation Technology 31:183–186
Zanini F, Klingemann A, Schlaepfer R, Schmidt BR (2008) Landscape effects on anuran pond occupancy in an agricultural countryside: barrier-based buffers predict distributions better than circular buffers. Canadian Journal of Zoology 86:692–699
Acknowledgments
We thank Tatsuya Amano and anonymous referees for their comments on the previous draft of this manuscript. This study was supported by the Global Environment Research Fund (F-072), Ministry for the Environment, Japan.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Rights and permissions
About this article
Cite this article
Kato, N., Yoshio, M., Kobayashi, R. et al. Differential Responses of Two Anuran Species Breeding in Rice Fields to Landscape Composition and Spatial Scale. Wetlands 30, 1171–1179 (2010). https://doi.org/10.1007/s13157-010-0103-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13157-010-0103-1