Abstract
The present paper deals with the investigation of abiotic and biotic influence on thermogenic travertine formation in Thermopylae hot springs, one of the largest active thermogenic travertine systems in Greece. Geological, mineralogical and microbiological data from three different types of travertines (cascades, terraces and fluvial crusts) revealed different cyanobacterial communities. Microscopic analysis of fresh and cultured material has shown that epilithic and endolithic cyanobacteria are almost the exclusive components of travertines’ photosynthetic microflora. Thirty-one (31) taxa of cyanobacteria are presented here, among them the frequently found, in such environments, Phormidium incrustatum and Aphanocapsa thermalis, as well as the taxonomically interesting diazotrophic morphotype identified as Chlorogloeopsis sp. Sampling sites I and II have similar formation conditions characterized by laminated travertines with low porosity and shrub lithotypes, with the cyanobacterium Leptolyngbya ercegovicii occupying an endolithic zone, while the upper part is occupied by colonial chroococcalean species. On the contrary, sampling site III is characterized by laminated travertine with fenestrial type porosity and absence of shrub lithotypes resulting in a completely different community of cyanobacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Travertine is the chemically precipitated continental carbonate deposit formed around springs, along streams, and occasionally in lakes, consisting of calcite and/or aragonite. The first and most significant chemical process of precipitation is the transfer (evasion or invasion) of carbon dioxide (CO2) from or to a groundwater source leading to calcium carbonate supersaturation, and finally to the nucleation and the formation of crystals (Pentecost et al. 1997; Fouke et al. 2000; Pentecost 2003, 2005; Okumura et al. 2011). The precipitation of calcite and/or aragonite crystals in travertines is basically induced by CO2 degassing, which elevates the pH and the mineral saturation state (Kitano 1963; Pentecost 1995).
Since the publication of Ferdinand Cohn’s pioneering work at Tivoli (Cohn 1864), it has been proven that bacteria and especially cyanobacteria play an important role in certain kinds of travertine formation (Pentecost 2005). Cyanobacteria are the major oxygenic microorganisms representing the most abundant phototrophic prokaryotes on travertines worldwide. Several species of cyanobacteria are widely distributed and often dominate on actively growing travertine surfaces playing a significant role in the biologically induced precipitation of CaCO3. This is presumably because these organisms are among the few which thrive in hot, sulfide-containing waters, while many species tolerate a wide range of light intensities, salinity and high pCO2 (Castenholz 2002; Banerjee et al. 2009).
The biologically induced mineralization (BIM) occurs through various metabolic processes, such as photosynthetic uptake of CO2 and/or HCO3 − by cyanobacteria, as well as ammonification, denitrification and sulfate reduction by other bacteria (Riding 2000; Beltrán-Magos et al. 2013). Especially for cyanobacteria, the following processes are known to contribute to the construction of carbonates: (a) increase of pH due to photosynthesis, (b) trap of CaCO3 grains by entangled filaments, (c) biogenic activity of some cyanobacterial species (e.g., Rivularia haematites) to secrete carbonate structures and (d) binding of CaCO3 grains in extracellular polymeric substances (EPS). EPS constitute a protective and adhesive material that anchors cells, colonies and filaments to the substrate, favoring sediment trapping. EPS possess the ability to concentrate Ca2+ cations favouring calcium carbonate encrustation by providing an ideal surface for adsorption of ions and mineral nucleation (Braissant et al. 2003; Beltrán-Magos et al. 2013). Furthermore, it is known that minerals originated through BIM nucleate and grow both extracellularly and also intracellularly. For example, calcite is capable of precipitating in Chlorogloea lithogenes sheath (Komárek and Montejano 1994).
Nevertheless, cyanobacteria play also a decisive role in the deconstruction of carbonate substrata, by weathering rocks and travertines (Schneider and Le Campion-Alsumard 1999). Lithobiontic cyanobacteria grow as epilithon and/or endolithon contributing to the biogenic and abiogenic deposition of CaCO3 as well as in the deconstruction of carbonate surfaces (Pentecost 2003, 2005; Pentecost and Whitton 2000; Whitton et al. 2012). The biocorrosive mechanism of lithobiontic cyanobacteria and especially of euendoliths is probably through the secretion of acidic substances or complexing agents such as extracellular polymers resulting in the production of little crystals, which are more susceptible to the inorganic process of dissolution (Schneider and Le Campion-Alsumard 1999). As a result, the cyanobacterial contribution to the formation of travertines is an adjunct activity of the two controversial processes: (a) construction and (b) deconstruction.
The abiotic and biotic influence on thermogenic travertine formation is an interdisciplinary subject and to study it, we have to investigate how geological and biological phenomena interact simultaneously in nature. For that reason, in the latest studies a combination of geological and biological aspects are combined to investigate the biotic influence on CaCO3 formation (Kandianis et al. 2008; Dupraz et al. 2009; Obst et al. 2009; Fouke 2011; Okumura et al. 2011, 2012; Kamennaya et al. 2012).
Greece offers an ideal place for the investigation of thermogenic travertine deposits, since they are scattered in many areas near hot springs (Gioni-Stavropoulou 1983; Orfanos 1985; Sfetsos 1988). Magmatic processes and active fault systems favor the rise of deep hydrothermal waters that are discharged at the surface as hot springs. The geochemical features of the majority of geothermal springs in Greece have been the focus of several studies (reviewed by Lambrakis and Kallergis 2005), but only recently have the active thermogenic travertine systems in Greece been geologically studied in detail (Kanellopoulos 2011, 2012, 2013; Winkel et al. 2013). Despite the fact that Greece is characterized by a great number of hot spring environments, the microflora of these unique ecosystems remains more or less unknown and the cyanobacteria of geothermal environments have received relatively little attention, as for example by Anagnostidis (1961, 1967) and Anagnostidis and Pantazidou (1988).
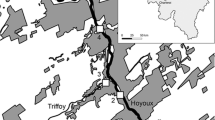
In Thermopylae area (lat. 38°47.5′–38°47.9′ and lon. 22°31.4′–23°32.4′, Fig. 1), one of the largest active thermogenic travertine-forming systems of Greece presents a great variety of morphological forms and lithotypes (Kanellopoulos 2012, 2013).
Geological map showing the study area and the localities sampled (after Kanellopoulos 2013)
The aim of the present study is to investigate the geological and biological aspects of active travertine deposits of hot springs (T = 41 °C) in Thermopylae (Greece) and examine the role that the cyanobacterial microflora played in their formation.
Geological setting
Thermopylae is located in the eastern part of central Greece (Fig. 1) and belongs to the western most geotectonic unit of the Internal Hellenides, the Sub-Pelagonian Unit (Aubouin 1959; Mountrakis 1986; Jolivet et al. 2013). The basement in Thermopylae area consists mainly of carbonate rocks (limestones and dolomites) of Middle Triassic–Middle Jurassic age. An ophiolitic sequence (peridotides, serpentinites, gabbros) is overthrusted on the carbonates. Fluvio-deltaic sediments of Neogene age fill the Sperchios graben and consist of alternations of marls, clays, sandstones and conglomerates (Philip 1974). The whole area is highly faulted due to extensional tectonics in the backarc region of the Hellenic subduction system (McKenzie 1970, 1972; Le Pichon and Angelier 1979; Jolivet et al. 2013).
There are two main sites in Eastern Central Greece with hot spring manifestations, namely Kamena Vourla and Thermopylae. These are the surface expression of an active hydrothermal field beneath the broad area, which also extends in the neighboring part of northern Euboea Island (e.g., Edipsos, Gialtra, Ilia; Fig. 1). The hydrothermal field expands around the volcanic center of Lichades (Kanellopoulos 2011). The volcanic islands of Lichades are located at the center part of North Euboea Gulf (Georgalas 1938) and consist of trachyandesite lava flows, dated at 0.5 Ma (Pe-Piper and Piper 2002). These volcanic rocks are located along a shear zone (Kranis 1999) indicating a tectonic control during their emplacement. Recent studies by Karastathis et al. (2011) prove the existence of a magma chamber at an estimated depth of 7–8 km in the North Evoikos Gulf.
Materials and methods
Hot spring and sampling sites
Hot spring water at Thermopylae has temperatures of 33–40.4 °C, pH of 5.9–6.2 and total dissolved solids (TDS) from 5.5 to 7.5 (g/L) and presents high content in Ca (up to 520 mg/L), S (up to 140 mg/L), Mg (220 mg/L) and Sr (up to 11.9 mg/L) (Kanellopoulos 2011).
Samples were collected from three sites (I–III). The sampling sites were selected at different distances from the hot spring, representing distinct environmental conditions with different cyanobacterial composition. Sites I and II (Fig. 2a, c) are located near the main outflow zone of the hot water. In both cases, the flow velocity is low and the physicochemical parameters of the water are similar (pH = 6.9, temperature = 30 °C, TDS = 8.2 g/L, salinity = 8.5 and EC = 14.7 mS/cm). The site III (Fig. 2f) is located at the main stream of the hot water. The flow velocity is fast and the physicochemical parameters of the water are slightly different (pH = 7.1, temperature = 38.2 °C, TDS = 7.9 g/L, salinity = 8.2 and EC = 14.2 mS/cm).
Morphologies and lithotypes of the studied travertines. a Overview image of the external wall of a cistern (site I), where, parallel layers of travertine have been deposited. An early stage cascade. b Section of sample from cascade (site I) showing characteristic lamination lithotypes. c Overview image of terraces at the Thermopylae (site II). d Sample from travertine terraces (site II) with height of dams up to 10 mm. e Section of sample from terracettes, where characteristic lamination lithotypes can be seen (site II). f Overview image of fluvial crusts in a stream (site III). g Details of fluvial crusts (site III). The crusts were formed above the old travertines (bedrock) as fine layers. h Section of sample from fluvial crusts (site III) showing lamination lithotypes with an abundance of fenestral-type porosity common throughout the area of Thermopylae
All samples were collected using sterilized equipment. In all samples the mineralogical composition, the mineral chemistry, the type of lithotypes, as well as the composition of cyanobacterial species were studied.
Mineralogical study
Samples from all studied sites were collected and analyzed at the laboratories of the Department of Geology and Geoenvironment, University of Athens.
The mineralogical composition was investigated mainly by X-Ray diffraction (XRD). XRD analyses were carried out using a Siemens Model 5005 X-ray Diffractometer, Cu Ka radiation at 40 kV, 40 nA, 0.020° step size and 1.0 s step time. The XRD patterns were evaluated using the EVA v.10.0 program of the Siemens DIFFRACplus and the D5005 software package.
Scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS) analysis were carried out using a Jeol JSM 5600 SEM instrument, equipped with an Oxford ISIS 300 OXFORD, with the following operating conditions: accelerating voltage 20 kV, beam current 0.5 nA, time of measurement 50 s and beam diameter 1–2 μm. The spectra were processed using the ZAF program (3 interaction). The microprobe analyses were conducted on polished sections of the samples after carbon coating. SEM images of the crystal structures were taken after gold coating of fragments.
Cultures and microscopy
Samples from all studied sites were collected and analyzed at the laboratories of the Department of Biology, University of Athens.
A portion of the sample was fixed with formaldehyde solution at a final concentration of 2.5 %, while another portion was used for culturing. Enriched cultures were obtained in flasks and Petri dishes with BG11 and BG 110 culture media (Stanier et al. 1971). Cultures were maintained in an incubator (SANYO, GALLENKAMP) under stable conditions and under natural diurnal cycle (north-facing window) at room temperature.
Both natural and cultured material was studied under a stereo-microscope (Stemi 2000C Zeiss, Germany) and under a high-resolution light microscope (Photomicroscope III, Zeiss, Germany). The observed cyanobacteria were identified using the classical literature such as (Geitler 1932, Desikachary 1959 and Komárek and Anagnostidis 1998, 2005) and many up to date papers. For SEM, fragments of the specimens were dehydrated in an alcohol series (30–100 %), critical point dried, gold coated and were observed at the above-mentioned scanning electron microscopy (SEM Jeol JSM 5600, Department of Geology and Geoenvironment).
Results
Mineralogical study
The main mineral phase identified by XRD analysis in all samples is calcite. It is usually present as rhombohedral crystals. Electron microprobe analyses indicate that in addition to Ca, the analyzed calcite contains up to 2.4 wt% Mg (Table 1), as has been also described by Kanellopoulos (2011, 2012).
Morphological types and lithotypes
The thermogenic travertines at Thermopylae are characterized by many morphological types and lithotypes (Kanellopoulos 2012, 2013). In all studied sites, the travertine are white to gray colored and present three different morphological types: cascade (site I), terraces (site II) and smooth fluvial crusts (site III).
Cisterns were constructed near the main stream to collect and store the hot water. When the main stream overflows or the cisterns overflow, hot water overtops the external walls of cisterns and seeps down with low flow velocity. In that site, approximately parallel layers in section were created, indicating a probably early-stage cascade (site I, Fig. 2a, b).
In site II, small pools with small depth and low flow velocity (terraces) exist. The slow passage of water through lakes means that CO2 evasion is reduced, while photosynthesis and evaporation become increasingly important as progenitors of carbonate precipitation. Over the rim and on the steep outside wall of the terrace (dam), water flows in a thin sheet resulting in the increase of the flow velocity. Morphological classification schemes for travertine terrace have been proposed by several authors (Bargar 1978; Bates and Jackson 1987; Pentecost and Viles 1994; Fouke et al. 2000). Using the classification suggested by Bargar (1978) and Fouke et al. (2000) in Thermopylae “microterracettes” of a few square centimeters or less, with maxima height of dams up to 10 mm, were identified (Fig. 2c, d). However, in the present paper the term “terrace” is used as a general term regardless of size, as suggested by Hammer et al. (2010).
In site III a range of superficial deposits formed inside the main stream of running hot water (Fig. 2f). They develop on a variety of structures, which are either smooth, or nodular and coralloid creating fluvial crusts. This morphological type is common in Thermopylae. Usually laminated smooth surfaces were developed above previous layers of travertine or around bedrock (Fig. 2g).
Concerning the lithotypes in sites I and II, the travertines show lamination with low porosity (Fig. 2b, e) and formation of shrubs, while in site III the travertines are laminated, without shrub formation and with an abundance of fenestral-type porosity (Fig. 2h).
Lamination (sites I, II and III) (Fig. 2b, e, h) was the common lithotype in our samples. The laminaes can usually be distinguished through eye observation consisting of gray- and white-colored layers with sometimes a last-top green color layer (Figs. 2e, g, 3c). Laminaes can reach a maximum of 1 mm thickness. Their magnified view shows fine-scale laminae of a few tens of micrometers (Fig. 2b). Shrub layers can be distinguished through eye observation between micritic-sized calcite laminaes in many cases (Fig. 2b, e, h).
Travertine formations and their cyanobacterial microflora under stereoscope (a–c) and SEM (d–i). a, b Laminated travertines (site I and II) with shrub lithotypes. a Details of the endolithic filaments of Leptolyngbya ercegovicii favoring travertine deconstruction. Scale bars 1 mm. c Fluvial crust with characteristic lamination (site III). Scale bar 1 mm. d, e Extracellular polymeric substances (EPS) favoring the crystal trapping. Scale bars 10 and 50 μm, respectively. f Rhombohedral crystals of calcite with distinct holes, some of them occupied by filamentous cyanobacteria (site II). Scale bar 10 μm. g Details of the calcified cyanobacterial sheaths in sites I and II. Scale bar 100 μm. h Heavily calcified tube formed by the cyanobacterium Phormidium incrustatum. Scale bar 10 μm. i Cyanobacterial filaments favoring crystal trapping. The arrow indicates a trace of lithobiontic cyanobacterium. j Diatom frustules embedded in EPS, some of them trapped by filamentous cyanobacteria
Travertine shrubs are observed in sites I and II. They are short, stubby, dense crystalline masses of calcium carbonate that expand upward by irregular branching. Shrub-like types could present considerably different morphologies. Chafetz and Guidry (1999) distinguished travertine shrubs into those whose outline is typical of the common garden-variety woody shrub or bush (bacterial shrubs, Chafetz and Folk 1984), to those that have regular geometric patterns (crystal shrubs) and to crystalline calcite fans (ray-crystal crusts, see also Folk et al. 1985). In the studied samples, shrubs usually had garden-variety woody shrub or bush patterns (bacterial shrubs, Fig. 3a). They also present irregular morphology without any specific crystallographic influence on their shape. Detailed study of the samples using SEM–EDS revealed that the shrubs were related with calcite tubes encrusting filaments (Fig. 3g, h).
Distribution of cyanobacteria based on morphology
Cyanobacteria were found to prevail at the hot spring and the outflows (downstreams). The predominance of the cyanobacteria is due to the reduced presence of eukaryotic algae such as diatoms. Microscopic analysis of fresh and cultured material from the three sampling sites revealed a total number of thirty-one (31) cyanobacteria (Table 2). The majority of the species identified are non-heterocytous filamentous cyanobacteria thriving at the mats’ surface and/or endolithic filaments distributed in the lower part of the travertines (see also Pentecost 2003). Heterocytous filaments with or without true branching have little representation with the exception of the genus Calothrix (site II), a commonly reported thermophilic genus (see also Pentecost 2003, 2005; Whitton et al. 2012).
At sites I and II, filaments of Leptolyngbya ercegovicii occupy an endolithic zone of 3–5 mm, while the upper part is occupied by colonial chroococcalean species such as Aphanocapsa thermalis, Ap. castagnei, Chroococcus minor, Ch. thermalis, Gloeocapsopsis crepidinum and Gl. dvorakii, Phormidium incrustatum is the most common carbonate-incrusted cyanobacterium found at sampling site II. It is noted that trichomes of Ph. incrustatum are surrounded by firm sheath of EPS, constituting the locus of intensive calcification (Fig. 3h). The diagenesis of micrite deposit of that particular species is similar to those observed by Golubic et al. (2008). Diatoms frustules or/and calcite crystals are usually observed embedded in EPS in both sites I and II (Fig. 3d, e, g, j).
At sampling site III, the upper part of the travertine formation is colonized by blue-green thin mats of Geitlerinema lemmermannii and Oscillatoria ornata, while thin vertical sections revealed calcite crystals attached to the filaments of Phormidium terebriforme and Spirulina subsalsa.
Discussion
Up to the present, it is well known that for about 3.5 billion years the global carbonate cycle has been regulated by photoautotrophic processes, with cyanobacteria being the dominant group with a decisive role in the construction and the deconstruction of carbonates (Schneider and Le Campion-Alsumard 1999).
This study examined the geological characteristics of thermogenic travertines in association with cyanobacterial communities at Thermopylae hot springs, Greece. A large thermogenic travertine deposit in this locality includes highly variable travertine textures and cyanobacteria species. Based on the associations among them, the findings could be useful to understand older formation processes for the different types of travertine. Such processes can be applied to laminated carbonates of the geological past, in which biogenicity remains controversial (Semikhatov et al. 1979; Buick et al. 1981; Lowe 1994; Grotzinger and Rothman 1996).
The thermogenic travertines of Thermopylae hot springs are formed dominantly by in situ precipitation. Travertines in sites I and II have similar formation conditions, i.e., low speed flow and low depth, resulting in the formation of laminated travertine with low porosity and shrubs lithotypes (Fig. 2a–e). The development of laminated lithotype may be due to inorganic processes, where the release of CO2 caused supersaturation of the solution and precipitation of CaCO3 (Jones et al. 2005) to biogenic processes (Chafetz and Folk 1984), or to the combination of multiple abiotic and biotic factors. Also, the shrub lithotypes identified in the studied samples have garden-variety woody shrub or bush patterns, which are characterized as bacterial shrubs as suggested by Chafetz and Folk (1984). Additionally, chroococcoid cyanobacteria such as Aphanocapsa thermalis, Gloeocapsa cf. violacea, Gloeocapsopsis crepidinum and Gl. dvorakii have been observed to thrive to the top layer of the shrubs, indicating the relation between the specific lithotype and cyanobacteria.
Detailed study of the samples using SEM–EDS indicated the presence of similar characteristic structures suggesting bio-mineralization processes by Microcoleus sociatus, Schizothrix cf. friesii at the upper part favoring sediment trapping by many ways and endolithic band consisting of Leptolyngbya ercegovicii at the inner part of the travertine (Fig. 3a, b) favoring the travertine deconstruction. Typical structures in both sites (I and II) are calcite tubes encrusting the filaments in different stages: during the early stage, calcite crystals, usually as micritic mud, precipitate within the outer sheath layer (Fig. 3d, e) due to photosynthetic bicarbonate uptake; while at a later stage solid calcite tubes are protruding vertically in direction to the surface. These structures were associated with the shrub lithotypes (Fig. 3g, h). After the decomposition of the organic material a solid tube remains, and the usually broken tube tip exposes the coalescing calcite crystals.
Travertine in site III is characterized by different formation conditions (e.g., fast speed flow, greater depth) compared to travertine from sites I and II. In site III, the laminated travertine displays a fenestrial type of porosity and no shrub lithotypes, as a result of a completely different cyanobacterial community. Detailed study of the site III samples using SEM–EDS suggests that no one of the above-mentioned phenomena of biomineralization at sites I and II took place here. In site III, characteristic structures attributed to bio-mineralization processes are holes in rhombohedral calcite crystals (Fig. 3f). In this sampling site with the highest temperature, cyanobacterial species that adapted to the extreme and specialized environment of hot springs (belonging to the order Oscillatoriales) were determined.
In conclusion, the formation of thermogenic travertine at Thermopylae shows a variation in morphological types, lithotypes and cyanobacterial species composition. The data presented show correlation between specific lithotypes, suggesting bio-mineralization processes and specific cyanobacteria species. The combination of the geological and biological data seems to suggest that the deposition of thermogenic travertine at Thermopylae (one of the larger active travertine-forming systems in Greece) is due to a combination of multiple abiotic and biotic factors. The open question that remains to be answered by future studies is to quantify the contribution of each factor in the deposition process. The combination of all this information will offer significant help to attain an in-depth understanding of carbonate formation in the geological past. To achieve that, it is necessary to investigate how geological and biological phenomena interact simultaneously in nature.
References
Anagnostidis K (1961) Untersuchungen über die Cyanophyceen einiger Thermen in Griechenland. Institut für Systematische Botanik und Pflanzengeographie der Universität Thessaloniki, 7:1–322, Taf. 1–38, Figs 1–285 (in Greek with German abstract)
Anagnostidis K (1967) Thermale und marine Spirulina Vegetation in Griechenland. Ein okologischer Vergleich. Verhandlungen der Internationale Vereinigung für theoretische und angewandte Limnologie 16:1565–1567
Anagnostidis K, Pantazidou A (1988) Endolithic cyanophytes from the saline thermal springs of Aedipsos, Hellas (Greece). Arch Hydrobiol/Algolog Stud 50–53(suppl. 80):555–559
Aubouin J (1959) Contribution à l’étude géologique de la Grèce septentrionale: Les confins de l’Epire et de la Thessalie. Ann Géol Pays Hellen 10:1–483
Banerjee M, Everroad RC, Castenholz RW (2009) An unusual cyanobacterium from saline thermal waters with relatives from unexpected habitats. Extremophiles 13:707–716
Bargar KE (1978) Geology and thermal history of Mammoth Hot Springs, Yellowstone National Park, Wyoming, U.S. Geol Surv Bull 1444:1–55
Bates RL, Jackson JA (1987) Glossary of geology, 3rd edn. American Geological Institute, Alexandria
Beltrán-Magos Y, Carmona J, Vilaclara G, Bojorge-García M (2013) Calcification of the filamentous cyanobacterium Blennothrix ganeshii in calcareous tropical streams of central Mexico region. Hidrobiológica 23:17–27
Braissant O, Cailleau C, Dupraz C, Verrecchia EP (2003) Bacterially induced mineralization of calcium carbonate in terrestrial environments: the role of exopolysaccharides and amino acids. J Sediment Res 73:485–490
Buick R, Dunlop JSR, Groves DI (1981) Stromatolite recognition in ancient rocks—an appraisal of irregularly laminated structures in an early Archean Chert-Barite Unit from North-Pole, Western-Australia. Alcheringa 5:161–181
Castenholz RW (2002) The long-term effects of UV exclusion on the microbial composition and photosynthetic competence of bacteria in hot spring microbial mats. FEMS Microbiol Ecol 39:193–209
Chafetz HS, Folk RL (1984) Travertines: depositional morphology and the bacterially constructed constituents. J Sediment Res 54:289–316
Chafetz HS, Guidry SA (1999) Bacterial shrubs, crystal shrubs, and ray-crystal shrubs: bacterial vs. abiotic precipitation. Sediment Geol 126:57–74
Cohn F (1864) Über die Entstehung des Travertin in den Wasserfällen von Tivoli. Neues Jb Min Geol Palaönt Stuttgart 40:580–610
Desikachary TV (1959) Cyanophyta. I.C.A.R. Monographs on Algae, New Delhi
Dupraz C, Reid RP, Braissant O, Decho AW, Norman RS, Visscher PT (2009) Processes of carbonate precipitation in modern microbial mats. Earth Sci Rev 96:141–162
Folk RL, Chafetz HS, Tiezzi PA (1985) Bizarre forms of depositional and diagenetic calcite in hot-spring travertines, central Italy, vol 36. Carbonate Cements, SEPM Special Publication, pp 349–369
Fouke B (2011) Hot-spring systems geobiology: abiotic and biotic influences on travertine formation at Mammoth Hot Springs, Yellowstone National Park, USA. Sedimentology 58(1):170–219
Fouke B, Farmer J, Des Marias D, Pratt L, Sturchio N, Burns P, Discipulo M (2000) Depositional facies and aqueous-solid geochemistry of travertine-depositing hot springs (Angel Terrace, Mammoth Hot Springs, Yellowstone National Park, U.S.A.). J Sediment Res A Sediment Petrol Process 70:565–585
Geitler L (1932) Cyanophyceae. In: Rabenhorst (Εd) Kryptogamenflora von Deutschland, Leipzig: Akademische Verlagsgesellschaft
Georgalas GC (1938) Le volcans des iles Likhades et de Hagios Ioannis (Kammena Vourla). Praktika Academia Athinion 13:86–98
Gioni-Stavropoulou G (1983) Inventory of hot and mineral springs of Greece, I, Aegean sea. Hydrological and Hydrogeological Investigation Report No. 39, IGME, Athens (in Greek)
Golubic S, Violante C, Plenkovic A, Grgasovic T (2008) Travertines and calcareous tufa deposits: an insight into diagenesis. Geologia Croatica 61:363–378
Grotzinger JP, Rothman DH (1996) An abiotic model for stromatolite morphogenesis. Nature 383:423–425
Hammer Ø, Dysthe DK, Jamtveit B (2010) Travertine terracing: patterns and mechanisms. Geol Soc Spec Pub 336:345–355
Jolivet L, Faccenna C, Huet B, Labrousse L, Le Pourhiet L, Lacombe O, Lecomte E, Burov E, Denèle Y, Brun J-P, Philippon M et al (2013) Aegean tectonics: strain localisation, slab tearing and trench retreat. Tectonophysics 597–598:1–33
Jones B, Renaut WR, Owen BR, Torfason H (2005) Growth patterns and implications of complex dendrites in calcite travertines from L`ysuhóll, Snæfellsnes, Iceland. Sedimentology 52:1277–1301
Kamennaya N, Ajo-Franklin C, Northen T, Jansson C (2012) Cyanobacteria as biocatalysts for carbonate mineralization. Minerals 2:338–364
Kandianis M, Fouke BW, Johnson R, Veysey J, Inskeep W (2008) Microbial biomass: a catalyst of CaCO3 precipitation in advectively dominated regimes. GSA Bulletin 120:442–450
Kanellopoulos C (2011) Geochemical research on the distribution of metallic and other elements in the cold and thermal groundwater, soils and plants in Fthiotida Prefecture and N. Euboea. Environmental impact. Ph.D. Thesis, University of Athens, Greece (in Greek with English abstract)
Kanellopoulos C (2012) Distribution, lithotypes and mineralogical study of newly formed thermogenic travertines in Northern Euboea and Eastern Central Greece. CEJG 4(4):545–560
Kanellopoulos C (2013) Various morphological types of thermogenic travertines in northern Euboea and eastern central Greece. Bull. Geol. Soc. Greece XLVII/3: 1929–1938
Karastathis VK, Papoulia J, Di Fiore B, Makris J, Tsambas A, Stampolidis A, Papadopoulos GA (2011) Deep structure investigations of the geothermal field of the North Euboean Gulf, Greece, using 3-d local earthquake tomography and Curie depth point analysis. J Volcanol Geoth Res 206:106–120
Kitano Y (1963) Geochemistry of calcareous deposits found in hot springs. J Earth Sci Nagoya Univ 1:68–100
Komárek J, Anagnostidis K (1998) Cyanoprokaryota 1. Teil: Chroococcales. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D (eds) Süsswasserflora von Mitteleuropa 19/1, Gustav Fischer, Jena-Stuttgart-Lübeck-Ulm
Komárek J, Anagnostidis K (2005) Cyanoprokaryota 2. Teil/2nd Part: Oscillatoriales. In: Büdel B, Krienitz L, Gärtner G, Schagerl M (eds) Süsswasserflora von Mitteleuropa 19/2, Elsevier/Spektrum, Heidelberg
Komárek J, Montejano G (1994) Taxonomic evaluation of several Chlorogloea species (Cyanoprocaryota) from inland biotopes. Arch Hydrobiol/Algolog Stud 74:1–26
Kranis H (1999) Neotectonic activity of fault zones in central-eastern mainland Greece (Lokris). Ph.D. Thesis, University of Athens, Greece (in Greek with English abstract)
Lambrakis N, Kallergis G (2005) Contribution to the study of Greek thermal springs: hydrogeological and hydrochemical characteristics and origin of thermal waters. Hydrogeol J 13:506–521
Le Pichon X, Angelier J (1979) The Hellenic arc and trench system: a key to the neotectonic evolution of the Eastern Mediterranean area. Tectonophysics 60:1–42
Lowe DR (1994) Abiological origin of described stromatolites older than 3.2 Ga. Geology 22:387–390
McKenzie D (1970) Plate tectonics of the mediterranean region. Nature 226:239–242
McKenzie D (1972) Active tectonics of the Alpide—Himalayan belt: the Aegean Sea and surrounding regions (Tectonics of the Aegean Region). Geophys J Roy Astr Soc 55:217–254
Mountrakis D (1986) The Pelagonian zone in Greece: a polyphase deformed fragment of the Cimmerian continent and its role in the geotectonic evolution of the Eastern Mediterranean. J Geol 94:335–347
Obst M, Wehrli B, Dittrich M (2009) CaCO3 nucleation by cyanobacteria: laboratory evidence for a passive, surface-induced mechanism. Geobiology 7:324–347
Okumura T, Takashima C, Shiraishi F, Nishida N, Yukimura K, Naganuma T, Koike H, Arp G, Kano A (2011) Microbial processes forming daily lamination in an aragonite travertine, Nagano-yu hot spring, southwest Japan. Geomicrobiol J 28:135–148
Okumura T, Takashima C, Shiraishi F, Akmaluddin Kano A (2012) Textural transition in an aragonite travertine formed under various flow conditions at Pancuran Pitu, Central Java, Indonesia. Sediment Geol 265–266:195–209
Orfanos G (1985) Inventory of hot and mineral springs of Greece, Peloponnesus, Zakynthos, Kythira. Hydrological and Hydrogeological Investigation Report No. 39, IGME, Athens (in Greek)
Pentecost A (1995) Geochemistry of carbon dioxide in six travertine-depositing waters of Italy. J Hydrol 167:263–278
Pentecost A (2003) Cyanobacteria associated with hot spring travertines. Can J Earth Sci 40:1447–1457
Pentecost A (2005) Travertine. Springer, Berlin, Heidelberg
Pentecost A, Viles HA (1994) A review and reassessment of travertine classification. Geogr Phys Quatern 48:305–314
Pentecost A, Whitton BA (2000) Cyanobacteria and limestone. In: Whitton BA, Potts M (eds) The ecology of cyanobacteria. Kluwer, Amsterdam, pp 257–279
Pentecost A, Bayari S, Yesertrner C (1997) Phototrophic microorganisms of the Pamukkale travertine, Turkey: their distribution and influence on travertine deposition. Geomicrobiology 14:269–283
Pe-Piper G, Piper D (2002) The igneous rocks of Greece, the anatomy of an orogen, Gebruder Borntraeger
Philip H (1974) Εtude néotectonique des rivages égéens en Locride et Eubée nord-occidentale (Grèce). de Montpelier, Thése doc sp, Acad
Riding R (2000) Microbial carbonates: the geological record of calcified bacterial-algal mats and biofilms. Sedimentology 47:179–214
Schneider J, Le Campion-Alsumard T (1999) Construction and destruction of carbonates by marine and freshwater cyanobacteria. Eur J Phycol 34:417–426
Semikhatov MA, Gebelein CD, Cloud P, Awramik SM, Benmore WC (1979) Stromatolite morphogenesis—progress and problems. Can J Earth Sci 19:992–1015
Sfetsos SC (1988) Inventory of hot and mineral springs of Greece, III, Continental Greece. Hydrological and Hydrogeological Investigation Report No. 39, IGME, Athens (in Greek)
Stanier RY, Kunisawa R, Mandel R, Cohen-Bazire G (1971) Purification and properties of unicellular blue green algae (Order Chroococcales). Bacteriol Rev 35:171–205
Whitton BA, Ward D, Castenholz R, Miller S (2012) Cyanobacteria in geothermal habitats. Ecology of cyanobacteria II. Springer, Netherlands, pp 39–63
Winkel L, Casentini B, Bardelli F, Voegelin A, Nikolaidis N, Charlet L (2013) Speciation of arsenic in Greek travertines: co-precipitation of arsenate with calcite. Geochim Cosmochim Ac 106:99–110
Acknowledgments
The authors would like to thank Assoc. Prof. Pantazidou Adriani of University of Athens, Faculty of Biology, Department of Ecology and Systematics, who provided insight and expertise that greatly assisted the research concerning cyanobacteria.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kanellopoulos, C., Lamprinou, V., Mitropoulos, P. et al. Thermogenic travertine deposits in Thermopylae hot springs (Greece) in association with cyanobacterial microflora. Carbonates Evaporites 31, 239–248 (2016). https://doi.org/10.1007/s13146-015-0255-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13146-015-0255-4