Abstract
A base line study was conducted to understand the geochemical variations of drip waters in Niah Great Cave, Sarawak, NW Borneo of Malaysia. Drip waters were collected at three different sites within the cave (D6: SW entrance enroute to painted Cave; D5: centre of the Great Cave, and D8: west mouth Great Cave entrance). The collected samples were analysed for temperature, pH, EC, major elements, non purgeable organic carbon, δ13C and δ18O. Mg/Ca and Sr/Ca ratios, ionic strength, partial pressure of CO2 and saturation indices of carbonate minerals have been used to dictate the geochemical signatures of drip water compositions. The data reveal a wide range of major ion chemistry at three different sites within the cave. The δ13C values in drip waters range from −10.58 to −10.09 ‰. The enriched and the depleted δ13C values are noted at the entrances of the cave due to degassing of water CO2 and warmer air results in lighter fractions of δ13C. The δ18O values range from −6.89 to –6.16 ‰. Minor δ18O deviation in the drip waters are due to the variation in drip rates as faster drip rates show depleted isotopic composition and vice versa. The spatial variability of drip water chemistry results due to water–rock interaction at various conditions of subsurface flow path. This study gives a basis for interpreting the varying controls on drip water chemistry in the cave.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cave drip water chemistry gains its own importance for interpreting the present day cave environmental condition to regulate the paleoclimate proxy-data extracted form speleothems composition (Baker et al. 1997). In karst environment, water chemistry can vary extremely over very short distances depending on the samples collected from an actively flowing conduit or from the rock matrix (Quinlan and Ewers 1985). Water chemistry can also be modified by CO2 outgassing, mineral dissolution and/or precipitation along the subsurface flow paths (Holland et al. 1964; Dreybrodt 1981; Herman and Lorah 1986). During low flow condition, water composition shifts towards concentrated endmembers and in the high-flow condition it appears to be diluted by rapid infiltrating fresh water endmembers (Mayer 1999). Many studies have been conducted to understand the controls on the geochemical evolution and spatial variability of cave drip waters (Tooth and Fairchild 2003; Musgrove and Banner 2004; McDonald et al. 2007).
Carbon isotopes in the cave system may indirectly indicate the vegetative change, climatic conditions and karst processes. Carbon isotope variations arise from a variety of causes such as soil respiration, fluid–rock interaction in the host limestone, degassing of CO2 and precipitation of calcite upflow from the speleothem drip site and calcite precipitation at the drip site (Jimenez de Cisneros and Caballero 2011). According to Li et al. (2011) the Furong Cave located in Chongqing of China shows that the δ18O (−11.5 to −8.2 ‰) and δ13C (−2.6 to 0.1 ‰) values of the bedrock around the Cave might be of marine biological origin during Cambrian. The variable δ13C values of active speleothem deposits from −11 to 0 ‰ could have been caused by kinetically mediated CO2 degassing processes. Carbon isotopes in drip water are affected by both Carbon isotope composition of soil air and host rock carbonate. It also strongly depends on the calcite dissolution system, i.e. open, closed and intermediate conditions (Fohlmeister et al. 2011). Li et al. (2012) found that in 54 soil samples collected from five soil profiles, δ13C of soil organic matters was −22 ‰, which could be attributed to the different transportation rates of stable carbon isotopes during the decomposition of plants and organic matters in soils. The average dissolved inorganic carbon δ13C value was −11 ‰, about 11 ‰ heavier than the δ13C of organic matters in soils, which proved that part of dissolved inorganic carbon in cave drip water was sourced from dissolution of inorganic carbonates. In Yuncheng Basin, China, δ13C values in groundwater ranging from −16.4 to −8.2 ‰ indicate that carbonate weathering is a significant source of dissolved inorganic carbon (Currell and Cartwright 2011).
Oxygen isotopes are useful as a paleoclimate proxy and the oxygen isotope composition of meteoric precipitation can exhibit systematic variation depending on various processes: (1) ambient temperature at the time of precipitation, (2) the amount of precipitation, (3) storm paths, (4) the distance of a precipitation event from its moisture source region in the ocean and (5) the ground surface elevation where precipitation occurs (Clark and Fritz 1997; Ingraham 1998). The isotopic composition of drip-waters also reflects other variables including atmospheric inputs, evaporation, soil profiles and local hydrogeology. The isotopic differences in δ18O between southern (−5.9 to −5.3 ‰) and northern groundwaters (−5.7 to −3.8 ‰) in Lakhssas Plateau, Morocco, are related to elevation that induced fractionation of oxygen isotope in recharge water that originated from coastal moisture (Ettayfi et al. 2012). It is also suggested that the high salinity in groundwater from the northern margin is related to the presence of schist rocks in these areas. The isotopic contents of groundwaters in the Souss Upstream Basin, southwestern Morocco, ranging from −8 to −5.2 ‰ for δ18O and from −52 to −34 ‰ for δD reveals a significant infiltration from surface water and a major recharge directly from fractures in the crystalline and limestone formations (Dindane et al. 2003).
The Great Cave of Niah in Sarawak is a famous archaeological site because they are one of the few places in south-east Asia with evidence of continuous human occupation during the late Pleistocene and early Holocene (Barker et al. 2002a, b). In the past two decades, numerous scientists have emphasized the archaeological importance of Niah Cave and human revolution in Sarawak region. Particularly, Harrisson (1958, 1959a, b, 1965, 1996) has initiated the study about the prehistory and continually concentrated on different aspects like radiocarbon dating, archaeological study, ethnological study, Stone Age culture, etc. Barker et al. (2000, 2001, 2002a, b, 2003, 2007) and Barker (2005) have discussed the archaeology at Niah Cave and also his group has done four seasons’ fieldwork at Niah to study the ‘human revolution’ in lowland tropical Southeast Asia. Barker and his group have also done a project on geomorphology of the Great Cave of Niah, Sarawak, as a part of the Niah Caves Project.
Gilbertson et al. (2005a, b) recorded the past human activity and geomorphological change in a guano-rich tropical cave mouth: initial interpretations of the Late Quaternary succession in the Great Cave of Niah, Sarawak. According to Hunt and Rushworth (2005a, b), influx of both sediment and pollen is controlled by two major factors: proximity to external wind-blown and wash transported inputs of silt, leaves and pollen and second, the strong patterned flux of guano in the interior of the cave. Hunt et al. (2007) has described stratigraphy, sedimentary and palynological evidence for changing environments at an area known as ‘Hell Trench in the west mouth of the Great Cave of Niah. Dykes (2007) studied about mass movements in cave sediments by performing a geotechnical investigation of a 42,000-year-old guano mudflow inside the Cave. The physical, hydrological and geotechnical properties of the material were determined to assess the stability of the slope which led to the conclusion that the climate had been wetter than it is at present. These findings have implications for the interpretation of sediment deposits in other relict caves.
Gillmore et al. (2005) have attempted a study on potential risk from 222radon posed to archaeologists and earth scientists in the Niah cave and suggested that the level of radon concentrations at the ground surface can exceed those of the surrounding area by a factor of >2×. This study also concluded that although radon concentrations in this famous cave are low by world standards, they may pose a health risk to both excavators and cave occupants to certain extent. Cobb et al. (2007) investigated the relationship between large-scale climate variability and rainfall along with cave drip water oxygen isotopic composition from Gunung Mulu and Gunung Buda National parks on intraseasonal to interannual timescales.
According to Bird et al. (2005), during the Last Glacial Maximum (LGM) in Borneo, offshore seawater temperature was about ~2 to 3 °C lower than that today, whereas air temperature was reduced by 6–7 °C. Also the estimated annual precipitation at LGM was reduced by about 30–50 % when compared with the present due to cooler annual air temperature which was associated with the reduction of precipitation (Kershaw et al. 2001; van der Kaars et al. 2000; Bird et al. 2005). Hunt et al. (2007) have discussed about biogeographical arguments, based on modern faunal distributions, and concluded that the ‘coastal’ lands of north and northwest Borneo, including Niah, may have remained amply humid during the late Pleistocene for lowland rainforest. Based on the above scenarios, there has been limited attempt to study the geochemistry of drip waters in Niah Great Cave. Hence, this study is a baseline attempt to understand few controls of drip water compositions in the cave.
Study area
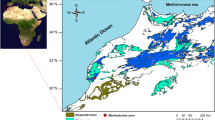
Niah Great Cave is one of the largest documented caves in Sarawak, NW of Borneo, Malaysia, and is the key archaeological site in Southeast Asia. The main interest in the cave has been the populations of cave swiftlets and bats (Harrisson 1959, 1996; Rabett et al. 2006). It lies in dense wet low land rainforest at the northeast corner of the Gunong Subis (N3°480; E113°470) (Fig. 1), an isolated group of tower karst massifs, located 64 km SSW of Miri and 11 km inland from the coast of South China Sea. The Gunong Subis has been developed in the algal patch reef facies of the Subis Limestone Member of the Tangap Formation, of Miocene age (Wilford 1964; Hazebroek and Morshidi 2001). This formation consists of different rock types such as calcareous shale, marl, greenish claystone and pure limestone beds (Hutchinson 2005). Near the cave mouth, sedimentation is dominated by wind-blown silt and airfall pollen, whereas the interior of the cave dominated by highly organic matters and largely composed of bird droppings (Hunt and Rushworth 2005a). Stratigraphy of Late Quaternary sedimentary sequence exposed in the West Mouth of the cave has been explained by Gilbertson et al. 2005a, b; Stephens et al. 2005. The West Mouth sediments are derived from the results of airfall, wash, fluvial, colluvial and mudflow processes, with episodic ponding and drainage (Gilbertson et al. 2005a, b). In this cave, about 3,200 m of passages have been identified which includes chambers up to 100 m in diameter and 70 m high. The morphology of the cave passages, with Scallops features, is consistent with the cave being of phreatic origin (Hunt and Rushworth 2005a, b). At the same time, the cave has been modified by subaerial processes. The west mouth of the Great Cave is a vast cave entrance (60 m high and 180 m wide) that opens about 12 m up the cliff-like side of a bedrock-floored gorge, which separates the massif containing the Great Cave from the main massif of the Gunong Subis.
Climate and rainfall
The climate of Sarawak is warm and humid throughout the year. The mean annual temperature at sea level is about 27 °C with a lapse rate of 0.5–0.7 °C/100 m elevation. The annual rainfall varies considerably both between sites and at the same site in different years. Rainfall data have been collected from January 1983 to February 2012 in the study area (Niah Forest Office). The average annual rainfall is about 2,813.5 mm per year. Higher rainfall is recorded during the month of December and lesser in June (Fig. 2). The annual rainfall is observed above average value in the south, west and interior of the State, north subcoastal lowlands and east of the Temabu Range in the northeastern uplands. Due to the variability and unpredictability of the rainfall between years, there is an underlying seasonality in rainfall patterns. However, high rainfall is expected from November to February, with a drier period from June to August. Seasonality is much more pronounced in the coastal and western parts of the State than in the northern and interior regions. Dominant moisture sources for Sarawak during the winter monsoon are the tropical western Pacific Ocean and the South China Sea, whereas during the summer monsoon it is from the Indian Ocean (Aggarwal et al. 2004).
Methods and materials
In order to assess the spatial variability, three drip water samples were collected from different sites within the cave (D6: SW entrance enroute to painted Cave; D5: center of the Great Cave; and D8: west mouth Great Cave entrance) (Fig. 1) during July 2011. Drip water samples, D6 and D8, with fast dripping rates of ~20–50 drips per minute (dpm) and for slow dripping site (~5–15 dpm), D5, were collected from the fractures of cave ceiling. pH, temperature and Electrical conductivity (EC) were determined in situ using portable pH and conductivity meter (Thermo Scientific Orion Star, 4 Star Plus Meter). The samples were collected in 250 ml polyethylene bottles and kept at a temperature of 4 °C until analysis. The collected samples were filtered using a preconditioned plastic Millipore filter unit equipped with a 0.45-μm filter membrane for further elemental analysis. HCO3 was analysed using titrimetry, and Ca, Mg, Na, K, Cl, SO4 and Sr by the ion chromatograph (IC, Metrohm 861). Duplicate samples were analysed to check the analytical precision and it is ±5 % for all hydrogeochemical variables. Dissolved organic carbon (DOC) data were analysed as non-purgeable organic carbon (NPOC) performed by the “Total Organic Carbon Analyser TOC-VCSH” (Shimadzu Corporation). Ionic strength (IS), Carbon dioxide partial pressure (Log pCO2) and saturation index (SI) of carbonate minerals were computed using the WATEQ4F (Hammarstrom et al. 2005).
X-ray diffraction (XRD) of the powder sample provides one of the easiest and semi- quantitative methods of identifying the minerals present in the rock samples. A representative powdered sample of bed rock (Limestone) collected in the cave was subjected to X-ray diffraction with scan angle (2θ) ranging from 5 to 89°, using a Phillip X’ Pert X-ray diffractometer.
For oxygen-18 isotope analysis, 25 ml of water samples were collected in airtight polyethylene bottles. The isotopic ratio was measured using Geo 20–20, Europa, mass spectrometer. For 18O/16O ratio measurement, 1 ml each of the water sample was flushed with 99.9 % pure CO2 gas at 1 bar pressure in a 5-ml capacity vial for 1 min and equilibrated for 8 h at 50 °C temperature. The equilibrated CO2 gas reflects the 18O/16O ratio of the water sample and the data acquisition system expresses the same ratio in permil (‰) variation with respect to an international standard, namely “Standard Mean Ocean Water” (SMOW). The results are expressed in δ values, which are in permil and are computed as follows: δ18O = (R sample− R standard/R standard) × 103, where R = 18O/16O. For Carbon isotope analysis, 0.1 ml of saturated HgCl2 solution was carefully added to the collected drip water samples to avoid algal growth. Then the δ 13C values were measured by isotope ratio mass spectrometer and given in ‰ with respect to PDB standard.
Results and discussion
Major ion chemistry
The concentrations of major dissolved constituents in the drip waters were quite variable at three different sampling points (Table 1). pH ranging from 6.65 to 6.92 shows near neutral in nature. EC ranges from 208 to 774 μS/cm and the higher value is noted in D8 (at the west mouth Great Cave entrance). The abundance of major ions are Ca > Na > Mg > K = HCO3 > SO4 > Cl in D5 and D6 drip waters; Ca > K > Na > Mg = HCO3 > SO4 > Cl in D8 drip water. In general, Ca and HCO3 are the dominant cation and anion, respectively, in the drip water composition, which is almost similar to that of other few karst region cave waters (Langmuir 1971; Jacobson and Langmuir 1974; Poth 1977).
Nitrate in cave waters can be derived from biogenic sediments through various processes as detrital material transported from the surface, excrement or guano produced by animals inside the cave and secondary material formed inside the cave (Khanaqa and Al-Manmi 2011). In Niah Great Cave, higher concentration of nitrate (16.47 mg/L) was observed in D6, which can be derived from soil leachate by recently recharged waters. NO3 is also derived from the degradation of organic matters present in the soil profile and also coated in the rock walls by bacterial action. This process was substantiated with the low Eh value observed in that location. Oxygen is the chief chemical species responsible for the Eh value in water. The increasing content of O2 in water also increases the values of Eh. So the depletion of oxygen in D6 shows that oxygen was consumed by the bacteria during this process (Akinbile and Yusoff 2011).
Phosphate ranges from 0.25 to 2 mg/L and the concentration of phosphate in the drip waters may be derived from the leaching of authigenic phosphate minerals that originate from the degradation of organic matters in the cave (Hutchinson 1950; Karkanas 2001). Authigenic phosphate minerals are good indicators of prior accumulation of phosphate-rich organic matter and to identify certain activity in open-air sites (Shahack-Gross et al. 2004).
The hydrochemical data have been plotted in Piper plot (Piper 1953) and all the samples are representing in Ca–HCO3 facies indicating recharged waters (Fig. 3). This type of water results from the dissolution of calcium-rich minerals such as calcite by water–rock interaction process (Freeze and Cherry 1979). The minerals present in the bed rock of Niah Cave were identified with semi-quantitative method (XRD) also reflecting the presence of large amount of Calcite with a significant high peak of Rhombohedral Calcite (Fig. 4). The other peak visible is that of Magnesite, Dolomite and Aragonite, with lesser amount of Quartz and Smithsonite. Gibbs plot (Gibbs 1970) is also used to understand and differentiate the processes control the drip water chemistry. In the plot, all the three samples fall in the water–rock interaction field suggesting that the weathering of rocks primarily controls the ion chemistry of drip waters (Fig. 5) as the bed rock is chiefly composed of carbonate minerals.
Total dissolved ion (TDI)
Total dissolved ion is used to understand the extent of reaction with bedrock minerals and thus is a rough indicator of residence time of water in the aquifer matrix (Mayer 1999). Drip waters range from 153.83 to 240.73 mg/L TDI. Samples collected in the three sites are inconsistent in TDI values. Higher TDI concentration was observed in D8 and significantly more concentrated ions indicate longer residence time in the rock matrix (Fig. 6). Lower TDI value was noted in D6 with less ionic concentrations except bicarbonate due to the flushing environment.
Ionic strength (IS)
Ionic strength is a measure of total concentration of ions which emphasizes increased contribution of species with charges greater than one to solution non-ideality (Domenico and Schwartz 1990):
where m 1 is the atomic/molecular weight and z 1 is the valance of the respective ion. The approximate value of ionic strength can also be computed from the specific conductance of the solution if this has been measured (Lind 1970). Domenico and Schwartz (1990) reported that ionic strength of fresh water is <0.005. In the present study, D6 water shows lower ionic strength (Fig. 7) and indicates newly recharged water into the rock matrix, whereas D5 and D8 water has higher ionic strength and reveals longer residence time within the rock matrix with high concentration (Chidambaram et al. 2010; Prasanna et al. 2010).
Mg/Ca and Sr/Ca
The rate of water residence time states the amount of water–rock interaction that occurs, with drier intervals resulting in longer residence time, more extensive water–rock interaction and higher Mg/Ca and Sr/Ca (Musgrove and Banner 2004). There are many controls on drip water Mg/Ca and Sr/Ca which include compositional variations of soils and bedrocks, the type of flow paths, amount of water–rock interaction and variable extents of prior calcite precipitation (PCP) (Fairchild and Tremble 2009). The water–rock interaction and PCP are two different processes and the amount of water–rock interaction that occurs is largely dictated by variable water residence time, whereas prior calcite precipitation can be dominantly dictated by variable water residence time or ventilation of cave-air CO2.
Drip water acquires the concentration of Mg and Sr from interaction with the soil and carbonate rocks that it moves through and attains higher Mg/Ca and Sr/Ca ratios (Musgrove and Banner 2004). Figure 8 in the present study reveals that D6 drip water has low Mg/Ca ratio and shows lesser water–rock interaction due to shorter residence time of water in the rock matrix, whereas D5 and D8 drip waters have higher Mg/Ca ratio and indicate relatively extensive water–rock interaction by longer residence time. The water–rock interaction curve in Fig. 9 demonstrating that D5, D6 and D8 drip waters experiences varying amounts of water–rock interaction. It is also shows good correspondence with observed trends of increasing Sr/Ca with decreasing Ca, which is common in calcite dominant karst aquifers.
Log pCO2
Log pCO2 values of the waters depends on various factors: (1) primary pCO2 of the infiltrating waters, which will be influenced by high pCO2 in soil zones (Pitty 1971), (2) buffering of CO2 in the aquifer (Atkinson 1977) and (3) degree of CO2 degassing (Toran and Roman 2006). Ventilation effects may limit the pCO2, particularly if there are large effluxes of CO2 rich water into the cave (Barker et al. 2000). The log pCO2 values range from −1.79 to −1.99 and individual samples show relatively significant variations in log pCO2. These values are higher in comparison with average atmospheric log pCO2 value of −3.5. The cave air pCO2 values reveal the pattern of circulation and mixing in the cave. The pattern is also related to the cave ventilation. The lowest pCO2 values were observed in D6 and D8 (Fig. 10), slightly lesser than the atmospheric value, revealing that the D6 water has just entered into the cave system or the residence time of this water in the rock matrix is considerably lesser or an open system substantiated by low TDI value. On the contrary, D8 water shows higher TDI value with lesser log pCO2 resulting in the loss of CO2 by degassing. The degassing possibly occurred along the fractures, cracks and fissures in the limestone leading to the near saturation of D8 water with respect to calcite.
The fast moving conduit water would to be expected to be at under saturation, and slow moving matrix liquid is believed to be at saturation (Toran and Roman 2006). The present data give the details about the contribution of rock matrix and drip water. The possible explanation is that the recharged water (D6 and D8) picked up excess CO2 over the atmospheric equilibrium value as it traveled through the soil profile above the cave. Subsequently, CO2 out gassing occurred when the matrix water entered the conduit.
Higher pCO2 value is noted in D5, the middle portion of the cave, because it is relatively shut off from the ventilation and does not experience as much air flow. This may also suggest that the additional CO2 has been acquired from the soils/sediments caps above the cave during the process of infiltration through the fractures of the rocks.
Saturation Index of Carbonate minerals
To determine the chemical equilibrium between minerals and waters, saturation indices of carbonate minerals were calculated using the following equation (Lloyd and Heathcode 1985):
where IAP is the ion activity product and Ks is the solubility product of the minerals.
The calculated values of SI for Calcite, Dolomite, Aragonite and Magnesite of the drip waters show that calcite and aragonite fall near saturation indicating precipitation (Fig. 11). The SI values of Dolomite and Magnesite fall under saturation showing dissolution effect (Prasanna et al. 2010). The out gassing of CO2 drives the carbonates equilibrium towards more calcite precipitation (Toran and Roman 2006):
Thus, bicarbonate is lost through the conversion of CO2 and subsequent out gassing and pH must rise to maintain the charge balance. Another way to explain this balance is that a decrease in pCO2 results in a decrease in H+ [pCO2 = (aHCO3 × aH)/(KHCO3 × Kco2)]. This pH increase further moves the reaction towards precipitation of calcite. However, out gassing occurs faster than CaCO3 precipitation, which leads to oversaturation (Freeze and Cherry 1979). Particularly, precipitation of calcite does not tend to occur until the saturation index is above 0.5 (Dreybrodt et al. 1992; Lorah and Herman 1988).
The log pCO2 versus SI of Calcite trend (Fig. 12) also supports the hypothesis that variations in out gassing are related to pathways and dissolution trends. D6 and D8 waters are closed to saturation and progressively decreasing in log pCO2 values due to CO2 out gassing. The out gassing exhibited a decrease in log pCO2 value and increase in SI of Calcite, whereas D5 was under saturated condition.
The SI of Magnesite is greater than the SI of Dolomite indicating that only excess of Mg is contributed to the formation of Dolomite in association with Ca. Moreover, the XRD data show that the bed rock is chiefly represented by Calcite and the process of attaining saturation of Dolomite and Magnesite is limited due to the availability of Mg from Magnesite and Dolomite in bed rock.
A mechanistic model for the precipitation of CaCO3 proposed by Plummer et al. (1978) considers the reaction between bulk solution CaHCO3 + and crystal growth of Calcite. Figure 13 shows the relationship between calculated SI of Calcite and the activity of the CaHCO3 + species in solution. A linear relationship between SI of Calcite and CaHCO3 + suggests that CaHCO3 + has an active role in the precipitation of calcite.
DOC (NPOC)
The level of dissolved organic carbon (DOC), also known as non-purgeable organic carbon (NPOC), in natural environments is comparable to the inhibition of calcite precipitation (Inskeep and Bloom 1986). But most calcite precipitation models do not account for organic matter inhibition. The available model proposed by Lebron and Suarez (1996) predict the calcite precipitation rate in the presence of DOC; they found that when the DOC ≥0.05 mM, the precipitation rate is essentially independent of the surface area of the calcite and dependent on the DOC concentration. They also determined that the calcite crystals were coated with an amount of DOC necessary to avoid precipitation of calcite by crystal growth and added to the solutions.
In this present study, an attempt was made to know the capability of DOC in the drip waters to inhibit the calcite precipitation. Figure 14 shows a negative linear relationship between DOC and SI of Calcite suggesting that the decrease in precipitation of calcite with increasing DOC. Hence the precipitation rate of calcite as a function of the DOC in the study area as inferred in the previous studies (Lebron and Suarez 1996).
Comparison of drip-rate with speleothem growth
According to the model proposed by Dreybrodt (1988), it is complicated to link the temporal variations of pCO2 between Ca concentration and saturation index, or between Ca concentration and predicted growth rate. But the effects of varying pCO2 will enhance the variability of growth rate with time. In the present study, samples that were collected in late summer (July 2011) could be represented by relatively poorly speleothem growth, because of slow drip rates, high pCO2 and comparatively lower Ca value observed at D5. Conversely, maximal growth rates reflecting high drip rate, low pCO2 values and high Ca were noted in D6 and D8. This statement has also been substantiated with DOC concentration in the drip waters, where D5 has higher DOC value and indicates slow crystal growth and lower values in D6 and D8 lead to faster growth rate (Lebron and Suarez 1996).
Carbon isotope
The carbon isotopic composition in the drip waters shows only a small variation, ranging from −10.58 to −10.09 ‰ of δ13C. Environmental local processes such as evaporation and changes in cave air pCO2 controlled by seasonal ventilation can influence the final δ13C values. The CO2-poor air, drawn into the cave through the entrance, enhances degassing of water CO2 and causes concomitant δ13C enriched carbonate deposition as noted in D6 (Spotl et al. 2005). D8 drip water has lighter fraction due to the reverse process as the air entering through D6 may pass through D5 and go out of the cave through D8 as observed in the field during the time of sampling. So the air coming out of the cave is warmer with slightly higher pCO2 than D6, which results in lighter fractions of δ13C (Fig. 15).
Diffuse water flow in karst aquifers would allow greater opportunity for interaction between the waters and the host limestone resulting in greater dolomite dissolution and calcite formation and there is a definite negative relationship noted between the δ13C values and the SI of Dolomite. The SI increases with the decrease in δ13C values. The enriched and the depleted δ13C values are noted at the entrance of the caves. It is also interesting to note that the saturation index of dolomite has an inverse linear relationship to δ13C (Fig. 16) than that of calcite.
Oxygen isotope
The mean rainfall δ18O value is −6.7 ± 2.8 ‰ in the northern Borneo region located near the study area (Cobb et al. 2007). The δ18O values of drip waters in Niah Great Cave range from −6.89 to −6.16 ‰. This suggests that the oxygen isotopic composition of the supplying water into the cave reflects an average rainfall condition. Cave drip water oxygen isotope composition is interpreted here as an annual rainfall δ18O signal as in Furong Cave of China (Li et al. 2011). The fact that the rainfall and drip water δ18O has almost the same average value, which indirectly indicates that minimal evaporation occurs between the ground surface and the drip water site. The lighter values are noted in the entrance samples D6 and D8. The D5 may be enriched as it is located in the centre of the cave and may be due to the variation in the over burden thickness or due to water flow directions. But still minor isotopic variations are also noted due to the variation in drip rates, as faster drip rates show depleted isotopic composition and vice versa (Cobb et al. 2007).
The fast and slow drip rates were indistinguishable and match with the mean rainfall δ18O value (−6.7 ± 2.8 ‰) in the nearby area (Cobb et al. 2007). The drip-rate is more responsive to specific recharge events than drip water δ18O, given that drip-rate is governed by instantaneous hydraulic pressure changes in the water flow pathway (Genty and Deflandre 1998; Baker and Brunsdon 2003), while drip water composition inferred to reflects a cumulative mixture of past rainfall events (Ayalon et al. 1998; Perrin et al. 2003; Cruz et al. 2005). Furthermore, the detailed investigation on drip rate, amount of rainfall, seasonal variation and humidity will help us to investigate the process responses for isotope fractionization and process governing the water chemistry of the Niah Great Cave.
Conclusions
This study reveals the geochemical variations between the drip waters collected from three different sites within the Niah Great Cave. Drip waters from the entrances of the cave shows considerably low ionic strength and lesser Log pCO2 values indicate newly recharged water from the soil leachate, with lesser residence time in the rock matrix. Whereas the drip water collected at the center of the cave has derived from intense water–rock interaction, gets more concentrated during the low-flow condition. CaHCO3 + species in the drip waters were confirmed as the controlling mechanism of calcite precipitation. SI of Calcite and Aragonite are near saturated leading to precipitation, whereas SI of Dolomite and Magnesite are under saturation state due to dissolution. The higher concentration of DOC has also found to decrease the rate of calcite precipitation and added to the solutions. Possible geochemical processes have been identified by using the co-variation of Mg/Ca and Sr/Ca. D6 drip water composition is derived with lesser water–rock interaction, whereas D5 and D8 drip waters are dictated by extensive water–rock interaction due to longer residence time. The fractionation of drip water δ13C depends on the degree of degassing of water CO2 associated with cave air temperature. Inverse relationship between δ13C and SI of Dolomite may results in greater dolomite dissolution and calcite formation due to water–rock interaction. Similar values of drip water δ18O and average annual rainfall δ18O indicates minimal evaporation. The lighter fractionation was observed in the entrance of the cave and enriched δ18O in the centre of the cave due to the variation in the over burden thickness or due to water flow directions. Though there may be other factors controlling the drip water chemistry, which have to be studied in detail by increasing the number of parameters analysed and the number of sampling locations.
References
Aggarwal PK, Frohlich K, Kulkarni KM, Gourcy LL (2004) Stable isotope evidence for moisture sources in the Asian summer monsoon under present and past climate regimes. Geophys Res Lett 31. doi:10.1029/2004GL019911
Akinbile CO, Yusoff MS (2011) Environmental impact of leachate pollution on groundwater supplies in Akure, Nigeria. Int J Environ Sci Dev 2(1):81–86
Atkinson TC (1977) Carbon dioxide in the atmosphere of the unsaturated zone: an important control of groundwater hardness in limestones. J Hydrol 35:111–123
Ayalon A, Bar-Matthews M, Sass E (1998) Rainfall-recharge relationships within a karstic terrain in the eastern Mediterranean semi-arid region, Israel: delta O-18 and delta D characteristics. J Hydrol 207:18–31
Baker A, Brunsdon C (2003) Non-linearities in drip water hydrology: an example from Stump Cross Caverns, Yorkshire. J Hydrol 277:151–163
Baker A, Barnes WL, Smart PL (1997) Variations in the discharge and organic matter content of stalagmite drip waters in Lower Cave, Bristol. Hydrol Process 11:1541–1555
Barker G (2005) The archaeology of foraging and farming at Niah Cave, Sarawak. Asian Perspect 44(1):90–106
Barker G, Barton H, Beavitt P, Chapman S, Derrick M, Doherty C, Farr L, Gilbertson D, Hunt C, Jarvis W, Krigbaum J, Maloney B, McLaren S, Pettit P, Pyatt B, Reynolds T, Rushworth G, Stephens M (2000) The Niah Caves Project: preliminary report on the first (2000) season. Sarawak Mus J 55(76):111–149
Barker G, Badang B, Barton H, Beavitt P, Bird M, Daly P, Doherty C, Gilbertson D, Glover I, Hunt C, Manser J, McLaren S, Paz V, Pyatt B, Reynolds T, Rose J, Rushworth G, Stephens M (2001) The Niah Cave Project: the second (2001) season of fieldwork. Sarawak Mus J 77:37–119
Barker G, Barton H, Beavitt P, Bird M, Daly P, Doherty P, Gilbertson D, Hunt C, Krigbaum J, Lewis H, Manser J, McLaren S, Paz V, Piper P, Pyatt B, Rabett R, Rose J, Rushworth G, Stephens M (2002a) Prehistoric foragers and farmers in Southeast Asia: renewed investigations at Niah Cave, Sarawak. Proc Prehistoric Soc 68:147–164
Barker G, Barton H, Bird M, Cole F, Daly P, Gilbertson D, Hunt C, Krigbaum J, Lampert C, Lewis H, Lloyd-Smith L, Manser J, McLaren S, Menotti F, Paz V, Piper P, Pyatt B, Rabett R, Reynolds T, Stephens M, Thompson G, Trickett M, Whittaker P (2002b) The Niah Cave Project: the third (2002b) season of fieldwork. Sarawak Mus J 78:87–177
Barker G, Barton H, Bird M, Cole F, Daly P, Dykes A, Gilbertson D, Hunt C, Krigbaum J, Lampert C, Lewis H, Lloyd-Smith L, Manser J, Menotti F, Paz V, Piper P, Pyatt B, Rabett R, Reynolds T, Stephens M, Thompson G, Trickett M (2003) The Niah Cave Project: the fourth (2003) season of fieldwork. Sarawak Museum Journal 58(ns79):45–120
Barker G, Barton H, Bird M, Daly P, Datan I, Dykes A, Farr L, Gilbertson D, Harrisson B, Hunt C, Higham T, Kealhofer L, Krigbaum J, Lewis H, McLaren S, Paz V, Pike A, Piper P, Pyatt B, Rabett R, Reynolds T, Rose J, Rushworth G, Stephens M, Stringer C, Thompson J, Turney C (2007) The ‘human revolution’ in lowland tropical Southeast Asia: the antiquity and behaviour of anatomically modern humans at Niah Cave (Sarawak, Borneo) J Hum Evol 52:243–261
Bird MI, Taylor D, Hunt CO (2005) Palaeoenvironments of insular southeast Asia during the last Glacial period: a savannah corridor in Sundaland. Quatern Sci Rev 24:2228–2242
Chidambaram S, Ramanathan AL, Prasanna MV, Karmegam U, Dheivanayagi V, Ramesh R, Johnsonbabu G, Premchander B, Manikandan S (2010) Study on the hydrogeochemical characteristics in groundwater, post- and pre-tsunami scenario, from Portnova to Pumpuhar, southeast coast of India. Environ Monit Assess 169:553–568
Clark ID, Fritz P (1997) Environmental Isotopes in Hydrology. Lewis Publishers, Boca Raton p 328
Cobb KM, Adkins JF, Partin JW, Clark B (2007) Regional-scale climate influences on temporal variations of rainwater and cave dripwater oxygen isotopes in northern Borneo. Earth Planet Sci Lett 263:207–220
Cruz FW, Burns SJ, Karmann I, Sharp WD, Vuille M, Cardoso AO, Ferrari JA, Dias PLS, Viana O (2005) Insolation-driven changes in atmospheric circulation over the past 116,000 years in subtropical Brazil. Nature 434:63–66
Currell MJ, Cartwright I (2011) Major-ion chemistry, δ13C and 87Sr/86Sr as indicators of hydrochemical evolution and sources of salinity in groundwater in the Yuncheng Basin, China. Hydrogeol J 19:835–850
Dindane K, Bouchaou L, Hsissou Y, Krimissa M (2003) Hydrochemical and isotopic characteristics of groundwater in the Souss Upstream Basin, southwestern Morocco. J Afr Earth Sc 36(4):315–327
Domenico PA, Schwartz FW (1990) Physical and chemical hydrogeology, 2nd edn. Wiley, New York, p 506
Dreybrodt W (1981) Mixing corrosion in CaCO3-CO2-H2O systems and its role in the karstification of limestone areas. Chem Geol 32:221–236
Dreybrodt W (1988) Process in Karst Systems. Springer, Berlin p 288
Dreybrodt W, Buhmann D, Michaelis J, Usdowski E (1992) Geochemically controlled calcite precipitation by CO2 outgassing: Field measurements of precipitation rates in comparison to theoretical predictions. Chem Geol 97:285–294. doi:10.1016/0009-2541(92)90082-G
Dykes A (2007) Mass movements in cave sediments: geotechnical investigation of a 42,000-year old guano mudflow inside Niah Great Cave, Sarawak, Borneo. Landslides. doi:10.1007/S10346-006-0077S
Ettayfi N, Bouchaou L, Michelot JL, Tagma T, Warner N, Boutaleb S, Massault M, Lgourna Z, Vengosh A (2012) Geochemical and isotopic (oxygen, hydrogen, carbon, strontium) constraints for the origin, salinity, and residence time of groundwater from a carbonate aquifer in the Western Anti-Atlas Mountains, Morocco. J Hydrol 438(439):97–111
Fairchild IJ, Tremble PC (2009) Trace elements in speleothems as recorders of environmental change. Quatern Sci Rev 28:449–468
Fohlmeister J, Scholz D, Kromer B, Mangini A (2011) Modelling carbon isotopes of carbonates in cave drip water. Geochim Cosmochim Acta 75(18):5219–5228. doi:10.1016/j.gca.2011.06.023
Freeze AR, Cherry JA (1979) Groundwater. Prentice-Hall Inc., Englewood cliffs, p 604
Genty D, Deflandre G (1998) Drip flow variations under a stalactite of the Pere Noel cave (Belgium). Evidence of seasonal variations and air pressure constraints. J Hydrol 211:208–232
Gibbs RJ (1970) Mechanisms controlling world water chemistry. Sci J 170:795–840
Gilbertson D, Bird M, Hunt C, McLaren S, Mani Banda R, Pyatt B, Rose J, Stephens M (2005a) Past human activity and geomorphological change in a guano-rich tropical cave mouth: initial interpretations of the Late Quaternary succession in the Great Cave of Niah, Sarawak. Asian Perspect 44(1):16–41
Gilbertson D, Bird M, Hunt C, Mclaren S, Mani Banda R, Pyatt B, Rose J, Stephens M (2005b) Past human activity and geomorphological change in a guano-rich trophical cave mouth: initial interpretations of the Late Quaternary succession in the Great Cave of Niah, Sarawak. Asian Prespect 44(1):16–41
Gillmore G, Gilbertson D, Grattan J, Hunt C, McLaren S, Pyatt B, Banda RM, Barker G, Denman A, Phillips P, Reynolds T (2005) The potential risk from 222radon posed to archaeologists and earth scientists: reconnaissance study of radon concentrations, excavations, and archaeological shelters in the Great Cave of Niah, Sarawak, Malaysia. Ecotoxicol Environ Safety 60:213–227
Hammarstrom JM, Seal RR II, Meier AL, Kornfeld JM (2005) Secondary sulfate minerals associated with acid drainage in the eastern US: recycling of metals and acidity in surficial environments. Geochemistry of Sulfate Minerals: A Tribute to Robert O. Rye. Paper 2. http://digitalcommons.unl.edu/usgsrye/2
Harrisson T (1958) The caves at Niah: a history of prehistory. Sarawak Mus J 12: 549–595 (new series)
Harrisson T (1959a) Radiocarbon datings from Niah: a note. Sarawak Mus J 9(13–14): 136–142
Harrisson T (1959b) New archaeological and ethnological results from Niah Caves, Sarawak. Man 59:53–69
Harrisson T (1965) 50,000 years of Stone age culture in Borneo. Smithsonian Inst Annu Rep 1964:521–530
Harrisson T (1996) The palaeoecological context at Niah Cave, Sarawak: evidence from the primate fauna. Bull Indo Pacific Prehistory Assoc 14:90–100
Hazebroek HP, Morshidi AKBA (2001) National Parks of Sarawak. Natural History Publications, Kota Kinabalu
Herman JS, Lorah MM (1986) Groundwater geochemistry in Warm River Cave, Virginia. Nat Speleological Soc Bull 48:54–61
Holland HD, Kirsipu TV, Huebner JS, Oxburgh UM (1964) On some aspects of the chemical evolution of cave waters. J Geol 72:36–67
Hunt CO, Rushworth G (2005a) Cultivation and human impact at 6000 cal yr BP in tropical lowland forest at Niah, Sarawak, Malaysian Borneo. Quatern Res 64:460–468
Hunt CO, Rushworth G (2005b) Airfall sedimentation and pollen taphonomy in the West Mouth of the Great Cave, Niah. J Archaeol Sci 32:465–473
Hunt CO, Gilbertson DD, Rushworth G (2007) Modern humans in Sarawak, Malaysian Borneo, during Oxygen Isotope Stage 3: palaeoenvironmental evidence from the Great Cave of Niah. J Archaeol Sci 34(11):1953–1969. doi:10.1016/j.jas.2007.02.023
Hutchinson GE (1950) Survey of contemporary knowledge of biogeochemistry: 3. The biogeochemistry of vertebrate excretion. Bulletin of the American Museum of Natural History, New York 96
Hutchinson CS (2005) Geology of North-West Borneo: Sarawak, Brunei and Sabah. Elsevier, Amsterdam
Ingraham NL (1998) Isotopic variations in precipitation. In: Kendall C, McDonnell J (eds) Isotope tracers in catchment hydrology. Elsevier, Amsterdam, pp 87–118
Inskeep WP, Bloom PR (1986) Kinetics of calcite precipitation in the presence of water soluble organic ligands. Soil Sci Soc Am J 50:1167–1172
Jacobson RL, Langmuir D (1974) Controls on the quality variations of some carbonate spring waters. J Hydrol 23:247–265
Jimenez de Cisneros C, Caballero E (2011) Carbon isotope values as paleoclimatic indicators. Study on stalagmite from Nerja Cave, South Spain. Carbonates Evaporites 26:41–46
Karkanas P (2001) Site formation processes in Theopetra Cave: a record of climatic change during the Late Pleistocene and Early Holocene in Thessaly, Greece. Geoarchaeology 16:373–399
Kershaw PA, Penny D, van der Kaars S, Anshari D, Thamotherampillai A (2001) Vegetation and climate in lowland southeast Asia at the Last Glacial Maximum. In: Metcalfe I, Smith JMB, Morwood M, Davidson I (eds), Faunal and Floral Migrations and Evolution in SE AsiaeAustralasia. A.A. Balkema, Lisse, pp 227–236
Khanaqa Polla A, Al-Manmi Diary A (2011) Hydrogeochemistry and Geomicrobiology of Darzila Spring in Sangaw, Sulaimaniyah, NE Iraq. Iraqi Bull Geol Min 7(3):63–76
Langmuir D (1971) The geochemistry of some carbonate groundwaters in central Pennsylvania. Geochim Cosmochim Acta 35:1023–1045
Lebron I, Suarez DL (1996) Calcite nucleation and precipitation kinetics as affected by dissolved organic matter at 25 °C and pH > 7.5. Geochem Cosmochim Acta 60:2765–2776
Li HC, Lee ZH, Wan NJ, Shen CC, Li TY, Yuan DX, Chen YH (2011) The δ18O and δ13C records in an aragonite stalagmite from Furong Cave, Chongqing, China: a 2000-year record of monsoonal climate. J Asian Earth Sci 40:1121–1130
Li TY, Li HC, Xiang XJ, Kuo TS, Li JY, Zhou FL, Chen HL, Peng LL (2012) Transportation characteristics of δ13C in the plants-soil-bedrock-cave system in Chongqing karst area. Sci China Earth Sci 55:685–694
Lind CJ (1970) Specific Conductance as a means of estimating ionic strength. US Geological Society, Professional paper, 700-D, P.D272–280
Lloyd JW, Heathcode JA (1985) Nature inorganic hydrochemistry in relation to groundwater. Oxford University Press, New York
Lorah M, Herman J (1988) The chemical evolution of a travertine depositing stream: geochemical process and mass transfer reactions. Water Resour Res 24:1541–1551
Mayer J (1999) Spatial and temporal variation of groundwater chemistry in Pettyjohns cave, northwest Georgia, USA. J Cave Karst Stud 61(3):131–138
McDonald J, Drysdale R, Hill D, Chisari R, Wong H (2007) The hydrochemical response of cave drip waters to sub-annual and inter-annual climate variability, Wombeyan Caves, SE Australia. Chem Geol 244:605–623
Musgrove M, Banner JL (2004) Controls on the spatial and temporal variability of vadose dripwater geochemistry: Edwards aquifer, central Texas. Geochim Cosmochim Acta 68:1007–1020
Perrin K, Jeannin PY, Zwahlen F (2003) Epikarst storage in a karst aquifer: a conceptual model based on isotopic data, Milandre test site, Switzerland. J Hydrol 279:106–124
Piper AM (1953) A graphic procedure I the geo-chemical interpretation of water analysis, USGS Groundwater Note no. 12
Pitty AF (1971) Rate of uptake of calcium carbonate in underground karst water. Geol Mag 108:537–543
Plummer LN, Wigley TML, Parkhurst DL (1978) The kinetics of calcite dissolution in CO2: water systems at 5–60 °C and 0.0–1.0 atm CO2. Am J Sci 278:179–216
Poth CW (1977) Summary groundwater resources report of Lancaster County, Pennsylvania: Pennsylvania Geological Survey, 4th series, Water Resources Report 43, p 80, 1 map
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2010) Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. Environ Monit Assess 168:63–90
Quinlan JF, Ewers RO (1985) Ground water flow in limestone terranes; strategy rationale and procedure for reliable and efficient monitoring of ground water quality in karst areas. In: Proceedings, Fifth national symposium on aquifer restoration and ground water monitoring. National Water Well Association, pp 197–234
Rabett RJ, Piper PJ, Barker G (2006) Bones from ‘Hell’: preliminary results of new work on the Harrisson faunal assemblage from the deepest part of Niah Cave, Sarawak. In: Bacus EA, Glover IC, Pigott VC (eds), Uncovering Southeast Asia’ past: selected papers from the 10th International Conference of the European association of Southeast Asian Archaeologists, pp 46–59
Shahack-Gross R, Berna F, Karkanas P, Weiner S (2004) Bat guano and preservation of archaeological remains in cave sites. J Archaeol Sci 31:1259–1272
Shurbaji ARM, Phillips F (1995) A numerical model for the movement of H2O, H-2, O-18, and (HHO)-H-2 in the unsaturated zone. J Hydrol 171:125–142
Spotl C, Fairchild IJ, Tooth AF (2005) Cave air control on dripwater geochemistry, Obir Caves (Austria): implications for speleothem deposition in dynamically ventilated caves. Geochim Cosmochim Acta 69:2451–2468
Stephens M, Rose J, Gilbertson DD, Canti M (2005) Micromorphology in cave sediments in the humid tropics: Niah Cave, Sarawak. Asian Perspect 44(1):42–55
Tooth AF, Fairchild IJ (2003) Soil and karst aquifer hydrological controls on the geochemical evolution of speleothem-forming drip waters, Crag Cave, southwest Ireland. J Hydrol 273:51–68
Toran L, Roman E (2006) CO2 outgassing in a combined fracture and conduit karst aquifer near Lititz Spring, Pennsylvania. Geol Soc Am Spec Pap 404:275–282
van der Kaars S, Wang X, Kershaw P, Guichard F, Setiabudi DA (2000) A late quaternary palaeoecological record from the Banda Sea, Indonesia: patterns of vegetation, climate and biomass burning in Indonesia and northern Australia. Palaeogeogr Palaeoclimatol Palaeoecol 155:135–153
Wilford GE (1964) The geology of Sarawak and Sabah Caves. Bull Geol Surv Borneo Region Malaysia 6:181
Acknowledgments
The authors thank the Department of Earth Sciences, Annamalai University, India, for water analysis. RN and MVP are also thankful to Curtin University, Sarawak, for the financial support through CSRF Project (CSRF 2087). RN is grateful to the Controller of National parks and nature reserves, Forest department, for granting permission to conduct research in Niah Great Cave. Pengarah, Jabatan Meterologi Malaysia, Cawangan Sarawak is gratefully acknowledged for providing rainfall data from Niah forest office. Special thanks to M.Sundararajan, National Institute for Interdisciplinary Science and Technology, CSIR, Kerala, India for the XRD analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasanna, M.V., Nagarajan, R., Chidambaram, S. et al. Drip water Geochemistry of Niah Great Cave, NW Borneo, Malaysia: a base line study. Carbonates Evaporites 29, 41–54 (2014). https://doi.org/10.1007/s13146-013-0164-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13146-013-0164-3