Abstract
Purpose
We aimed to evaluate the relation between visceral fat volume and fluorodeoxyglucose (FDG) uptake of the liver measured by maximum or mean standardized uptake value.
Methods
We retrospectively analyzed 96 consecutive records of positron emission tomography/computed tomography (PET/CT) performed for cancer screening between May 2011 and December 2011. Subjects were divided into 2 groups according to Hounsfield unit (HU) of the liver comparing with that of the spleen. The control group (20 women, 56 men) demonstrating HU of the liver equal or greater than that of the spleen included 76 patients, while the fatty liver group (2 women, 18 men) showing HU of the liver less than that of the spleen included 20 patients. We compared FDG uptake of the liver and visceral fat volume between two groups. We evaluated correlation of hepatic FDG uptake measured by maximum or mean standardized uptake value (SUV) with visceral fat volume and attenuation.
Results
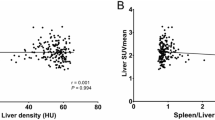
The fatty liver disease group showed higher aspartate aminotransferase (AST)of (24.42 ± 7.22, p = 0.012), alanine aminotransferase (ALT) of (25.16 ± 11.68, p = 0.001), body mass index (BMI) of (24.58 ± 3.29, p = 0.021), and visceral fat volume (3063.53 ± 1561.43, p = 0.011) than the control group. There were no statistically significant differences of mean standardized uptake value of the liver (liver SUVmean) (2.73 ± 0.19, p = 0.723), maximum standardized uptake value of the liver (liver SUVmax) (3.39 ± 0.53, p = 0.8248) and liver SUVmean/spleen SUVmean (1.13 ± 0.10, p = 0.081) between the two groups. Strong correlations were shown between liver SUVmean and BMI (r = 0.609, p < 0.001) and between liver SUVmean and visceral fat volume (r = 0.457, p < 0.001). Liver SUVmax was also strongly correlated with BMI (r = 0.622, p = 0.001) and visceral fat volume (r = 0.547, p < 0.001). There was no significant association of mean attenuation value of the liver (liver HUmean) with liver SUVmean (r = -0.003, p = 0.979) or liver SUVmax (r = -0.120, p = 0.244).
Conclusion
Hepatic FDG uptake quantified as SUVmean or SUVmax is not correlated with hepatic steatosis but with visceral fat volume in cancer screening.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obesity has become a public health problem in the world. Dietary excess and obesity are associated with the accumulation of lipid into adipocytes and the expansion of the adipose tissue [1]. Excess fat of the body correlates with increased mortality and risk for disorders like diabetes, hypertension and atherosclerosis of vessels [2–4]. Distinguishing accumulation of visceral fat and subcutaneous fat has become important, which can be measured by current imaging techniques. The amount of visceral fat has been known to be more strongly associated with metabolic risk factors than is subcutaneous or total body fat [5].
A hexokinase mediated phosphorylation takes place in 18F-Fluorodeoxyglucose (FDG) using positron emission tomography/computed tomography (PET/CT), while few remain unphosphorylated and may be transported out of the cell [6]. The liver can be an exception to this trapping mechanism, which means hepatic tissue demonstrates an increased activity of glucose-6-phosphatase and thus actively metabolises FDG dependent on insulin levels and sensitivity [6]. Hepatic FDG uptake has been described as a reference for equivocal FDG accumulation of the abdomen in the literature [7]. However, Lin et al., have indicated that the liver cannot be used as a comparator of extrahepatic foci of FDG uptake in patients with fatty liver disease [8]. A previous study by Abele et al., demonstrated there is no association between attenuation and hepatic FDG uptake [9]. However, the correlation with visceral fat volume has not yet been determined.
Normal physiologic FDG uptake of the liver is usually heterogenous, which makes interpretation difficult for nuclear physicians. We hypothesized there may be a relationship between hepatic FDG uptake and visceral fat volume. In this study, we evaluated the correlation of hepatic FDG uptake measured by maximum or mean standardized uptake value (SUV) with visceral fat volume and attenuation.
Materials and Methods
Patients
We retrospectively analyzed 96 consecutive records of PET/CT performed for cancer screening between May 2011 and December 2011. Subjects with malignancy or known liver diseases which can affect hepatic FDG uptake (hepatitis [10], lymphoma [11] and hepatic angiosarcoma [12]) were excluded. Blood sampling was done to check aspartate arminotransferase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH). The height and the weight were measured, and the body mass index (BMI, kg/m2) was calculated as weight in kilograms divided by the square of the height in meters. Subjects were divided into two groups according to the HU of the liver (48.65 ± 7.55 HU) compared with that of the spleen (42.98 ± 7.16 HU). The control group (20 women, 56 men) demonstrated HU of the liver equal or greater than that of the spleen (76 patients), while the fatty liver group (2 women, 18 men) showed HU of the liver less than that of the spleen (20 patients). This study was approved by the clinical research ethics committee of Pusan National University Hospital.
18F -FDG PET/CT
Standard patient preparation included at least 8 h fasting and a serum glucose level of less than 120 mg/dL before 18F-FDG administration. 18F-FDG PET/CT imaging was performed 60 min after injection of 3.7 Mbq/kg of body weight (0.1 mCi/kg) of 18F-FDG. The SUV was calculated using the body weight. Patients were hydrated with 500 ml of water by mouth before the PET/CT imaging. At 60 min after administration of 18F-FDG, a low-dose area from the base of the skull to the proximal thighs was performed for the purpose of attenuation correction and precise anatomical localization.
All patients were examined on a dedicated PET/CT scanner (Biograph40, SIEMENS, Knoxville, TN, USA). Emission scan time per bed position was 3 min; six bed positions were acquired. PET data were obtained using a high resolution whole body scanner with an axial field of view of 21.6 cm. The average axial resolution varied between 2.0 mm full width at half maximum (FWHM) in the center and 2.4 mm at 28 cm. The average total PET/CT examination time was 20 minutes. The CT scan was obtained with a tube voltage of 120 kVp and a tube current of 170 mAs. Attenuation correction was performed for all patients with iterative reconstruction. PET/CT images were analyzed in three different planes (transverse, coronal and sagittal). PET/CT images were interpreted visually by two nuclear physicians. In the event of disagreement, a consensus was established.
Measurement of Hepatic Parameters
A circular region of interest (ROI) (>2 cm) was placed in the right lobe of the liver, avoiding vessels, bile ducts, calcifications, and artifacts. Mean and maximum standardized uptake value (SUVmean and SUVmax), mean attenuation value by Hounsfield unit (HUmean) of the liver were measured for each ROI.
Measurement of Visceral Fat Volume
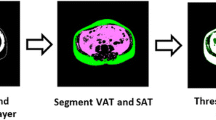
Visceral fat tissue boundaries were defined as intra-abdominal fat bound by parietal peritoneum or transversalis fascia, excluding vertebral column and paraspinal muscles by setting the attenuation values for the volume of interest (VOI) within a range of -250 to -50 HU (window center, -150 HU). Abdominal fat compartments were manually drawn on 20 axial slices of each subject, which defined the outer border of the volume. Visceral fat tissue was reconstructed using a CT software (SIEMENS, Syngo CT basic evaluation), and visceral fat volume was calculated. Gas and intestinal contents were excluded. (Fig. 1)
Statistics
Data were analyzed by MedCalc v.9.3 software (MedCalc, Mariakerke, Belgium). Significance in values of variables between the two groups was tested by the Mann-Whitney test. Pearson correlation was used to analyze the degree of association between hematological indices, BMI, visceral fat volume and FDG uptake. Results were considered statistically significant when a p-value was less than 0.05.
Results
Patient Characteristics
Patient characteristics are summarized in Table 1. Age (mean ± SD, 51.58 ± 8.45 years, p = 0.730), gender (p = 0.213), ALP (164.61 ± 50.95 IU/L, p = 0.943), LDH (341.67 ± 70.74, p = 0.061), weight (69.69 ± 10.72, p = 0.281), and height (168.28 ± 7.78, p = 0.394) were not significantly different between groThe fatty Fatty liver disease group showed higher AST (24.42 ± 7.22, p = 0.012), ALT (25.16 ± 11.68, p = 0.001), BMI (24.58 ± 3.29, p = 0.021), visceral fat volume (3063.53 ± 1561.43, p = 0.011) than the control group. There were no statistically significant differences of liver SUVmean (2.73 ± 0.19, p = 0.723), liver SUVmax (3.39 ± 0.53, p = 0.8248) and liver SUVmean/spleen SUVmean (1.13 ± 0.10, p = 0.081) between the two groups.
Correlation Between Hepatic FDG Uptake and BMI, Visceral Fat Volume, Liver HUmean
Liver SUVmean was strongly correlated with BMI (r = 0.6090, p < 0.0001) and visceral fat volume (r = 0.457, p < 0.001). Liver SUVmax was also strongly correlated with BMI (r = 0.622, p = 0.001) and visceral fat volume (r = 0.5469, p < 0.001). However, there was no significant association of liver HUmean with liver SUVmean (r = -0.003, p = 0.979) or liver SUVmax (r = -0.120, p = 0.244). Scatter diagrams and regression lines of each variable are presented in Fig. 2.
Correlation Between Hepatic FDG Uptake and Liver Function Test
Among the results from the liver function tests, there were statistically significant positive correlations between AST and liver SUVmean (r = 0.242, p = 0.018), and liver SUVmax (r = 0.249, p = 0.014). Also, positive correlation was found between ALT and liver SUVmax (r = 0.211, p = 0.039). Table 2 summarizes correlations between FDG uptake of the liver and the liver function test. Scatter diagrams and regression lines of each variable are shown in Fig. 3.
Discussion
The purpose of this study was to evaluate factors influencing FDG uptake of the liver in healthy subjects. In this study, there was no significant difference of liver SUVmean and liver SUVmax between controls and fatty liver groups. Results of the present study are similar with those of the earlier study [9]. However, Abele et al., did not evaluate differences of AST, ALT and visceral fat volume between groups. In this study, AST of four subjects (4.17 %) and ALT of four subjects (4.17 %) showed higher values than the normal range (AST, 5-40 IU/L; ALT, 7-56 IU/L). Although most of the AST and ALT values of subjects were in normal ranges, there were significant differences between groups. In our study, the difference of liver SUVmean/spleen SUVmean between groups was not significant. However, Qazi et al., showed liver SUVmax/spleen SUVmax ratio of the fatty liver group was lower than that of the control [7]. SUVmax is the most commonly used parameter to evaluate tumors, while SUVmean may be a more robust parameter to measure FDG uptake of a large organ like the liver [9].
In the present study, there was no significant association of liver HUmean with liver SUVmean and liver SUVmax, which is similar with that of Abele et al., [9] in that hepatic steatosis does not appear to have any effect on FDG uptake of the liver.
However, correlations between BMI and liver SUVmean and between BMI and liver SUVmax were statistically significant in our study. Previous study has also reported BMI was the strongest determinant of liver FDG uptake [13]. They recommended screening for the metabolic syndrome in the subject with high FDG uptake of the liver [11].
No previous study has documented the correlation between visceral fat volume and FDG uptake of the liver. BMI and waist circumference are commonly used as surrogate measures of adiposity. We drew ROI of visceral fat area to measure the volume of each subject directly. Visceral fat accumulation has been known to play a role in steatosis and fibrosis in the pathogenesis and prognosis of nonalcoholic fatty liver disease [13]. Visceral obesity induces a chronic low-grade inflammatory state, because chemokines released by hypertrophied adipocytes induce macrophage accumulation in visceral fat tissue [14]. Infiltrated macrophages produce pro-inflammatory cytokines (tumor necrosis factor-α, interleukin-6, macrophage chemoattractant protein-1, resistin and leptin) and nitric oxide, and these inflammatory changes induce adipokines dysregulation [14, 15]. Altered long-term expression of liver metabolic enzymes may be critical in the transition to the chronic inflammatory state [16]. Fontana et al., demonstrated that visceral fat is an important source of IL-6 production in obese people [17]. IL-6 concentrations in the portal vein were much higher than in the peripheral artery blood, and there was a significant correlation between IL-6 concentrations of the portal vein which drains visceral fat and systemic C-reactive protein (CRP) concentrations [17, 18]. FDG uptake of the liver may be induced by an inflammatory state of the liver. Also, it has been known that FDG uptake in fat is very low. FDG uptake of nonfatty tissues may be influenced by the distribution and volume of fatty tissue. These may explain potential mechanisms of strongly significant correlations between liver SUVmean and visceral fat volume and between liver SUVmax and visceral fat volume.
Among the liver function tests, significant correlations between AST and liver SUVmean and between liver SUVmax and AST, ALT were found. Lin et al., [19] demonstrated positive correlations between AST, ALT and FDG uptake of the liver. As elevation of serum aminotransferases indicates hepatocellular injury, correlations between liver function tests and FDG uptake of the liver may reflect the inflammatory state.
The current study has several limitations. First, this is a retrospective study with a small sample size. As Korean national insurance does not cover the use of PET/CT for cancer screening, we had difficulties collecting subjects in this study. Second, because the subjects in our study performed PET/CT for cancer screening, we could not evaluate the correlation between cytokines, CRP of blood samples and FDG uptake of the liver directly. Third, as not all subjects had blood sampling for lipid profiles, we could not evaluate the relation between FDG uptake of the liver and lipid profiles.
Conclusion
FDG uptake of the liver quantified as SUVmean or SUVmax is not correlated with hepatic steatosis but with visceral fat in cancer screening.
References
Shoelson SE, Herrero L, Naaz A. Obesity, inflammation, and insulin resistance. Gastroenterol. 2007;132(6):2169–80.
Larsson B, Svärdsudd K, Welin L, Wilhelmsen L, Björntorp P, Tibblin G. Abdominal adipose tissue distribution, obesity, and risk of cardiovascular disease and death: 13 year follow up of participants in the study of men born in 1913. Br Med J (Clin Res Ed). 1984;288(6428):1401–4.
Lapidus L, Bengtsson C, Larsson B, Pennert K, Rybo E. Sjöström L. Distribution of adipose tissue and risk of cardiovascular disease and death: a 12 year follow up of participants in the population study of women in Gothenburg, Sweden. Br Med J (Clin Res Ed). 1984;289(6454):1257–61.
Ducimetiere P, Richard J, Cambien F.The pattern of subcutaneous fat distribution in middle-aged men and the risk of coronary heart disease: the Paris prospective study. Int J Obes. 1986;10(3):229–40.
Boyko EJ, Leonetti DL, Bergstrom RW, Newell-Morris L, Fujimoto WY. Visceral adiposity, fasting plasma insulin, and blood pressure in Japanese-Americans. Diabetes Care. 1995;18(2):174–81.
Kanstrup IL, Klausen TL, Bojsen-Møller J, Magnusson P, Zerahn B. Variability and reproducibility of hepatic FDG uptake measured as SUV as well as tissue-to-blood background ratio using positron emission tomography in healthy humans. Clin Physiol Funct Imaging. 2009;29(2):108–13.
Khandani AH, Wahl RL. Applications of PET in liver imaging. Radiol Clin North Am. 2005;43(5):849–60.
Lin CY, Lin WY, Lin CC, Shih CM, Jeng LB, Kao CH. The negative impact of fatty liver on maximum standard uptake value of liver on FDG PET. Clin Imaging. 2011;35(6):437–41.
Abele JT, Fung CI. Effect of hepatic steatosis on liver FDG uptake measured in mean standard uptake values. Radiol. 2010;254(3):917–24.
Oh M, Baek S, Lee SO, Yu E, Ryu JS. A case of acute Q fever hepatitis diagnosed by F-18 FDG PET/CT. Nucl Med Mol Imaging. 2012 Epub.
Kaneko K, Nishie A, Arima F, Yoshida T, Ono K, Omagari J, et al. A case of diffuse-type primary hepatic lymphoma mimicking diffuse hepatocellular carcinoma. Ann Nucl Med. 2011;25(4):303–7.
Oe A, Habu D, Kawabe J, Torii K, Kawamura E, Kotani J, et al. A case of diffuse hepatic angiosarcoma diagnosed by FDG-PET. Ann Nucl Med. 2005;19(6):519–21.
Eguchi Y, Mizuta T, Sumida Y, Ishibashi E, Kitajima Y, Isoda H, et al. The pathological role of visceral fat accumulation in steatosis, inflammation, and progression of nonalcoholic fatty liver disease. J Gastroenterol. 2011;46 Suppl 1:70–8.
Kamada Y, Takehara T, Hayashi N. Adipocytokines and liver disease. J Gastroenterol. 2008;43(11):811–22.
Qian Y, Fan JG. Obesity, fatty liver and liver cancer. Hepatobiliary Pancreat Dis Int. 2005;4(2):173–7.
Sato N. Central role of mitochondria in metabolic regulation of liver pathophysiology. J Gastroenterol Hepatol. 2007;22 Suppl 1:S1–6.
Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007;56(4):1010–3.
Schenk Jr WG, McDonald JC, McDonald K, Drapanas T. Direct measurement of hepatic blood flow in surgical patients: with related observations on hepatic flow dynamics in experimental animals. Ann Surg. 1962;156:463–71.
Lin CY, Ding HJ, Lin T, Lin CC, Kuo TH, Kao CH. Positive correlation between serum liver enzyme levels and standard uptake values of liver on FDG-PET. Clin Imaging. 2010;34(2):109–12.
Acknowledgements
This study was supported by a Pusan National University two year Research Grant.
Conflict of Interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pak, K., Kim, SJ., Kim, I.J. et al. Hepatic FDG Uptake is not Associated with Hepatic Steatosis but with Visceral Fat Volume in Cancer Screening. Nucl Med Mol Imaging 46, 176–181 (2012). https://doi.org/10.1007/s13139-012-0143-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13139-012-0143-y