Abstract
Sea level change influences biodiversity of endemic cave fauna to varying degrees. In anchialine systems, a marine layer flows under less saline layers, each with differing associated fauna. We assess the role of present and historic (last glacial maximum – 18,000 years ago) distance from the ocean in determining species richness and phylogenetic diversity patterns for two groups of anchialine crustaceans: the marine-restricted Remipedia and a subset of groundwater-inhabiting atyid shrimp with greater tolerance for salinity variation. We calculated species richness and phylogenetic diversity per cave based on records of remipede and atyid diversity at 137 locations in the Yucatán Peninsula, Caribbean, Australia, and the Canary Islands. After calculating the distance of each cave’s surface opening from the past and present shoreline, we evaluated how species richness and phylogenetic diversity change with distance from the present and historic ocean. Remipede species richness and phylogenetic diversity declined rapidly with distance from the ocean. Ninety-five percent of the remipedes surveyed were located within 7 km of the present ocean and 18 km of the historic ocean. Atyid species richness and phylogenetic diversity declined more slowly with distance from the ocean than that of remipedes. Atyid shrimp were also distributed over a broader range: 95 % were located within 100 km of the present ocean and 240 km of the historic ocean. Our findings indicate that coastal geomorphology and salinity tolerance influence a clade’s distribution with respect to its distance from the ocean. We also report a possible latent response to sea level change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anchialine habitats are physically complex, being dependent on input from both overlying terrestrial (meteoric water) and tidally influenced, yet land-locked marine environments. It is difficult to determine connectivity of anchialine fauna in present and past timescales for several reasons. First, these habitats often extend below the surface through porous rock as cave complexes whose extent we are only beginning to understand (Iliffe 2000). While anchialine systems occur as pools and caves in coastal regions worldwide, this underground setting with its local physico-chemical gradients shelters a unique and novel stygobiont fauna whose members often have limited distributions and, therefore, infrequent sightings (Iliffe and Kornicker 2009). Second, anchialine fauna are often separated by salinity-stratified water layers (Iliffe and Kornicker 2009). For example, while many caridean shrimp and amphipods are restricted to the meteoric waters above the halocline (Debrot et al. 2003; De Grave et al. 2008), other arthropods such as remipedes are restricted to the underlying marine component of anchialine systems (Mejía–Ortíz et al. 2007; Neiber et al. 2011). Still other crustaceans, such as some atyid shrimp, are found in both marine and less saline layers (Sanz and Platvoet 1995; Alvarez et al. 2005; Hunter et al. 2008, Pakes pers. observ.) The effect of salinity tolerance on species connectivity and distribution in these complex systems is likely great, but as yet untested.
Similarly, the impact of sea level fluctuations on anchialine water layers and their associated cave fauna can be weakened or intensified by the cave’s topography (Iliffe 2000). As the meteoric and marine layers track sea level through cave passages, the availability of connections between successive levels of the cave complex determines a taxon’s ability to track their niche through time. Furthermore, distance to the ocean and the strength of tidal currents will also interact with cave topography to affect dispersal distances (Christman and Culver 2001). Sea level changes have been especially acute during the last 18,000 years, fluctuating over 100 m (Miller et al. 2005). These changes altered the distribution of plants and animals (e.g., Erwin et al. 2006; Legra et al. 2008; Vaselli et al. 2008; Pyenson and Lindberg 2011; Woodroffe and Murray-Wallace 2012; Niemiller et al. 2013). Falling sea levels dry out formerly available aquatic habitats, resulting in migration or extinction (Jablonski 1985; Finnegan et al. 2012). As sea level recedes, emerging land patches interrupt continuous passages of water, causing fragmentation of species ranges, which may subsequently drive evolutionary divergence and speciation (Jaume et al. 2008; Zaksek et al. 2009; Fiser et al. 2012). Sea level rise has equally important effects; marine transgressions flood habitats and allow organisms to disperse into new locales, sometimes connecting formerly separated habitats and taxa (Smith 2001; Cromer et al. 2005; Zaksek et al. 2007; Botello and Alvarez 2010). Meteoric inputs in themselves can also be altered by climate change (e.g., temperature, precipitation) and urbanization (e.g., pollution, water diversion projects, etc.). While marine inputs are thought to be less susceptible to urbanization than meteoric inputs (pollution and ocean acidification excepted), they may be especially affected by increasing global sea temperatures, which could cause admixture between previously stratified water layers in the cave.
Here we test whether the distance between anchialine caves and their marine inputs influences species richness and phylogenetic diversity patterns for two groups of anchialine stygobiont crustaceans: the marine-restricted Remipedia and a subset of groundwater-inhabiting, salinity-tolerant atyid shrimp. This approach requires taxon and habitat continuity since the last glacial maximum (LGM) (Miller et al. 2005). Distances between caves and coast were calculated for present sea level and sea level (-120 m) during the LGM. We then compare how species richness and phylogenetic diversity change with distance from the present and historic coastlines. By integrating deeper time sea level data we attempt to understand how previous perturbations may have shaped current distributions in these unique ecosystems. Our results provide a perspective (based on historical performance) from which to evaluate ecological traits and behaviors in light of future global change.
Materials and methods
Taxa
Remipedes are stygobiont crustaceans that are hypothesized to be the sister taxon of the Hexapoda (= Collembola, Protura, Diplura, and Insecta) (Koenemann et al. 2007; Neiber et al. 2011). Multiple remipede species are found on the Caribbean/Atlantic islands (especially in the Bahamas) and Mexico’s Yucatán Peninsula, and single species are found on Cape Kimberly in Western Australia, and the Canary Islands, Spain (Fig. 1). Putative fossil remipedes are known from the Carboniferous of North America: Tesnusocaris goldichi Brooks 1955, from the upper Mississippian of Texas (328.3-318.1 mya) and Cryptocaris hootchi Schram 1974, from the Pennsylvanian of Illinois (314.6 - 306.9 mya), USA (Brooks 1955; Emerson and Schram 1991). Both localities reflect open marine depositional environments and do not appear to be fossil cave deposits (Schram 1974; Emerson and Schram 1991).
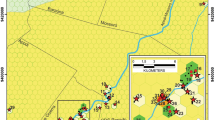
Remipede species richness in (a) the Caribbean, (b) Canary Islands, (c) Western Australia, and (d) global richness. Species richness by cave correlates with size of white circles. For (d), only the caves with the highest richness in the region are shown. The present-day coastlines are outlined in black, and the land masses exposed 18 kya are illustrated in dark gray. Bathymetry is shown in light gray. The Bahamas had the highest species richness with up to eight species per cave. Richness is limited to one species per cave in Western Australia and two species per cave in the Canary Islands. All analyses were performed in ArcMap using the Plate-Carree projection system
In contrast to remipedes, members of the Atyidae occur worldwide. Cave dwelling atyids vary from facultative stygobionts, with well-developed eyes and body pigmentation, to obligate stygobionts, with highly reduced eyes and lacking pigment (von Rintelen et al. 2012). Atyid shrimp are found in freshwater, brackish, and anchialine habitats in Caribbean, Mexican, Australian, and southern European submerged karst systems (Zaksek et al. 2007; Page et al. 2008) (Fig. 2). Molecular data suggest a likely sister taxon relationship with Xiphocaris, and several distantly related clades in stygobiotic habitats appear to have evolved independently (von Rintelen et al. 2012). Fossil freshwater atyid shrimp are first reported from Lower Cretaceous (145.5-99.6 mya) deposits in Spain and Brazil (von Rintelen et al. 2012). Martin and Wicksten (2004) hypothesize that the atyids expanded from fully marine to freshwater habitats during the Jurassic (199.6-145.5 mya).
Atyid species richness in (a) the Yucatan Peninsula, (b) the Caribbean, (c) southern Europe, (d) Australia, and (e) global species richness. Species richness by cave correlates with the size of white circles. For (e), only the caves with the highest richness in the region are shown. The present-day coastlines are outlined in black, and the landmasses exposed 18 kya are illustrated in dark gray. Bathymetry is shown in light gray. Mexico had the highest atyid species richness, with up to two species per cave. Atyid species were found near the coasts and up to several hundred kilometers inland. All analyses were completed in ArcMap using the Plate-Carree projection system
Sister taxon relationships and branch lengths from available molecular phylogenetic analyses of atyid shrimp and remipedes (e.g., Page et al. 2008; Neiber et al. 2011; Botello et al. 2013; Hoenemann and Neiber 2013) suggest local diversification, while branch lengths suggest that these diversifications were substantially earlier than the LGM.
Distance and diversity analyses
Our analyses are limited to anchialine caves in regions where remipedes and atyid shrimp taxa are present (Electronic Supplementary Material, Table 3). A total of 137 caves were identified within these regions. Caves in these regions were present before the last sea level low stand, providing suitable habitat for anchialine fauna (Kensely and Williams 1986; Humphreys 1999; Walker et al. 2008; van Hengstrum et al. 2009; 2012; Denniston et al. 2013). Remipedes occurred in 41 (30 %) of these caves and atyid shrimp in 67 (50 %). However, both taxa co-occurred in only six (4 %) of these caves. Thirty-five (26 %) of these caves are reported to contain neither remipedes nor atyid shrimp. Maps of each region were generated with ArcMap 10.1 and using the NOAA ETOPO1 Global Relief Model (Amante and Eakins 2009) in the World Plate-Carree projected coordinate system. The linear distance from each cave opening to the present-day coastline was measured. This distance was then re-measured after sea level was digitally lowered to reconstruct each region’s coastline 18,000 years ago (18 kya) during the LGM, when sea level was 120 m lower than the present (Miller et al. 2005). Although cave networks often extend well beyond their openings, the limitations of SCUBA surveys restrict data collection to about a 0.8 km of cave openings (called cenotes), and the opening is assumed to be a proxy of the actual surveyed area. Here, we noted when groups of cenotes are known via cave networks as systems (systemas), potentially facilitating further inland occurrences of remipede and atyid taxa.
The relationship between species richness, defined as the total number of remipede or atyid species at a cave locality, and distance of the cave from the ocean was fitted to a Poisson distribution (chi-squared test). To compare the relationship between diversity and distance for each group at modern and 18 kya sea levels, the species richness value of each cave in the Poisson distribution was normalized by square root transformation and then multiplied by distance of that point from the ocean. This produced a weighted distribution of diversity and distance. The relative distance that species richness spreads inland, or the species diversity envelope, was then calculated as standard deviations of this weighted distribution. Phylogenetic diversity was calculated as the total branch lengths of all species present at a cave locality, thereby giving a measure of genetic divergence between the species in a cave. For remipedes we used the 50 % majority-rule consensus tree of COI sequence data presented by Neiber et al. (2011). For atyid shrimp, we used the Bayesian tree based on rrnL, COI, cyt b, LSU, SSU and histone H3A sequences published by Botello et al. (2013).
Results
Remipedes
Caves inhabited by remipedes were significantly closer to the shoreline than caves without remipedes (Fig. 3a), both at present-day sea level (t-test, p < 0.001) and at paleo-sea level (t-test, p < 0.001). Ninety-five percent of the caves with remipedes occur within 7 km of the present-day coastline and within 18 km of the historic coastline (Table 1). These caves were also typically located on coastlines where the nearshore bathymetry is steep, so these locations thereby remain near marine water sources during sea level regressions (Fig. 1). On average, caves with remipedes occurred 1.24 km (±1.73) from the present-day shoreline, significantly closer than caves without remipedes, which occurred 44.37 km (±33.20) from the present-day shoreline (t-test, p < 0.001). Comparisons of these cave distances at 18 kya showed similar patterns (t-test, p < 0.001) (Fig 3a).
Average distance (km) of remipede and atyid cave faunas from the present-day shoreline and the estimated paleo-shoreline of the last glacial maximum (-120 m). Black bars = taxon present; gray bars = taxon absent; error bars = standard error; asterisks (*) denote statistical significance (t-test, p < 0.001). (a) Remipedes, (b) atyids
Remipede species richness and phylogenetic diversity were highest in caves closest to the ocean, with as many as eight species per cave and a phylogenetic diversity of 2.76 changes per base pair (Fig. 4a). Both species richness and phylogenetic diversity declined as distance from the ocean increased. Both species richness and phylogenetic diversity resembled Poisson distributions, declining rapidly with distance from the present ocean (χ2 = 1.390, p < 0.01; χ2 = 0.112, p < 0.001, respectively, Fig. 4c-d), decreasing to an average of only one species per cave within 5 km of the present-day ocean (Fig. 4a). The width of the species richness envelope was 1.724. During the LGM, remipede species richness and phylogenetic diversity declined more slowly with distance from the paleo-shoreline, producing a wider richness envelope of 4.711 (χ2 = 9.543, p < 0.05; χ2 = 6.155•104, p < 0.001, respectively, Fig. 4b). The phylogenetic diversity envelope for present-day distances was also very narrow compared to that estimated for the paleo-shoreline (Table 1).
Remipede species richness (gray circles, left axis) and phylogenetic diversity (black diamonds, right axis) plotted against distance from shoreline: (a) Recent, (b) 18 kya. Poisson distributions for remipede species richness (dashed gray line) and phylogenetic diversity (solid black line): (c) Recent, (d) 18 kya. Atyid species richness (gray circles, left axis) and phylogenetic diversity (black diamonds, right axis) plotted against distance from shoreline: (e) Recent, (f) 18 kya. Poisson distributions for atyid species richness (dashed gray line) and phylogenetic diversity (solid black line): (g) Recent, (h) 18 kya. All eight plots show Poisson distributions (p < 0.001)
Atyids
Caves with atyids were not limited to coastlines with narrow continental shelves (Fig. 2). Atyid shrimps ranged from within a few kilometers of the present-day shoreline to 127 km inland. Caves with atyids were found significantly farther from the ocean than caves without atyids at the present-day sea level (t-test, p < 0.001, Fig. 3b) and the paleo-sea level (t-test, p < 0.001, Fig. 3b). Ninety-five percent of atyid-present caves were found within 100 km of the present ocean and within 240 km of the paleo-shorelines (Table 1).
Atyid species richness did not change significantly with distance from the modern or paleo-shoreline (Fig. 4e-f). Typically, one to two species per cave were found within 100 km of the present shoreline and 240 km of the paleo-shoreline, decreasing to one species per cave further inland (Fig. 4e-f). This distribution also resembled a non-random Poisson distribution for the present-day (χ2 = 7.916•1017, p < 0.001, Fig. 4g) and for the paleo-shoreline (χ2 = 5.977•10112, p < 0.001, Fig. 4h).
Despite the high number of species in the Caribbean region, phylogenetic diversity remained below 0.05 changes per base pair in most caves (Table 2). Phylogenetic diversity for present-day (χ2 = 2.572•1090, p < 0.001, Fig. 4e-f) and paleo-shorelines (χ2 = 6.700•10233, p < 0.001, Fig. 4g-h) also showed Poisson distributions. Phylogenetic diversity remained low at all distances from the coast, never rising above 0.144 changes per base pair in a single cave. Species richness and phylogenetic diversity envelopes for paleo-shorelines were more than twice as wide as envelopes for the present day (Table 1).
Discussion
The recent distributions of remipedes and atyids in anchialine cave systems show significantly different patterns. As shown above, atyids were found in caves more than 70 km inland (Figs. 3b and 5a). In contrast, most living remipedes are found in caves within 7 km of the coast (Fig. 3). During the LGM, 95 % of these locales would have resided within 18 km of the coastline, a distance well within the range seen today in Yucatan systems that contain remipedes (Fig. 5b). This congruence suggests that horizontal and vertical connections in these cave systems provided sufficient connectivity for remipede populations to track the halocline during sea level change, thereby reducing local extirpations (see also Iliffe 2000:72). In addition, the steeper the geomorphology of the adjacent continental shelf, the further inland populations can be maintained (see below).
Scatterplot of the distances of cave openings from the current coastline and the coastline 18,000 years ago during the last glacial maximum. Analysis limited to caves in regions where both remipedes and atyid shrimp co-occur (Table 2). Box in the lower corner of (a) is enlarged to show finer scale remipede distributions in (b). Regressions. (a) Shrimp: y = 0.2376x + 10.0617, r2 = 0.4297; Neither: y = 0.2610x + 9.0827, r2 = 0.4568. (b) Remipede: y = -0.3130x + 0.3040, r2 = 0.6379
Anchialine habitats that require the interface of fresh and salt water can be likened to intertidal communities, where organisms utilize specific zones in a relatively limited space and must track their habitats on short- and long-term scales or face extirpation (Vaselli et al. 2008; Iliffe and Kornicker 2009). During the last 2.5 mya of global cooling, eustatic sea level changes have occurred about every 20-40 kyr with amplitudes as large as 140 m (Miller et al. 2005). Today’s remipede populations are present in areas where the distance to the ocean did not greatly change during the most recent sea level regression (Fig. 5). This distribution suggests that if more distant populations were established during earlier transgressive periods [e.g., +20 m at 120 kya (Miller et al. 2005)], they did not survive when sea level fell because of an inability to access a suitable habitat. Tracking the anchialine niche during sea level changes would necessitate continuous horizontal and vertical cave passageways in the system through which remipedes could locate the halocline (Fig. 6). Where the sea floor drops off steeply, the coastline remains relatively local, and the halocline would move primarily vertically when sea level receded. The lower portions of the cave networks must still remain in contact with marine waters, allowing the anchialine habitat to persist in that location, albeit further from the surface (Fig. 6, left). Where nearshore bathymetry is more gradually sloped, the coastline moves further away during a sea level regression, resulting in a drop in tidal exchange of marine water (Iliffe and Kornicker 2009). If the sea level drop is great enough, a cave’s marine layer may be no longer be available, eliminating the anchialine habitat as freshwater invades the cave (Fig. 6, right).
Profile of cave networks in a karst platform with a steep shelf at left and shallow shelf at right. (a) Past glacial maximum (18 kya), (b) current sea level. Double arrows represent vertical connections between horizontal cave passages. Dashed lines represent upper layers of meteoric groundwater and marine layers, which change as sea level rises from (a) -120 m to (b) 0 m, creating variation in connectivity of cave passage. On the edges of continental plates (left side of diagram), passages remain vertically connected throughout the rock profile as sea level falls. On more gradual slopes (right side of diagram), vertical connections are less common, causing breaks in anchialine cave networks as sea level falls
In contrast to Remipedia, members of Atyidae inhabit non-anchialine habitats worldwide and are capable of living in the meteoric groundwater (von Rintelen et al. 2012). As cave networks are depleted of their marine layer during sea level regressions, some atyids persisted in the meteoric groundwater. Furthermore, invasion and reinvasion from nearby freshwater streams via the meteoric groundwater likely also facilitate the gradual population of caves after sea level changes. The widespread distribution of the clade may be explained by local habitat use of cave atyids. While the majority of Typhlatya spp., for example, have been reported in freshwater, a third from caves in the Bahamas, Turks and Caicos, Ascension Island, the Galapagos, and Bermuda (Sanz and Platvoet 1995; Alvarez et al. 2005; Hunter et al. 2008) have been reported in brackish or fully marine passageways. Furthermore, Typhlatya pearsei individuals have been observed to swim between water layers in Mexican systems. These salinity tolerances may buffer atyid shrimp against extirpation due to sea level rise.
Our results have implications for predicting how species distributions will change under different environmental conditions. With sea level rise, coastal habitats such as the intertidal zone are predicted to move inland, forcing many species to migrate or adapt to new conditions (Galbraith et al. 2002). We expect anchialine species will also need to migrate or adapt as intruding marine waters move the halocline further inland or climate change leads to different precipitation regimes. Furthermore, species richness and phylogenetic diversity declined with distance from the coast for both remipedes and atyids, but the decline was much slower in atyids than remipedes. The differences in distribution and biodiversity between these two anchialine taxa likely result from the very different evolutionary histories of the two groups – remipedes remain constrained to their pleisomorphic marine habitat, while the extant atyid shrimp lineage has become established in meteoric and other low salinity habitats. Such different evolutionary histories (and the traits they produced) are present in every anchialine community. Although these taxa share a unique habitat today, predicting the future distributions of anchialine faunal assemblages as if they have a single common origin or climate change response will not likely lead to an efficient conservation strategy.
If sea level rises as much as the predicted 1.4 m in the next century (Rahmstorf 2007), the location of the marine-meteoric interface will likely move further from the coast as marine waters move inland. The availability of these habitats for anchialine faunas inland will be controlled, in part, by geology and existing karst network topology. In many regions coastal development has modified and will continue to modify the coastline, also making it difficult to predict where suitable habitats will remain as sea level rises (Galbraith et al. 2002). As presented above, remipedes and atyids would be expected to respond differently to this shift because of their restriction to different water layers in anchialine environments and the abiotic and biotic factors with which they interact.
Our results also suggest that concentrating conservation efforts on cenotes and cave systems within 7 km of the estimated coastline for the year 2100 in the Bahamas and Yucatán, Mexico, would maintain higher anchialine diversity through the next major global perturbation. This hypothesis is supported by the presence of the greatest diversity of remipedes and atyid shrimp over the last glacial and interglacial (Holocene) cycle in this region with respect to coastline. Such sites should therefore have a higher priority than more distant locales. While these examples only address a small subset of the anchialine fauna, they demonstrate the need to incorporate the different physiological tolerances of each taxon when predicting future habitat distributions. We also emphasize the importance of an integrative approach to global change issues, which includes historical factors [see also Smith (2001); Jackson et al. (2001), and Pyenson and Lindberg (2011)]. An approach integrating multiple temporal and spatial scales is needed to understand a world being rapidly perturbed by biotic and abiotic processes.
References
Alvarez, F., Iliffe, T. M., & Villalobos, J. L. (2005). New Species of the Genus Typhlatya (Decapoda: Atyidae) from Anchialine Caves in Mexico, the Bahamas, and Honduras. Journal of Crustacean Biology, 25, 81–94.
Amante, C., & Eakins, B.W. (2009). ETOPO1 1 Arc-Minute Global Relief Model: Procedures, Data Sources and Analysis. NOAA Technical Memorandum NESDIS NGDC-24.
Botello, A., & Alvarez, F. (2010). Genetic variation in the stygobiotic shrimp Creaseria morleyi (Decapoda: Palaemonidae), evidence of bottlenecks and re-invasions in the Yucatan Peninsula. Biological Journal of the Linnean Society, 99, 315–325.
Botello, A., Iliffe, T. M., Alvarez, F., Juan, C., Pons, J., & Jaume, D. (2013). Historical biogeography and phylogeny of Typhlatya cave shrimps (Decapoda: Atyidae) based on mitochondrial and nuclear data. Journal of Biogeography, 40, 594–607.
Brooks, H. K. (1955). A crustacean from the Tesnus Formation (Pennsylvanian) of Texas. Journal of Paleontology, 29, 852–856.
Christman, M. C., & Culver, D. C. (2001). The relationship between cave biodiversity and available habitat. Journal of Biogeography, 28, 367–380.
Cromer, L., Gibson, J. A. E., Swadling, K. M., & Ritz, D. A. (2005). Faunal microfossils: Indicators of Holocene ecological change in a saline Antarctic lake. Palaeogeography Palaeoclimatology Palaeoecology, 221, 83–97.
De Grave, S., Cai, Y., & Anker, A. (2008). Global diversity of shrimps (Crustacea : Decapoda : Caridea) in freshwater. Hydrobiologia, 595, 287–293.
Debrot, A. O. (2003). The freshwater shrimps of Curacao, West Indies (Decapoda, Caridea). Crustaceana, 76, 65–76.
Denniston, R. F., Asmerom, Y., Lachniet, M., Polyak, V. J., Hope, P., An, N., et al. (2013). A Last Glacial Maximum through middle Holocene stalagmite record of coastal Western Australia climate. Quaternary Science Reviews, 77, 101–112.
Emerson, M. J., & Schram, F. R. (1991). Remipedia Part 2 Paleontology. Proceedings of the San Diego Society of Natural History, 7, 1–52.
Erwin, R. M., Sanders, G. M., Prosser, D. J., & Cahoon, D. R. (2006). High tides and rising seas: potential effects on estuarine waterbirds. Studies in Avian Biology, 32, 214–228.
Finnegan, S., Heim, N. A., Peters, S. E., & Fischer, W. W. (2012). Climate change and the selective signature of the Late Ordovician mass extinction. Proceedings of the National Academy of Sciences, 109, 6829–6834.
Fiser, C., Blejec, A., & Trontelj, P. (2012). Niche-based mechanisms operating within extreme habitats: a case study of subterranean amphipod communities. Biology Letters, 8, 578–581.
Galbraith, H., Jones, R., Park, R., Clough, J., Herrod-Julius, S., Harrington, B., et al. (2002). Global climate change and sea level rise: Potential losses of intertidal habitat for shorebirds. Waterbirds, 25, 173–183.
Hoenemann, M., Neiber, M. T., Humphreys, W. F., Iliffe, T. M., Li, D., Schram, F. R., & Koenemann, S. (2013). Phylogenetic analysis and systematic revision of Remipedia (Nectiopoda) from Bayesian analysis of molecular data. Journal of Crustacean Biology, 33, 603–619.
Humphreys, W. F. (1999). Physico-chemical profile and energy fixation in Bundera Sinkhole, an anchialine remipede habitat in north-western Australia. Journal of the Royal Society of Western Australia, 82, 89–98.
Hunter, R. L., Webb, M. S., Iliffe, T. M., & Bremer, J. R. A. (2008). Phylogeny and historical biogeography of the cave-adapted shrimp genus Typhlatya (Atyidae) in the Caribbean Sea and western Atlantic. Journal of Biogeography, 35, 65–75.
Iliffe, T. M. (2000). Anchialine cave ecology. In H. Wilkens, D. C. Culver, & W. F. Humphreys (Eds.), Ecosystems of the World (pp. 59–76). Amsterdam: Elsevier.
Iliffe, T. M., & Kornicker, L. S. (2009). Worldwide diving discoveries of living fossil animals from the depths of anchialine and marine caves. Smithsonian Contributions to the Marine Sciences, 38, 269–280.
Jablonski, D. (1985). Marine regressions and mass extinctions: a test using modern biota. In J. W. Valentine (Ed.), Phanerozoic diversity patterns: profiles in macroevolution (pp. 335–354). Princeton: Princeton University.
Jackson, J. B. C., Kirby, M. X., Berger, W. H., Bjorndal, K. A., Botsford, L. W., Bourque, B. J., et al. (2001). Historical overfishing and the recent collapse of coastal ecosystems. Science, 293, 629–638.
Jaume, D., Boxshall, G. A., & Gracia, F. (2008). Stephos (Copepoda: Calanoida: Stephidae) from Balearic caves (W Mediterranean). Systematics and Biodiversity, 6, 503–552.
Kensley, B., & Williams, D. (1986). New Shrimps (Families Procarididae and Atyidae) from a Submerged Lava Tube on Hawaii. Journal of Crustacean Biology, 6, 417–437.
Koenemann, S., Schram, F. R., Hoenemann, M., & Iliffe, T. M. (2007). Phylogenetic analysis of Remipedia (Crustacea). Organisms Diversity & Evolution, 7, 33–51.
Legra, L., Li, X., & Peterson, A. T. (2008). Biodiversity consequences of sea level rise in New Guinea. Pacific Conservation Biology, 14, 191–199.
Martin, J. W., & Wicksten, M. K. (2004). Review and redescription of the freshwater atyid shrimp genus Syncaris Holmes, 1900, in California. Journal of Crustacean Biology, 24, 447–462.
Mejía–Ortíz, L. M., Yánez, G., & López–Mejía, M. (2007). Echinoderms in an anchialine cave in Mexico. Marine Ecology, 28(s1), 31–34.
Miller, K. G., Kominz, M. A., Browning, J. V., Wright, J. D., Mountain, G. S., Katz, M. E., et al. (2005). The Phanerozoic record of global sea-level change. Science, 310, 1293–1298.
Neiber, M. T., Hartke, T. R., Stemme, T., Bergmann, A., Rust, J., Iliffe, T. M., et al. (2011). Global Biodiversity and Phylogenetic Evaluation of Remipedia (Crustacea). PLoS ONE, 6, e19627.
Niemiller, M. L., McCandless, J. R., Reynolds, R. G., Caddle, J., Near, T. J., Tillquist, C. R., et al. (2013). Effects of climatic and geological processes during the Pleistocene on the evolutionary history of the northern cavefish, Amblyopsis spelaea (Teleostei: Amblyopsidae). Evolution, 67, 1011–1025.
Page, T. J., Cook, B. D., von Rintelen, T., von Rintelen, K., & Hughes, J. M. (2008). Evolutionary relationships of atyid shrimps imply both ancient Caribbean radiations and common marine dispersals. Journal of the North American Benthological Society, 27, 68–83.
Pyenson, N. D., & Lindberg, D. R. (2011). What happened to gray whales during the Pleistocene? The ecological impact of sea-level change on benthic feeding areas in the North Pacific Ocean. PLoS ONE, 6(e21295), 1–14.
Rahmstorf, S. (2007). A Semi-Empirical Approach to Projecting Future Sea-Level Rise. Science, 315, 368–370.
Sanz, S., & Platvoet, D. (1995). New perspectives on the evolution of the genus Typhlatya (Crustacea, Decapoda): first record of a cavernicolous atyid in the Iberian Peninsula, Typhlatya miravetensis n. sp. Contributions to Zoology, 65, 79–99.
Schram, F. R. (1974). Paleozoic Peracarida of North America. Fieldiana Geology, 33, 95–124.
Smith, F. (2001). Historical regulation of local species richness across a geographic region. Ecology, 82, 792–801.
van Hengstrum, P. J., & Scott, D. B. (2012). Sea-level rise and coastal circulation controlled Holocene groundwater development in Bermuda and caused a meteoric lens to collapse 1600 years ago. Marine Mircopaleontology, 90–91, 29–43.
van Hengstrum, P. J., Reinhardt, E. G., Beddows, P. A., Schwarcz, H. P., & Gabriel, J. J. (2009). Foraminifera and testate amoebae (thecamoebians) in an anchialine cave: Surface distributions from Aktun Ha (Carwash) cave system, Mexico. Limnology & Oceanography, 54, 391–396.
Vaselli, S., Bertocci, I., Maggi, E., & Benedetti-Cecchi, L. (2008). Assessing the consequences of sea level rise: effects of changes in the slope of the substratum on sessile assemblages of rocky seashores. Marine Ecology Progress Series, 368, 9–22.
von Rintelen, K., Page, T. J., Cai, Y., Roe, K., Stelbrink, B., Kuhajda, B. R., et al. (2012). Drawn to the dark side: A molecular phylogeny of freshwater shrimps (Crustacea: Decapoda: Caridea: Atyidae) reveals frequent cave invasions and challenges current taxonomic hypotheses. Molecular Phylogenetics and Evolution, 63, 82–96.
Walker, L. N., Mylorie, J. E., Walker, A. D., & Mylorie, J. R. (2008). The caves of Abaco Island, Bahamas: keys to geologic timelines. Journal of Cave and Karst Studies, 70, 108–119.
Woodroffe, C. D., & Murray-Wallace, C. V. (2012). Sea-level rise and coastal change: the past as a guide to the future. Quaternary Science Reviews, 54, 4–11.
Zaksek, V., Sket, B., & Trontelj, P. (2007). Phylogeny of the cave shrimp Troglocaris: Evidence of a young connection between Balkans and Caucasus. Molecular Phylogenetics and Evolution, 42, 223–235.
Zaksek, V., Sket, B., Gottstein, S., Franjevic, D., & Trontelj, P. (2009). The limits of cryptic diversity in groundwater: phylogeography of the cave shrimp Troglocaris anophthalmus (Crustacea: Decapoda: Atyidae). Molecular Ecology, 18, 931–946.
Acknowledgements
MM thanks members of the “Cave Team” and Lindberg Lab for their support and feedback. We thank T. Iliffe, L. Mejía-Ortíz, and J. Yager for supplying and verifying taxon localities in the Electronic Supplementary Material, Table 3. Use of GIS software and technical assistance was provided by the University of California Berkeley Geospatial Information Facility. This is publication number 2045 from the University of California Museum of Paleontology.
Ethics Statement
All methods comply with the current laws of the USA, the country in which this research was performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 149 kb)
Rights and permissions
About this article
Cite this article
Moritsch, M.M., Pakes, M.J. & Lindberg, D.R. How might sea level change affect arthropod biodiversity in anchialine caves: a comparison of Remipedia and Atyidae taxa (Arthropoda: Altocrustacea). Org Divers Evol 14, 225–235 (2014). https://doi.org/10.1007/s13127-014-0167-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-014-0167-5