Abstract
Reflection spectra obtained from hyperspectral imaging can be used as a bio-optical taxonomic identification tool if the pigment composition and the corresponding optical absorption signatures of an organism are known. In this study we elucidate species-specific absorption and corresponding reflection signatures of marine organisms and discuss optical fingerprints from underwater hyperspectral imaging (UHI) for future automated identification of organisms on the seafloor. When mounted on underwater robots, UHI has the potential to be a time- and cost-efficient identification and mapping method covering large areas over a short time. Hyperspectral imaging in vivo and in situ were used to obtain species-specific reflection signatures (optical fingerprints). High performance liquid chromatography, liquid chromatography–mass spectroscopy and nuclear magnetic resonance were used for pigment identification and to obtain species-specific absorption signatures of four marine benthic species; the spoonworm Bonellia viridis, and the sponges Isodictya palmata, Hymedesmia paupertas and Hymedesima sp. Species-specific optical fingerprints based on a UHI-based reflectance signature were verified successfully in the organisms investigated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, there is international recognition that, in order to allow effective management of marine nature and sound decision making (Buhl-Mortensen and Buhl-Mortensen 2008; Boyd et al. 2006; Sakshaug et al. 2009), there is a need to map large areas of the seafloor using new underwater platforms such as gliders, autonomous underwater vehicles (AUV) and remotely operated vehicles (ROV, Sørensen et al. 2012; Johnsen et al. 2013a, b). A newly developed technique called underwater hyperspectral imaging (UHI) has been described (Johnsen et al. 2013b) that can be deployed on ROV or AUV to create object of interest (OOI) maps of benthic organisms. In contrast to multispectral imagers, which are sensitive in only a few selected wavebands, hyperspectral imagers (HI) measure the spectral upwelling radiance Lu(λ) or reflectance R (λ) per image pixel of bio-geo-chemical OOI (Klonowski et al. 2007; Volent et al. 2007; Johnsen et al. 2009, 2013b). The HI consist of a spectrometer equipped with a charge coupled device (CCD) as a detector, which makes it possible to reconstruct detailed images of a large area of interest at high spectral (1 nm) and spatial (1 cm) resolution. The UHI is an active optical sensor equipped with an underwater housing, using its own light sources, in contrast to passive remote sensing from satellites using the Sun as a light source (Johnsen et al. 2013b). Former studies on marine organisms have shown that reflectance signatures obtained from HI and UHI are inversely related to the specific absorption signature of the organism (Volent et al. 2007, 2009; R. Pettersen et al. unpublished). Therefore, HI can be used as a bio-optical taxonomic tool in the same manner as organism-specific absorption signatures have been used (Roy et al 2011; Pettersen et al. 2011). HI and UHI can be used as a taxonomical identification tool to make optical fingerprints of marine organisms only if the pigment composition and corresponding absorption signature of the organism is known and can be used to verify the reflectance signature (R. Pettersen et al. unpublished). By creating a spectral library of organisms with verified reflectance signatures, mapping of the seafloor with UHI can be made more efficient.
One of the taxa examined in this study was Bonellia viridis Rolando, 1821, from the phylum Echiura (Fig. 1a) and is distributed in the Atlantic, Indian and Pacific Oceans (Wesenberg-Lund 1939). It contains the green pigments bonellin and neobonellin as its main pigments (Agius et al. 1979). Bonellin is the main pigment in the proboscis of the worm and is found to be responsible for masculinisation in B. viridis (Baltzer 1931; Cariello et al. 1978; Agius et al. 1979). Neobonellin is a biologically active derivative of bonellin that is found in the body skin of B. viridis (Cariello et al. 1978). Both neobonellin and bonellin are known to act as an embryotoxic agent in echinoderms and ascidians (Cariello et al. 1978; De Nicola Giudici 1984). Another taxa examined was the orange sponge Isodyctia palmata (phylum porifera, class of demospongiae, Ellis and Solander 1786, Fig. 1b), which is distributed in the boreal and sub-arctic North Atlantic having only a few predators (nudibranchs and littorinid snails); crabs and other invertebrate predators find the sponge distasteful (Appeltans et al. 2012). To our knowledge, the pigment composition in I. palmata has not been revealed. The second sponge investigated was Hymedesmia paupertas (phylum porifera, class of demospongia, Bowerbank 1864, Fig. 1c)—a common species in the north Atlantic, most often with a deep blue colour (Appeltans et al. 2012). The main pigment isolated from H. paupertas is lactarazulene (Fehler 2005), which has also been isolated (and identified) from the edible fungus Lactarius deliciosus (Sorm et al. 1954). The last organism investigated—a bright red colored Hymedesmia sp., probably a Hymedesmia (Stylopus) coriacea (Fristedt 1885, Fig. 1d)—was also from the family Hymedesmiidae.
The main aim of this study was to use HI with reflectance [R(λ)] per image pixel for taxonomic identification verified by organism-specific absorption characteristics. Pigment investigation was also conducted due to the incomplete pigment composition knowledge of the chosen organisms. The goal was to evaluate if UHI-based identification can be used to identify and detect species-specific spectral signatures (absorption and reflectance), or optical fingerprints of marine organisms that can be used as verification for future automated identification of marine organisms.
Materials and methods
Sampling and in vivo absorption spectra
All four species was sampled with a Sperre SUB-fighter 7500 remote operated vehicle (ROV) deployed from the NTNU research vessel RV Gunnerus. Co-ordinates for sampling locations are given in EU89-UTM sone 33 formats. B. viridis at 224 m depth in Trondheimsfjorden, Norway [7037347.67 (e), 565129.70 (n)], I. palmata at 42 m depth outside Stavøya, Norway [7051622.65 (e), 52494.75 (n)], H. paupertas were sampled at Stokkbergneset, Norway at 75 m depth [70381350.07 (e), 545394.57 (n)] and Hymedesmia. sp at 40 m depth at Stokkbergneset, Norway [7038151.13 (e), 545340.15 (n)]. Immediately after collection, half the sample was ground and placed on Whatman GF/F glass fibre filters on neutral plastic petri dishes for in vivo (n = 4) absorption measurements using a Unicam UV 500 spectrophotometer (http://www.thermoscientific.com/uv-vis). The other half of the tissue was frozen for later pigment investigation.
In vitro absorption and pigment extraction
Tissue for pigment extraction was crushed into small fragments with a mortar before liquid–liquid extraction was performed. First, the crushed tissue was mixed with acetone (20 ml) and placed in a Bransonic 221 ultrasound bath (48 kHz and 50 W) for 15 min, embedded in cold water (3 °C) in the dark. The acetone suspension was centrifuged in a Hermle Z230 centrifuge at 2,500 rpm (600 g) for 5 min and re-filtered with a RC 0.20 μm PFTE membrane filter to avoid debris. Water (100 ml) was added to the extract and acetone removed with vacuum suction. The water phase was transported to a separation funnel and extracted with dichloromethane (3 × 10 ml). The extraction procedure was conducted three times to make sure that as much as possible of the pigment was washed out of the water phase, which could be detected by looking at the colour of the extract. The dichloromethane extracts were dried with nitrogen gas and then dissolved in methanol for in vitro absorbance and mass spectrometric (MS) analysis. In vitro absorbance characteristics (200–750 nm) of the methanol extract were measured with a Unicam spectrophotometer in a 1-cm path-length quartz cuvette with n = 1 due to limitation of sample tissue. In addition, H. paupertas and Hymedesmia sp. acetone extracts were dried with nitrogen gas and dissolved in methanol and prepared for MS analysis. Hymedesmia paupertas was also extracted with methanol for high performance liquid chromatography (HPLC) for possible detection of chlorophylls that are not detected in the liquid chromatography-mass spectrometry (LC-MS) method used.
High performance liquid chromatography
Pigments were analysed by a Hewlett Packard 1100 Series HPLC system equipped with a quaternary pump system, injector, auto sampler, Waters Symmetry C8 column (150 × 4.6 mm, 3.5 μm particle size) and a diode array absorbance detector (400–700 nm). The auto sampler drew 77 μL from each sample vial and 23 μL water, which were mixed automatically five times before injection. The water was added to increase the polarity in order to improve separation of chlorophyll carbons. In the mobile phase, solvent 1 was a mixture of methanol:acetonitrile:aqueous pyridine (0.25 M pyridine) in the ratio 50:25:25 (v/v), solvent 2 was acetonitrile:acetone in the ratio 80:20 (v/v) and solvent 3 (methanol) was used for cleaning the system before and after the analysis. Analytic separation was performed using the method of Zapata et al. (2000).
Mass spectrometric analysis
The MS analyses were performed with an Agilent liquid chromatography-diode array detector-quadrupole time-of-flight-mass spectrometer model 6520 (LC-DAD-QTOF-MS) with ionisation mode atmospheric pressure chemical ionisation (APCI) and a Zorbax SB C8 column of dimensions 4.6 × 150 mm. The mobile phases were methanol-water (50:50) in the first mobile phase bottle (A) and dichloromethane-methanol-water (45:50:5) in the second mobile phase bottle (B). Flow was 1 ml/min, 2 μl extract were injected for each sample and the gradient was set to: 0–2 min 0 % B, 2–10 min 0–10 % B, 10–12 min 100 % B and 12.1–20 min 0 % B. The Agilent MassHunter qualitative analysis software was used to assign accurate masses to the pigments observed in the diode array chromatogram. Accurate mass search with a +/– 10 ppm window was performed to tentatively identify the unknown pigments. The online version of the Dictionary of Natural Products (DNP) database (http://www.chemnetbase.com) containing structure information for over 226,000 natural products was used.
NMR analysis of Hymedesmia paupertas
NMR spectra were recorded on a Bruker Avance 600 MHz instrument fitted with a TCI cryoprobe. The chemical shifts are reported relative to trimethylsilane (TMS) for both 1H and 13C. To aid in peak assignments, the 2D NMR techniques COSY, NOESY, HSQC and HMBC were used.
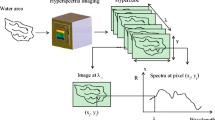
In vivo and in situ reflectance from hyperspectral imaging
In vivo upwelling radiance Lu(λ) (raw data) and relative R(λ) (spectra corrected for lamp source and inherent optical properties (IOP), i.e. of water, phytoplankton, coloured dissolved organic matter (CDOM) and total suspended matter (TSM) (R. Pettersen et al. unpublished), were measured with a custom made HI consisting of a solid-state push-broom HI, 2 cm long, 4 cm wide, 600 g, with power requirement of 12 V, 139 mA (Sigernes et al. 2000; Johnsen et al. 2013b). Frame rate was set to 25 images per second and the camera was equipped with a 12-mm Schneider (f:1.2) front lens with aperture set to 2.8 to enhance depth of field. The spectral resolution was 1 nm in the region 420–680 nm. The spatial resolution perpendicular to the moving direction was 2.06 mm/pixel. Data from the HI and UHI was stored as video files (avi-format) from a digital video recorder (Archos AV-400, 13 Mb min–1, comprising 25 images at 300 wavelengths giving an image cube of 7,500 slit images s–1). All four species were sampled and measured on land with a white spectrally neutral, polyethylene plastic plate for light source and ambient light correction. B. viridis, I. palmata and Hymedesmia. sp. were measured with the HI placed on a tripod equipped with a panorama head. The distance between the HI and the OOI was 1.5 m. HI measurements of B. viridis were made outdoors using the sun as a light source while I. palmata and Hymedesmia sp. were scanned inside the lab with two halogen lamps (Green force 35 W halogen) as a light source. For HI measurements of H. paupertas, the HI was fitted to a Leitz Leica MS5 stereomicroscope (1–80×) according to Volent et al. (2009). The light source was a Leitz CLS 150X halogen lamp emitted through two optical fibre bundles (Volent et al. 2009). A white, spectrally neutral, polyethylene reference plate was placed beside or under the OOI and used to correct for the spectral lamp output and ambient light. The same reference plate was used for the underwater HI in situ (see below) to correct for the lamp, ambient light and IOP according to Johnsen et al. (2013b).
In situ reflection measurements of Isodictya palmata were made with the HI placed in a custom-made underwater house, depth rated to 500 m using a BK7 glass as optical window (Johnsen et al. 2013b). The settings for HI were the same as for the in vivo measurements. Hopavågen—a landlocked bay at Agdenes in central Norway (63°35′32.94″N, 9°32′28.49″E)—was used as the experimental site. Scuba divers placed a custom-made cart running over a “railway” divided into four sections (1 × 0.6 m each), in a downhill slope at 4–5 m depth (for more details see Johnsen et al. 2013b). Isodyctia palmata together with an IOP reference standard was placed in the centre of a railway section by scuba divers. The cart was equipped with a halogen and an LED lamp (Green force 35 W halogen lamp and a Quadristar P4 LED lamp,) and the UHI was positioned with the slit direction perpendicular to the moving direction. The UHI swath width (view-angle) at 1.35 m distance (optically 1.0 m in water) to the sponge was 40 cm and spectral resolution 1 nm in the region 420–680 nm. In situ UHI mapping of the sponge was done by sliding the cart up and down the railway at a speed of 7 cm s–1 with the help of an electrical winch powered by a 12 V car battery and a 5 mm thick wire of 50 m in length connecting the cart and the winch on land. Correspondingly, an underwater UHI cable of 50 m was connected to the UHI for on-line monitoring, recording, exposure control and data transfer and storage using a digital video recorder (Archos AV-400) powered by a 12 V battery.
The reflectance spectra R(λ) of a given OOI was obtained by using Lu raw (λ) (raw spectra with light source) and Lu light (λ) denoting light source spectrum from reference plate in Eq. 1.
HI and UHI data was processed with the custom made yaPlaySpecX software described in Volent et al. (2007, 2009). In the yaPlaySpecX program, radiance spectra can be extracted as single spectra from single pixels (single pixel spectra) or as average spectra from the amount of pixels defined by user (running mean spectra). For radiance spectra in this research, running mean spectra with n = 50 of each organism were extracted.
Results and discussion
Bonellia viridis
Pigment analysis (LC-MS) detected only one pigment in B. viridis, and surprisingly this was not the expected bonellin, but a derivative of bonellin called neobonellin. Neobonellin (pigment 1) with maximum optical density [OD(λ)max] at 392 nm, 494 nm, 521 nm, 586 nm and 640 nm was identified from the body skin of B. viridis with LC-MS analysis and found to contribute to the deep green colour of the animal (Fig. 2a, Table 1). The in vivo and in vitro OD absorption signatures (Fig. 2b) corresponded to the absorption spectra of neobonellin from 400 to 650 nm (in vivo and in vitro was only measured from 400 to 700 nm) except a shoulder at 488 nm that is not shown or reported for the neobonellin absorption spectra and a OD(λ)max at 538 nm that correspond both to the reported absorption max of neobonellin (536 nm) and bonellin (539 nm) absorption spectra but was not evident in the absorption spectra from LC-MS analysis. The bonellin absorption spectrum has been reported to have OD maxima at: 394–488 (shoulder)–494–521–539–590–(620) and 641 nm (Cariello et al. 1978), indicating that the shoulder at 488 nm in the in vivo and in vitro spectra were due to bonellin. LC-MS analysis did not detect bonellin, probably because the proboscis, which is the main source of bonellin, was not included in the extraction, only the body skin, which is the main source of neobonellin. When HI measurements were conducted, the animal had withdrawn its proboscis (probably because of being negatively phototactic) so that only the body skin was used also for HI reflection spectra. Therefore, the location of pigments in the OOI and also the behaviour and movement pattern of sessile and none sessile OOI is important knowledge and needs to be taken into consideration when using absorption and reflection signatures as taxonomic identification tools. For organisms living on the sea bottom, in situ UHI measurements have short exposure times and therefore animals that are negatively phototactic would not have time to withdraw their appendages or perform an escape. Sessile organisms that, by chance, have withdrawn their coloured appendages, have other body parts that can be used for identification. For burrowing organisms and organisms living within the sediments, the UHI technique might not be applicable.
a, b Bio-optical signatures from B. viridis. a Specific absorbance spectra of neobonellin (pigment 1) detected from liquid chromatography–mass spectroscopy (LC-MS) analysis of the spoon worm B. viridis. b Absorbance spectra in vivo OD(λ) and in vitro OD(λ) plotted against reflection spectra in vivo R(λ) from the hyperspectral imager (HI), illustrating that the OD(λ) spectra are inversely related to the R(λ) spectra. The in vitro OD(λ) curve has been interrupted from 630 nm–642 nm (marked with: = =) due to saturation of the detector in the spectrophotometer. The spectra have been scaled to 1 at their highest OD (λ) and R(λ) for easier comparison
The HI reflection spectra of B. viridis were found to be high in the 420–480 nm wavelength area with a R(λ)max at 441 nm (Fig. 2b). The corresponding low absorption in the same wavelength area led to the conclusion that, for B. viridis, R(λ) and OD(λ) spectra were inversely related.
Isodictya palmata
Five pigments (Fig. 3a) detected with LC-MS analysis of I. palmata are listed in Table 1. Four of the five pigments were tentatively identified by DNP search while the fifth (Pigment 3, Table 1) did not get any hits and was presumed unknown. The DNP database returned three hits for the first of the five pigments detected: 2,6-benzothiazolediol and two derivatives of the same component (Pigment 2, Table 1, Fig. 3a). The ppm error for this pigment was higher than the 10 ppm window usually used for DNP searches, but the pigment was considered interesting for this organism (see following “Results and Discussion”).
a, b Bio-optical signatures from I. palmata. a Specific absorbance spectra of tentatively identified pigments and unidentified pigments 2–6 (Table 1) detected by LC-MS analysis. b The absorbance spectra in vivo OD(λ) and in vitro OD(λ) plotted against reflection spectra in vivo R(λ) ) and in situ R(λ) from the HI, illustrating that the OD(λ) spectra are inversely related to the R(λ) spectra. The spectra have been scaled to 1 at highest OD(λ) and R(λ) for easier comparison
Derivatives of 2,6 benzathiazolediol are known to be produced by the bacteria Micrococcus sp., which has been isolated from the tropical sponge Tedania ignis (Stierle et al. 1988). Sponges are known to be hosts for symbiotic microorganisms and, since all sponges are filter feeders, they filtrate and extract microorganisms from the water. Microorganisms that resist the digestive process and immune response of the host sponge can successfully inhabit the sponge (Wilkinson 1987; Lee et al. 2001). Some species of Micrococcus are pigmented and a large number of heterotrophic bacteria are also able to synthesise carotenoides (Hailian et al. 2006) and may therefore contribute to the colour of the host organism. Further analysis is needed to make a definite identification of the pigment found, but it is highly likely that 2,6 benzathiazolediol and derivatives can be found in I. palmata. DNP search of pigment 4 returned five hits (Table 1), of which the purine pigment erinacean with an absorption max at 272 nm in methanol is the most likely identity. The pigment detected from I. palmata had an OD(λ)max at 278 nm (Fig. 3a). Erinacean was isolated for the first time from the Antarctic sponge Isodictya erinacean (Topsent 1916; Moon et al. 1998) and found to be bioactive by being weakly antibiotic and cytotoxic (Moon et al 1998; Bandaranayake 2006). Cytotoxic substances in sponges are presumed to be a part of the defence mechanism to prevent predation from other benthic species (Moon et al. 1998), indicating that I. palmata has both physical (spicules) and chemical defence mechanisms. The other hits from the DNP were Asperopterin B isolated from Aspergillus oryzae; 1,3,9-trimethyl-8-nitrosoisoxanthine isolated from Cucumaria frondosa; and two derivatives of Pyrimido[5,4-e]-1,2,4-triazine-3,5,7-triol: 4-Methylfervenulone isolated from Streptomyces spp. and 2-methylfervenulone. Also for this pigment, further analysis is needed for definite identification, but it is highly likely that the pigment is erinacean because it has been isolated previously from a species in close relation to I. palmata. Calicogorgin B (Pigment 5) isolated from the gorgonian Calicogorgia sp. was also identified tentatively in I. palmata (Table 1, Fig. 3a). In Calicogorgia sp., calicogorgin B has been reported to possess lethal and repellent activities against predatory gastropods (Ochi et al. 1992); this pigment might be one reason why I. palmata has few predators. The absorption of calicogorgin B in the visible part of the wavelength spectrum with a OD(λ)max at 404 nm, indicates that this pigment is a main contributor to the orange colour of the sponge. A derivative of aspergamide B was the last pigment detected from I. palmata (Pigment 6, Table 1, Fig. 3a). One biological source of aspergamide B is the fungus Aspergillus sp., which is found everywhere in the marine and terrestrial environment and is probably taken up by filtration of water.
The in vivo and in vitro OD(λ) spectra showed absorbed in the UV region (<389 nm) (Fig. 3b), corresponding to the specific absorption of the pigments detected in Fig. 3a. There was also a broad absorbance band in the visible part of the wavelength spectra at 400–450 nm that corresponded to the specific absorption of pigment 5 (Fig. 3a). OD(λ) spectra decreased in the 450–500 nm areas and, correspondingly, the in vivo and in situ R(λ) spectra from HI measurements increased (Fig. 3b), making the OD(λ) and R(λ) spectra from I. palmata inversely related. The in vivo and in situ HI R(λ) spectra were measured with different distances and medium between the sponge and the HI detector; in vivo measurements were taken in air with a microscope and in situ measurements were taken under water at a distance of 1.35 m between OOI and UHI. The shape of the R(λ) spectra show that the distance and inherent optical properties in the water did not have a significant impact on the reflection signature when a reference plate was used for correction. These results are in accordance with those of Johnsen et al. (2013b) and R. Pettersen et al. (unpublished) and show that HI and UHI has the potential to be an accurate taxonomic tool.
Hymedesmia paupertas
An accurate mass search in DNP indicated that pigment 7 (Table 1) detected from HPLC and LC-MS was lactarazulene, with OD(λ)max at 291, 376 and 605 nm. Absorbance spectra (350–700 nm) of lactarazulene from HPLC had only two OD(λ)max peaks at 376 nm and 605 nm (Fig. 4a). The corresponding absorbance spectra of lactarazulene detected from LC-MS was taken from the UV region to the green part of the visible spectrum, i.e. 220 nm–500 nm with OD(λ)max at 291 nm and an OD(λ)max at 376 nm was measured (Fig. 4a). Pigment 7 suspected to be lactarazulene was purified and submitted to NMR analysis. Proton and carbon-13 NMR shifts were recorded and assigned unambiguously to lactarazulene with the help of 2D NMR methods. Additionally, the proton NMR shifts were compatible with those in the literature (Takekuma et al. 1988). Carbon-13 NMR shifts in CDCl3 have, to our knowledge, not been reported previously. However, the reported 13C chemical shifts in C6D6 show close resemblance to our findings (Fehler 2005) (Table 2). There were several other pigments separated by HPLC that absorb in wavebands that potentially could be contributing to the colour of the sponge, but not all were identified (pigments 10–12, Table 1, Fig. 4b). Chlorophyll a [Chl a, OD(λ)max 431 nm and 663 nm, pigment 8] and diatoxanthin [OD(λ)max 454 nm and 484 nm, pigment 9] were identified by HPLC (Table 1), but the concentration of these and the unidentified pigments were trace amounts compared to the concentration of lactarazulene. These results show that lactarazulene is the main pigment responsible for the blue colour of the sponge, whereas no epifauna were registered on the sponge. It is most likely that Chl a and diatoxanthin have entered the sponge matrix through filtration of water containing phytoplankton.
a–c Bio-optical signatures from Hymedesmia paupertas. a Specific absorbance spectra of lactarazulene (pigment 7) detected with high performance liquid chromatography (HPLC) and LC-MS. The absorbance spectra of lactarazulene from LC-MS analyses has been scaled to 1 at highest OD(λ) for easier comparison. b Specific absorbance spectra of chlorophyll a, diatoxanthin and three unidentified pigments (pigment 8–12) detected with HPLC. c Absorbance spectra in vivo OD(λ) and in vitro OD(λ) plotted against reflection spectra in vivo R(λ) from the HI, illustrating that the OD(λ) spectra are inversely related to the R(λ) spectra. The spectra have been scaled to 1 at highest OD(λ) and R(λ) for easier comparison
In vivo and in vitro absorbance spectra indicated high absorbance at wavelengths <380 nm and high absorbance in a broad wavelength band from 600 to 650 nm (Fig. 4c), corresponding to the absorbance spectra of lactarazulene. The HI R(λ) spectra were high for the 430–575 nm wavelength area with a R(λ)max at 475 nm, the OD(λ) for in vivo, in vitro and lactarazulene were correspondingly low in the same area (Fig. 4c) and show that R(λ) and OD(λ) for H. paupertas were inversely related. Chl a, diatoxanthin and the unidentified pigment (pigment 10–12) absorbs in the 400–500 nm wavelengths (Fig. 4b), but does not seem to have a significant impact of the reflectance, probably due to its low concentration.
Hymedesmia sp.
From the red coloured Hymedesmia sponge, four pigments were detected by LC-MS analysis (Fig. 5a). Following a DNP search to identify the pigments, pigment 13 had no hits in the database and remained unidentified (Table 1). The DNP database search returned three hits for pigment 14, 2(3H)-benzothiazolethione and two derivatives of the same compound (Fig. 5a). Like 2,6-benzothiazolediol and its derivatives from I. palmata, 2(3H)-benzothiazoletione has been isolated from Micrococcus sp. in Tedania ignis and this might therefore be the source of the pigment isolated from Hymedesmia sp. 13-Propanoyloxylupanine (Pigment 15)—a derivative of 13-hydroxylupanin isolated from the plant genus Lupinus sp.—was also detected in Hymedesmia sp., absorbing in the ultraviolet part (ODmax 284 nm). Pigment 16 detected from Hymedesmia sp. absorbs in the range of 400–550 nm with a OD(λ)max at 486 nm (Fig. 5) indicating that this is the main pigment responsible for the red colour of the sponge. The DNP database returned four hits for the pigment and three for potential pigment candidates have been isolated previously from the marine environment (Table 1). Tedanin has been isolated from the sponges Tedania digitata and Clathria frondifera, isotedanin from the sponge Agelas mauritiana (Britton et al. 2004), 3,3′-dihydroxyleprotene from marine bacteria and 3.3.4.4′-tetrahydro-β,β-carotene-2.2′-dione from Carausius morosus (Phylum Arthropoda); this latter compound is most likely the pigment responsible for the colour of Hymedesmia sp. as it has been isolated from the marine environment before, but further analysis is needed to have certain identification.
a, b Bio-optical signatures from Hymedesmia sp. a Specific absorbance spectra of tentatively identified pigments 14–16 and unidentified pigment 13 (Table 1) detected from LC-MS analysis. b The absorbance spectra in vivo OD(λ) and in vitro OD(λ) plotted against reflection spectra in vivo R(λ) ) from the HI, illustrating that the OD(λ) spectra are inversely related to the R(λ). The spectra have been scaled to 1 at highest OD(λ) [except in vitro OD, which have been scaled to 1 at 471 nm, which is the highest OD(λ) for the indicated pigment absorbing in the visible spectrum] and R(λ) for easier comparison
The in vitro OD(λ) spectra corresponded to the absorption of pigments detected from LC-MS analysis in Fig. 5a with OD(λ)max at 284, 343, 411 and 471 nm. The in vivo OD(λ) spectra were not as pronounced as the in vitro spectra but had clearly a ODmax at 485 nm corresponding to pigment 16.
Most of the pigments detected from Hymedesmia sp. absorbed in the UV region, below the detection limit of the HI, as was the unidentified pigment 13 (Table 1, Fig. 5a). HI R(λ) spectra were inversely related to the OD(λ) spectrum of Hymedesmia sp. (Fig. 5b).
This study has initiated a spectral library of verified bio-optical signatures measured with HI and UHI. Objects of interest were identified using three different approaches: (1) traditional taxonomy based on morphology, needle preparation and colour (OOI was first sampled and then identified); 2) chemo-taxonomy (pigment isolation and characterisation, HPLC, LC-MS-QTOF, and NMR); and (3) bio-optical identification (HI and spectrophotometry). Morphological-feature-based taxonomy is time-consuming and expensive and misidentification can occur due to inexperience or the presence of morphological features that are not always species-specific. Chemo- and bio-optical taxonomy has been used for more than three decades for phytoplankton and macroalgae (Roy et al. 2011), but obtaining pigment composition and corresponding optical signatures is quite a new approach for benthic animals (Elde et al. 2012). Bio-optical identification (absorbance, reflectance and fluorescence exCitation spectra) has been used widely for both remote sensing and for in situ monitoring of micro- and macroalgae (reviewed by Johnsen et al. 2011). Hyperspectral imaging is a new identification technique for shallow-living marine organisms, corals, seagrass and micro- and macro-algae (Klonowski et al. 2007; Volent et al. 2007). UHI has the potential to be a more automated method when used as a sensor on underwater robots such as ROV and AUVs (Moline et al. 2005; Johnsen et al. 2013b). The use of HI as a taxonomic identification tool can be used unsupervised by discriminating between unknown OOI, or in supervised classification (as in this study) identifying and mapping an OOI with a known pigment signature. One problem that might arise, especially for organisms with a pigment content influenced by pigmented heterotrophic bacteria, is that the abundance and species distribution of pigmented microbes are influenced heavily by environmental properties such as temperature, salinity, nutrient level and competition for resources (Hailian et al. 2006). Therefore, a given taxa may have diverse colorations and optical signature that may not be the same over any given distinct geographical and ecological areas; information of this sort also needs to be incorporated in the spectral library for world wide use. Also, many organisms have pigments absorbing in the UV part of the electromagnetic spectrum and it is possible that these pigments would be better suited to separate visibly similar coloured organisms. Extending the pigment search area into shorter wavelengths increases the opportunity to find species-specific absorbance traits (marker pigments).
The prototype HI sensor used in this study had low sensitivity at wavelengths below 420 and above 680 nm, while several of the pigments that characterised the OOI absorb below 420 nm into the UV region. A more UV and infrared (IR) sensitive sensor, with higher spatial and radiometric resolution has been developed and used with success on a ROV covering large areas of seafloor for habitat mapping and also creating geo-localised seafloor maps (Johnsen et al. 2013a, b; Ludvigsen et al. 2013), but even more sensitive sensors needs to be developed to ensure more reliable and powerful identification and mapping tools for all kinds of marine organisms.
Concluding remarks
The results of this study show that absorbance spectra [OD (λ) and HI spectra (R(λ)] are inversely related for the organisms investigated, and hence R(λ) images can be used as bio-optical taxonomic identification tools. If the pigment composition of the OOI is known, together with corresponding optical properties and a knowledge of additional environmental factors affecting the pigment signature in an organism, then UHI can be a powerful identification and mapping tool for OOI on the seafloor. The construction of a spectral library of verified reflection signatures from marine organisms would enable automated identification of the sea floor. This study implies that if there is detailed knowledge of species-specific pigment composition and corresponding optical fingerprints, an UHI-based seafloor mapping may, in addition to identifying and mapping OOI, also indicate their chemical composition. Chemical imaging may augment information regarding the physiological state of an organism, for bioprospecting, and can be a helpful tool in environmental management and decision-making. To reach these goals, the HI technique needs to be further developed regarding sensor sensitivity and corresponding light sources at shorter (UV) and longer (IR) wavelengths for better separation of organisms with pigments absorbing at wavelengths beyond the visible range (400–700 nm).
References
Agius, L., Ballantine, J. A., Ferrito, V., Jaccarini, V., Murray-Rust, P., Pelter, A., et al. (1979). The structure and physiological activity of bonellin- a unique chlorin derived from Bonellia viridis. Pure and Applied Chemistry, 51, 1847–1864.
Appeltans, W., Bouchet, P., Boxshall, G.A., De Broyer, C., de Voogd, N.J., Gordon, D.P., (Eds.) (2012). World Register of Marine Species. Accessed at http://www.marinespecies.org on 20 March 2013.
Baltzer, F. (1931). Sipuncula. Handbuch der Zoologie, 11, 15–61.
Bandaranayake, W. M. (2006). The nature and role of pigments of marine invertebrates. Natural Product Reports, 23, 223–255.
Boyd, S.E., Coggan, R.A., Birchenough, S.N.R., Limpenny, D.S., Eastwood, P.E., Foster Smith, R.L., et al. (2006). The role of seabed mapping techniques in environmental monitoring and management. Science Series Technical Report no.127. Cefas. http://www.cefas.co.uk/Publications/techrep/techrep127.pdf. Accessed 20 March 2013.
Britton, G., Liaaen-Jensen, S., Pfander, H., Mercadante, A. Z., & Egeland, E. S. (2004). Carotenoids, Handbook. Basel: Birkhäuser. 647p.
Buhl-Mortensen,L., & Buhl-Mortensen, P. (2008). Mareanotokt 2008104 OG 2008114. Samlet toktrapport fra bunnkartlegging i TromsøII og Nordland. Havforskningsinstituttet.http://www.imr.no/publikasjoner/andre_publikasjoner/toktrapporter/2009/Toktrapport_nr_4_09_mareano.pdf/nb-no. (In Norwegian) Accessed 1 March 2013
Cariello, C., De Nicola Giudici, M., Zanetti, L., & Prota, G. (1978). Neobonellin, a new biologically active pigment from Bonellia viridis. Experienta, 34(11), 1427–1429.
De Nicola Giudici, M. (1984). Defence mechanism of Bonellia viridis. Marine biology, 78, 271–273.
Elde, A. C., Pettersen, R., Bruheim, P., Järngren, J., & Johnsen, G. (2012). Pigmentation and spectral absorbance signatures in deep-water corals from the Trondheimsfjord, Norway. Marine Drugs, 10(6), 1400–1411.
Fehler, K. (2005). Isolirung und strukturaufklärung von marinen kaltwasserschwämmen und korallen. http://ediss.sub.uni-hamburg.de/volltexte/2005/2570/pdf/Dissertation_Fehler.pdf
Hailian, D., Nianzhi, J., Yohua, H., & Yonghhui, Z. (2006). Diversity and distribution of pigmented heterotrophic bacteria in marine environments. FEMS Microbiology Ecology, 57, 92–105.
Johnsen, G., Volent, Z., Sakshaug, E., Sigerne, F., & Pettersson, L. H. (2009). Remote sensing in the Barents Sea. In E. Sakshaug, G. Johnsen, & K. Kovacs (Eds.), Ecosystem Barents Sea (pp. 139–166). Trondheim: Tapir Academic.
Johnsen, G., Moline, M., Pettersson, L. H., Pinckney, J., Pozdnyakov, D. V., Egeland, E., et al. (2011). Optical monitoring of phytoplankton bloom pigment signatures. In S. Roy, C. Llewellyn, E. Egeland, & G. Johnsen (Eds.), Phytoplankton pigments: Updates on characterization, chemotaxonomy and applications in oceanography (Vol. 14, pp. 538–581). Cambridge: Cambridge University Press.
Johnsen, G., Ludvigsen, M., Sørensen, A. J., Moline, M. A. (2013a). Underwater hyperspectral imagery for identification, mapping and monitoring of bio-geo-chemical feautures on the sea floor. Blue Photonics 3. Paper 6960. European Optical Society.
Johnsen, G., Volent, Z., Dierssen, H., Pettersen, R., Ardelan, M. V., Søreide, F., et al. (2013b). Underwater hyperspectral imagery to create biogeochemical object of interest maps of seafloor. In J. Watson & O. Zielinski (Eds.), Subsea optics and imaging. Cambridge: Woodhead.
Klonowski, W. M., Fearns, P. R., & Lynch, M. J. (2007). Retrieving key benthic cover types and bathymetry from Hyperspectral Imagery. Journal of Applied Remote Sensing, 011505, 1–21.
Lee, Y. K., Lee, J.-H., & Lee, H. K. (2001). Microbial symbiosis in marine sponges. Journal of Microbiology, 39(4), 254–264.
Ludvigsen, M., Sørensen, J. A., Ødegård, Ø., Johnsen, G., Lågstad, P. A. (2013). Scientific operations combining ROV and AUV in the Trondheimsfjord. Oceans. Bergen 10–13 June 2013.
Moline, M. A., Blackwell, S. M., von Alt, C., Allen, B., Austin, T., Case, J., et al. (2005). Remote Environmental Monitoring Units: an autonomous vehicle for characterizing coastal environments. Journal of Atmospheric and Oceanic Technology, 22(11), 1797–1808.
Moon, B., Baker, B. J., & McClintock, J. B. (1998). Purine and nucleoside metabolites from the Antarctic sponge Isodictya erinacea. Journal Natural Products, 61, 116–118.
Ochi, M., Yamada, K., Kawakami, H., Tatsukawa, A., & Kotsuki, H. (1992). Calicogorgins A, B and C, Three bioactive Sphinganine derivatives from the gorgonian calicogorgia sp. Tetrahedron letters, 33(49), 7531–7534.
Pettersen, R., Johnsen, G., Berge, J., & Hovland, E. K. (2011). Phytooplankton chemotaxonomy in waters around the Svalbard archipelago reveals high amounts of Chl b and precence of gyroxanthin-diester. Polar Biology, 34, 627–635.
Roy, S., Llewellyn, C. A., Egeland, E. A., & Johnsen, G. (2011). Phytoplankton pigments (p. 845p). Cambridge: Cambridge University press.
Sakshaug, E., Johnsen, G., & Kovacs, K. (2009). Ecosystem Barents Sea (p. 587p). Trondheim: Tapir Academic.
Sigernes, F., Lorentzen, D. A., Heia, K., & Svenoe, T. (2000). Multipurpose spectral imager. Applied Optics, 39, 3143–3153.
Sorm, F., Benesová, V., & Herout, C. (1954). Uber die struktur des lactarazulens und des lactaroviolins. Collection of Czechoslovak Chemical Communications, 19, 357–364.
Stierle, A. C., Cardellina, J. H., & Singleton, F. L. (1988). A marine micrococcus produces matabolites ascribed to the sponge Tedania ignis. Experientia, 44(11–12), 1021.
Sørensen, A. J., Dukan, Ludvigsen, M., Fernandez, D. D., & Candeloro, M. (2012). Development of dynamic positioning and tracking system for the ROV Minerva. In G. Roberts & B. Sutton (Eds.), Further advances in unmanned marine vehicles (p. 39p). UK: IET.
Takekuma, S., Matsubara, Y., Yamamoto, H., & Nozoe, T. (1988). Autoxidation of Solid Guaizaulene and of the Solution in DMF in the Presence of bace or acid: a comparative study of the product distribution. Bulletin of the Chemical Society of Japan, 61, 475–481.
Volent, Z., Johnsen, G., & Sigernes, F. (2007). Kelp forest mapping by use of airborne hyperspectral imager. Journal of Applied Remote Sensing, 1, 011503–011521.
Volent, Z., Johnsen, G., & Sigernes, F. (2009). Microscopic hyperspectral imaging used as a bio-optical taxonomic tool for micro- and macroalgae. Applied Optics, 48, 4170–4176.
Wesenberg-Lund, E. (1939). Pølseorme (p. 58p). København: Gads.
Wilkinson, C. R. (1987). Significance of microbial symbionts in sponge evolution and ecology. Symbiosis, 4, 135–146.
Zapata, M., Rodríguez, F., & Garrido, J. L. (2000). Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reverse-phase C8 column and pyridine containing mobile phases. Marine Ecology Progress Series, 195, 29–45.
Acknowledgments
The research was founded by The Norwegian University of Science and Technology (NTNU) and Statoil contract number 4501535437 “Underwater Hyperspectral Imager” patent number: US 8502974 B2 (R.P & G.J). Thanks are due to Hans Tore Rapp, University of Bergen for identification of sponges and to the crew of RV Gunnerus and NTNU-AUR-Lab engineer Robert Staven for sampling of material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pettersen, R., Johnsen, G., Bruheim, P. et al. Development of hyperspectral imaging as a bio-optical taxonomic tool for pigmented marine organisms. Org Divers Evol 14, 237–246 (2014). https://doi.org/10.1007/s13127-013-0163-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-013-0163-1