Abstract
The monophyly of the panpulmonate, usually marine benthic Sacoglossa—and its basal division into shelled Oxynoacea and shell-less Plakobranchacea—is undisputed, but family relationships are in doubt. Of particular interest is the potentially basal plakobranchacean family Platyhedylidae, comprising morphologically aberrant members lacking head tentacles or body appendages. Herein we re-describe the type species of the genus Gascoignella, G. aprica Jensen, 1985, from Hong Kong. Morphological data was generated by three-dimensional reconstruction from serial semi-thin sections using Amira software. Our microanatomical results largely confirm the original description. The anterior digestive system is sacoglossan-like but modified, e.g. the ascus is not demarcated externally and pharyngeal pouches are lacking. The digestive gland is bipartite, with two rami separated by a longitudinal, muscular, median septum, but fused in the rear end. The postpharyngeally situated nerve ring contains fused cerebropleural ganglia; the short visceral loop has three ganglia. Two major cerebral nerves were identified as rhinophoral and labiotentacular nerves, innervating sensory areas on the head velum. Gascoignella aprica is a hermaphrodite with a truly androdiaulic genital system of which some originally ambiguous characters are clarified. Bursa and prostate insert into a fertilization chamber proximal to a sac-like albumen gland and a tubular mucus gland. The cephalic copulatory apparatus contains a penis armed with a short and straight stylet and an accessory gland of unclear function; the presumed mode of sperm transfer is discussed. A well-developed heart and a large H-shaped kidney are present; the nephroduct opens into the intestine. Epidermal glands and body tissues are described for the first time. The presence of a unique longitudinal, median septum is considered diagnostic for Platyhedylidae, multiple further apomorphies are given. Morphological evidence supports the molecular phylogenetic hypothesis that the Platyhedylidae could be a basal non-shelled sacoglossan lineage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Molecular phylogenetic studies (e.g. Klussmann-Kolb et al. 2008; Dinapoli and Klussmann-Kolb 2010; Jörger et al. 2010) challenged conventional ideas on euthyneuran gastropod phylogeny. Rather than being divided into monophyletic Opisthobranchia and Pulmonata (e.g. Gosliner 1994; Wägele et al. 2008), previous ”opisthobranch orders“ are distributed over the “new euthyneuran tree” (Schrödl et al. 2011a). The Panpulmonata (Jörger et al. 2010) comprise traditional pulmonates, but also previously lower heterobranch Pyramidellidae and Glacidorbidae, and two “opisthobranch orders”, i.e. Acochlidia and Sacoglossa. The Acochlidia are a modestly diverse taxon of mainly tiny mesopsammic marine slugs (Schrödl and Neusser 2010), but also brackish-water, limnic, and even amphibious species exist (Neusser and Schrödl 2007, 2009; Neusser et al. 2011b; Brenzinger et al. 2011a). The second group, Sacoglossa, are marine or brackish water species, suctorially feeding on algae (Jensen 1981, 1993a,b,c, 1997). The greenish body colouration of most members derives from the chloroplasts of their prey (Clark et al. 1990). The globally known roughly 300 species of Sacoglossa are usually characterised by having an ascus, i.e. a muscular sac storing worn radula teeth, a uniseriate radula used for piercing algae, and an esophageal pouch (Jensen 1991, 1996; Mikkelsen 1996; Wägele et al. 2008).
Because some members share a shell-less body, a uniseriate radula with dagger-like teeth and an androdiaulic genital system, Acochlidia were thought to be closely related with Sacoglossa (e.g. Gosliner 1994; Sommerfeld and Schrödl 2005). The acochlidian Ganitidae with a sacoglossan-type radula, however, were shown to be derived rather than basal acochlidians in both morphology-based and molecular phylogenetic analyses (Jörger et al. 2010; Schrödl and Neusser 2010), and acochlidian and sacoglossan androdiaulic systems differ regarding the relative insertion of the vas deferens, which is proximal in sacoglossans and distal in acochlidians (Schrödl et al. 2011a). The single described mesopsammic sacoglossan species Platyhedyle denudata Salvini-Plawen, 1972, was originally thought to be acochlidian due to features such as a worm-like body with reduced head tentacles, and having separate cerebral and pleural ganglia in a postpharyngeal central nervous system. However, later studies by Jensen (1985), Wawra (1988) and Rückert et al. (2008) showed distinct morphological differences, proved its sacoglossan nature and explained similarities by habitat-induced convergence. In fact, Sacoglossa clustered as sister of various clades with interstitial members (Rhodopemorpha, philinoglossid cephalaspideans and Acochlidia) in a morphocladistic analysis by Wägele and Klussmann-Kolb (2005). This association was shown to be an artefact by all available molecular data (Jörger et al. 2010; Wilson et al. 2010; Schrödl et al. 2011a). Recently, the new family Aitengidae was established for some amphibious “bug-eating slugs” that show mixed sacoglossan and acochlidian features (Swennen and Buatip 2009). However, computer-aided microanatomical three-dimensional (3D) reconstructions and multi-locus sequence marker analysis place the Aitengidae within Acochlidia (Neusser et al. 2011b).
Alternative hypotheses on the origin of Sacoglossa include descendance from cephalaspidean euopisthobranchs. The infaunal sacoglossan Ascobulla, having large radula teeth with long triangular cusps for piercing algae and an ascus for storing worn teeth, apart from pharynx features, closely resembles Cylindrobulla—an equally infaunal genus with small teeth with short triangular cusps and a poorly developed ascus (Jensen 1995, 1996). The latter genus was either considered member of cephalaspideans (Jensen 1996), or the most basal sacoglossan offshoot (i.e. sister of all other sacoglossans) (Mikkelsen 1996, 1998). Molecular data, however, show that Cylindrobulla is an ingroup member of panpulmonate oxynoacean sacoglossans (Mikkelsen 1998; Maeda et al. 2010; Jörger et al. 2010; Göbbeler and Klussmann-Kolb 2011; Neusser et al. 2011b), implying that similarities with euopisthobranch cephalaspideans, such as the head shield used for digging in sand, are secondarily derived (see Malaquias et al. 2009; Brenzinger et al. 2012). “Rampant parallelism” as stated already by Gosliner (1981) to be typical for opisthobranchs thus expands to all euthyneurans and molecular phylogeny shows that even more features are concerned (Schrödl et al. 2011a).
Recent multi-locus studies all place Sacoglossa within Panpulmonata, usually in a rather basal position. Sacoglossa were either recovered as sister to intertidal limpet-shelled Siphonarioidea (Klussmann-Kolb et al. 2008; Dinapoli and Klussmann-Kolb 2010; Jörger et al. 2010), as sister to all non-siphonarioidean panpulmonates (Göbbeler and Klussmann-Kolb 2011), or as sister to those panpulmonates that still bear an operculum (estuarine Amphiboloidea plus parasitic marine Pyramidellidae plus limnic Glacidorbidae) in some analyses by Jörger et al. (2010). The latter relationship, but under a traditional Opisthobranchia concept (for a rebuttal see Schrödl et al. 2011b), was recovered also by mitochondrial genomic sequence data and the group called Siphoglossa (Medina et al. 2011). Siphoglossa, however, were not recovered monophyletic in a comprehensive mitogenomic approach by Stöger and Schrödl (2012).
In addition to the unique ascus there may be more features apomorphic for sacoglossans, but this depends on the assumed origin and inclusiveness of the group. Kleptoplasty, with non-functional chloroplasts stored in body tissue was reconstructed as sacoglossan synapomorphy by Maeda et al. (2010), and retained (or lost) among shelled Oxynoacea. Functional kleptoplasty, retaining chloroplasts and using photosynthetic carbohydrates, according to Händeler et al. (2009), evolved within another major sacoglossan subclade called Plakobranchoidea, comprising sea slugs with dorsolateral prolongations of the foot edges (parapodia). Sacoglossans show a head-foot with usually one pair of longitudinally enrolled tentacles (rhinophores), except for some derived plakobranchaceans that have digitiform or bifid tentacles, or none at all, like intertidal Gascoignella and interstitial Platyhedyle; the latter genera combined into Platyhedylidae (Jensen 1985, 1996; Rückert et al. 2008).
The aberrant family Platyhedylidae comprise very few members, i.e. the mesopsammic Mediterranean Platyhedyle denudata Salvini-Plawen, 1973, the intertidal Gascoignella aprica Jensen, 1985, and two further species from intertidal mudflats of Thailand. Swennen (2001) described G. nukuli and G. jabae based mainly on external and radular rather than microanatomical details. While G. nukuli externally resembles other Platyhedylidae regarding the absence of tentacles and body processes, and in having a pair of digestive gland rami fusing in the rear part, G. jabae differs externally, showing a pair of posterior, elongated cerata. A fourth record of a putative yet unidentified “Gascoignella sp.” depicted in Gosliner et al. (2008: p. 65), rather appears to be an Aiteng. In having the head-foot separated from the visceral sac, shell-less Platyhedylidae (except G. jabae) resemble the shelled Oxynoacea. In morphocladistic analyses, aberrant Platyhedylidae were recovered as rather basal plakobranchacean offshoot (Jensen 1996), with exact systematic position uncertain, or forming an internal Plakobranchoidean branch (Mikkelsen 1998). Molecular multi-locus analyses showed G. nukuli in a basal plakobranchacean position (Jörger et al. 2010). Recent analyses additionally including P. denudata recovered monophyletic Platyhedylidae as a basal plakobranchacean branch (Neusser et al. 2011b). However, taxon sampling is not yet dense enough to reveal the exact origin and inner phylogeny of platyhedylids. Thus, morphology is currently the only available source of information on all known members of this fascinating sacoglossan family, and should be studied and interpreted in the light of modern euthyneuran tree hypotheses.
Gascoignella aprica Jensen, 1985, was described as the type species for the genus Gascoignella in the newly established family Gascoignellidae by Jensen (1985). Going into considerable morphological detail, the more or less conventional nature of the digestive system was recognised, while the complex reproductive system was apparently distinct from any other sacoglossan. The combination of reduced external and complicated reproductive characters did not fit into traditional sacoglossan family level classification. Jensen (1985) also recognised external similarities between Gascoignella aprica and the former acochlidian Platyhedyle denudata, such as the shared presence of a longitudinal septum separating the left and right halves of the visceral cavity. Main distinctive features were the fused versus separate cerebral and pleural ganglia, the absence versus presence of spicules and hermaphroditism versus dioecy. Jensen (1985) doubted P. denudata to be the single sacoglossan with separate cerebropleural ganglia, and predicted that, if ganglia were shown to be fused, the families Platyhedylidae and Gascoignellidae could be merged. Ultimately, Jensen’s (1985) assumption was confirmed by a series of increasingly more detailed morphological re-examinations. The original description of Platyhedyle denudata by Salvini-Plawen (1973) was based on preserved material and squeezing techniques. Wawra (1988, 1991) corrected and supplemented it based on observations of living specimens and paraffin/paraplast based histological serial sections (7–8 μm). Huber (1993) re-examined its central nervous system and showed P. denudata to have the usual sacoglossan type cerebropleural ganglia. Rückert et al. (2008) used resin based histology with computer-aided reconstruction of all major organ systems from serial semithin histological sections (1.5 μm). Central nervous and other features of P. denudata were checked, confirmed or corrected and supplemented by details undetectable from thicker paraffin/paraplast slides. In general, recent 3D microanatomical approaches have shown earlier original anatomical descriptions to be erroneous to a surprising extent, especially referring to small euthyneuran species (e.g. Neusser et al. 2006, 2009b; Neusser and Schrödl 2007; Brenzinger et al. 2012). Careful histological analyses and reconstructing 3D models using AMIRA are well-suited to reveal detailed, accurate and reproducible data (DaCosta et al. 2007), and were applied successfully to lower heterobranchs (e.g. Brenzinger et al. 2011b; Haszprunar et al. 2011), Nudibranchia (DaCosta et al. 2007; Martynov et al. 2011), and Cephalaspidea (Golding 2010; Brenzinger et al. 2012).
This study thus uses modern semithin histological analyses and computer-aided 3D modelling with AMIRA software to explore the microanatomy of Gascoignella aprica in depth. Material collected at the type locality in Hong Kong were embedded in resin and cut into semithin sections. Our aims were to (1) check and supplement the original description of G. aprica, especially focussing on details of the taxonomically and phylogenetically important central nervous and reproductive systems, (2) compare this information with literature data on the microanatomy of Platyhedyle denudata, (3) find potential synapomorphies and evaluate the origin of the supposedly basal sacoglossan family Platyhedylidae on the basis of our novel morphoanatomical data.
Material and methods
The two specimens of G. aprica used in this study were collected by K.R.J. in a tidal mudflat in Tsim Bei Tsui, Deep Bay, Hong Kong in April 1998. They were found crawling on an exposed algal mat at low tide during the day. They were relaxed in an isotonic solution of magnesium chloride, fixed in 4 % neutral formaldehyde and stored in 75 % ethanol. The fixed specimens were stored at the Zoological Museum in Copenhagen (ZMUC, unnumbered; section series 4F3 and 4F4). Specimens were postfixed by transferring them into a 0.01 M cacodylate buffer solution with 1 % osmium tetroxide for 1.5 h, and eluted in cacodylate buffer. Both specimens of G. aprica were decalcified in 1 % ascorbic acid, dehydrated in a graded acetone series (30, 50, 70, 90 and 100 %) and transferred into Spurr’s low viscosity epoxy resin (Spurr 1969). Resin blocks were transformed into ribboned serial sections (1.5 μm) using a diamond knife (Diatome Histo Jumbo, http://www.emgrid.com.au) installed on a rotation microtome (Microm HM 360, Zeiss, Jena, Germany), according to standard procedures (Henry 1977; Ruthensteiner 2008). Slices were stained using Richardson’s stain (Richardson et al. 1960), a 1:1 mixture of methylene blue-azure II stain and distilled water and sealed with Araldit resin (Romeis 1989) under coverslips.
Sections of specimen 4F4 were photographed using a Leica DMRD microscope, and a Spot Insight Color digital camera, (Diagnostic Instruments, http://www.spotimaging.com) and Spot 3.1 software (Diagnostic Instruments). Pictures were saved as .tif at a resolution of 1600 × 1200 pixels and 24-bit colour depth.
The reconstruction of all major organ systems of specimen 4 F4 was done using Amira 5.2.0 (Visage Imaging, Berlin, Germany) following basically the procedures described by Neusser et al. (2006) and Ruthensteiner (2008). Sections of specimen 4 F3 were used for comparison.
An interactive 3D model (accessible through the supplementary material) was compiled according to the procedure described by Ruthensteiner and Heß (2008).
Results
General morphology
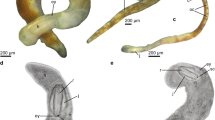
The body (length 2.54 mm) of Gascoignella aprica appears dorsoventrally flattened. It consists of an anterior head-foot complex and a posterior visceral mass, separated from each other by a muscular, transversal diaphragm that forms an externally visible groove (mdg, Fig. 1a). There are no rhinophores, cerata or parapodia. The head-foot complex features a cephalopedal groove, in which the mouth is situated. The body cavity of the head-foot comprises digestive organs (oral tube, oral glands, pharynx, salivary gland and esophagus) as well as the postpharyngeal CNS, a prostate gland and the male copulatory complex (Fig. 1b). The foot is short, wide, ciliated and does not reach far under the visceral sac.
a–e. Anatomical overview, three dimensional (3D) reconstruction of pedal gland and semithin cross-sections of pedal gland and epidermis of Gascoignella aprica Jensen, 1985. a–c 3D reconstructions: a external morphology of specimen 4F4, right view. b Overview of microanatomy showing internal organ systems, right view. c Pedal gland, ventrally encompassing other organs of the head-foot complex. d Cross section of the pedal gland ventral of the central nervous system. e Dorsal epidermis of the visceral sac, * Voluminous “type1” cells, ** greenish “type2” cells. a Anus, bc bursa copulatrix, brm buccal retractor muscle, cns central nervous system, dgl digestive gland, es esophagus, ey eye, ft foot, gcp cephalopedal groove, gg complex of genital glands, go gonad, hf head-foot complex, ht heart, kd kidney, lsg groove caused by longitudinal septum, mdg groove caused by muscular diaphragm, ot oral tube, p penis, pc pericardium, pg pedal ganglion, pgd pedal gland duct, pgl pedal gland, pl pigment layer, psg penial sheath gland, rs radula sheath, sgl salivary glands, subg subintestinal ganglion, usd unconfirmed salivary duct, vg visceral ganglion, vs visceral sac. Bars a, b, c 200 μm; d 50 μm; e 25 μm
The visceral sac is in large part divided longitudinally by a vertical muscular septum. This so-called median septum (Rückert et al. 2008) is less distinct in the anterior part of the visceral sac, where it is penetrated by the intestine, the anterior anastomosis of the digestive gland and the gonoduct. Posteriorly, the septum is penetrated by a second anastomosis of the digestive gland. The septum creates an externally visible, longitudinal furrow on the dorsal side of the visceral sac. The intestine is connected to the digestive gland. The latter is a massive, split organ, which fills in large part both ventral sides of the visceral sac. The dorsal part of the visceral sac is filled with the gonad and a bursa on the left, and an arrangement of male and female glands on the right, i.e. prostate and two nidamental glands. Externally, apart from the mouth, three more openings are visible: the male genital opening under the right eye, the female genital opening laterally in the groove caused by the diaphragm, and the anus lateroventrally on the visceral sac.
Epidermis
The epidermis is rather smooth, consisting mainly of glandular structures and interspersed tegmental cells (Fig. 1e). It is mostly 35–40 μm thick, except for the groove caused by the muscular diaphragm, where the epidermis is thinner (ca. 10 μm). The epidermis of the dorsal and lateral sides of the head-foot and the visceral mass is pigmented. The yellow-to-brownish stained pigment granules are situated apically in the epidermal cells, forming a smooth surface of ca. 8 μm thickness.
There are at least three types of glandular cells in the epidermis:
“Type 1”- glands constitute the largest part of the epidermis. These glands appear to a great extent optically empty (i.e. white), but almost all of them contain spherical to longish, light gray stained and amorphous structures (Figs. 1e and 11e). The cells are bottle-shaped (diameter up to 35 μm) and open to the exterior via an apical pore. This type of gland is prevalent in the dorsal and lateral sides of the visceral-sac and the head-foot, and less frequent in the foot sole and ventral side of the visceral sac.
“Type 2”-glands are medium-sized (diameter up to 20 μm), monocellular and spherical. The light-blue-stained cytoplasm of their cells encloses a greenish stained, amorphous content. The nucleus is stained dark blue. These cells occur in the dorsal and lateral epidermis exclusively. Most of them open to the exterior via a digitiform duct and an apical pore.
“Type 3”-glands (not shown) are formed by accumulations of cells with a diameter of up to 25 μm. These cells contain a blue-stained nucleus and are filled with spherical, violet-stained vesicles. Thin ducts connect these glands to the outside.
In contrast to the dorsal and lateral epidermal cells, the ones of the foot show a dense apical ciliation. The “type 3”-glands prevail in the foot-area.
Anterior pedal gland
In the head-foot complex, a massive, sac like, light violet stained pedal gland is present (Figs. 1c, d, 2b, 7c and 11e). It opens to the outside via a broad, tubular connection (Ø up to 80 μm) to the mouth area, ventrally of the oral tube (Fig. 1c). The gland extends ventrally of the pharynx, the pedal ganglia and the esophagus. The latter two structures are encompassed laterally by the gland. This anterior pedal gland is 320 μm long and has a maximum width of 425 μm. The glandular cells are filled with small spherical granules and stained in different nuances of purple. In the posterior part of the gland, parallel structures with a filamentous appearance can be found. They are stained dark purple (Figs. 7c and 11e).
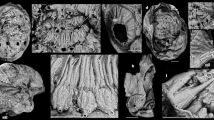
Semithin cross- (a–c) and longitudinal-sections (d) of the digestive system of G. aprica. a Cross section at the middle of the buccal mass; arrows pigment granules. b Posterior end of the buccal mass; note origin of the radula (*) and sourrounding odontoblasts. c Esophagus and esophageal diverticulum, interconnection of the salivary glands (*). d Longitudinal section of the buccal mass of specimen 4 F3 showing the ascending limb of the radula. alr Ascending limb of the radula, amu ascus muscle, brm buccal retractor muscle, dlr descending limb of the radula, es esophagus, esd esophageal diverticulum, og1 oral gland 1, og2 oral gland 2, oph odontophore, pgl pedal gland, phc pharyngeal cavity, psg penial sheath gland, r radula, rn rhinophoral nerve, rs radula sheath, sgl salivary glands, sm septate muscle, vd vas deferens, vn1 visceral nerve 1, wt worn teeth. Bars a–c 50 μm, d 100 μm
Musculature
Body musculature consists of blue staining muscle fibers that either extend through the body or are associated closely to specific organs. A thin sheath of muscle fibers (about 3 μm thick) is situated just below the epidermal basal lamina. Numerous muscle fibers pervade the head-foot area in dorsoventral orientation and cross on the ventral side, forming a basket-like web, in which the inner organs are located. The most complex muscular structures in the head-foot are the buccal mass and the penis (see digestive and genital system, respectively).
There is a paired buccal retractor (about 15 μm high and 25 μm wide) which splits up into two strands anteriorly; one part inserts close to the connection of oral tube and pharynx, the other is attached to the posterior portion of the buccal mass, ventral of the ascus. Further posteriorly the buccal retractor (Figs. 1d and 2b) connects to the diaphragm. This is a transversal, muscular structure, dividing the visceral sac from the head-foot. The diaphragm is up to 20 μm thick and penetrated by the esophagus, the aorta, the vas deferens and some nerves (see central nervous system). Additionally, a paired muscle runs from the anterior part of the buccal mass to the base of the bulge-like structures in the oral tube (not shown).
The median septum, which divides the visceral sac into a right and a left part, is not as distinct as, and thinner (up to 5 μm) than the diaphragm. However, the separation of the digestive gland in a left and a right ramus clearly shows its presence (see Figs. 3 and 4b). Two muscular layers arise from the left and right ventral side of the visceral sac and form the median septum, creating a ventral, hemolymph-filled space between their bases.
Schematic overview over the digestive system of G. aprica. Asterisk indicates position of the longitudinal, median septum. a Anus, bm buccal mass, d diaphragm, dgl digestive gland, es esophagus, esd esophageal diverticulum, it intestine, mo mouth opening, og1 oral gland 1, og2 oral gland 2, ot oral tube, pgo opening of the unknown salivary duct to the pedal gland duct, sgd salivary gland duct, sgl salivary gland, sto stomach, usd unconfirmed salivary duct
a–d 3D reconstruction of the digestive system of G. aprica. a Localization of the digestive system in the specimen, right view. b Ventral view of the digestive system, note branches of the digestive gland, separated by the longitudinal median septum (*). c Buccal apparatus and associated organs, right view. d Digestive system, digestive gland transparent, right posterolateral view, note connection of unconfirmed salivary duct and pedal gland duct (*). a Anus, amu ascus muscle, as ascus, bm buccal mass, dgl digestive gland, es esophagus, esd esophageal diverticulum, it intestine, mo mouth opening, og1 oral gland 1, og2 oral gland 2, ot oral tube, pgd pedal gland duct, ph pharyngeal cavity, r radula, rs radula sheath, sgd salivary gland duct sgl salivary glands, sto stomach, usd unconfirmed salivary duct. Bars a, b, d 200 μm; c 100 μm
Digestive system
The mouth is situated medioventrally in the groove between the head and the foot (Fig. 4a, b). The short oral tube possesses thick columnar epithelial cells with a basal lying nucleus. Close to the pharynx, the oral tube widens, and the wall of the tube forms two lateral notches. These notches comprise bulge-like structures, which enclose the entrance to the pharynx.
Two pairs of adjacent but separate oral glands (Figs. 2a, 3 and 4d) seem to secrete into the posterior lumen of the oral tube, close to the entrance of the pharynx. Both glands (Fig. 2a) contain small, granular vesicles, but they differ in staining, location and size. The bigger glands are stained light grayish, and posses big, darker gray stained nuclei. Due to their unknown function and homology, we name them “oral gland 1”. The second pair of glands (“oral gland 2” herein) contains vesicles of different staining, from grayish to dark blue. Nuclei are visible, but they are much smaller than the ones of “oral gland 1”.
The large and bulbous buccal mass (Fig. 4c) is a prominent structure of the head-foot complex (350 μm long, 190 μm wide and 245 μm high). The pharyngeal cavity (Figs. 2a, d and 4c) is connected anteroventrally to the oral tube and lined with a thin, homogeneously blue stained cuticle. In cross section, the cavity appears nearly triangular in its anterior part, and flattens progressively towards the connection with the esophagus on the posterodorsal side of the pharynx (Fig. 2a). The cavity is encompassed dorsally by a thick and massive muscular structure of alternating bands of different orientation. This septate muscle (Gascoigne 1979) is horseshoe-shaped. On its median sides, facing the odontophore, cells filled with pigment granules are situated. On the ventral side of the cavity, the wedge-shaped, muscular odontophore carries the monostichous radula, and separates the latter in an ascending and a descending limb. The teeth of the radula are formed within the radula sheath by odontoblasts, surrounding the origin of the ascending limb (see Fig. 4c). The odontoblasts appear as dark blue, distinctly bordered cells, with an amorphous, light gray content and dark blue nuclei (Fig. 2b, d). There are 11 teeth in the ascending limb; the descending limb of the radula is surrounded by a muscular layer and carries 9–10 teeth. The descending limb leads to the ascus which is situated between the ascending and the descending limb. The ascus (Fig. 4c) is an epithelium-lined, sac-like structure on both sides of the descending limb. In this structure (110 μm long, 125 μm wide and 70 μm high), used teeth are stored in a heap without orientation (Fig. 2b). The ascus is surrounded by odontophoral musculature in the posterior part of the pharynx and therefore might be hardly discernable in a macroscopic dissection.
The paired, flat and elongated salivary glands are situated on both sides of the esophagus. They are interconnected in the space between esophagus and esophageal blind sac (Figs. 2c and 3). The right salivary gland is markedly longer (390 μm) than the left one (260 μm). The former encompasses the whole anterior half of the esophagus while the latter does not reach that far anterior. The salivary glands have a central, not ciliated lumen surrounded by dark stained, glandular mass. The cells are orientated radially around the lumen and contain colourless vesicles as well as purple or dark blue stained ones (Figs. 2c and 7b). Both salivary glands are connected to the pharynx via the thin salivary ducts (Ø 15 μm) that emerge on the anterior tip of the glands and enter the pharyngeal cavity right and left lateral of the esophagus (Figs. 4c and 7b). According to the different size of the glands, the left salivary duct is much longer than the right one. Before they enter the pharynx, the ducts widen to a bulbous structure (Ø 25 μm). An additional duct was found to emerge from the left salivary gland. This duct (Ø 20–25 μm) is strongly coiled and runs anteriorly alongside the pharynx (Fig. 4d), until it connects with the opening of the pedal gland (see above). Due to its unclear affiliation and function, the duct is called “unconfirmed salivary duct” herein.
The esophagus (Figs. 2c, 3 and 4d) emerges from the posterodorsal area of the pharynx. It consists of three specifiable parts. The most anterior portion is a short, narrow, ciliated duct that abruptly widens into a voluminous, bulbous, tubular structure, lined with an epithelium consisting of large, elongated cells. The interior is slightly filled with granules of different size, shape and staining properties. Shortly afterwards, the esophagus is constricted vertically and divided into the left lateral esophageal diverticulum (Figs. 2c and 4d), and the right lateral part, that is tubular and narrows progressively on its way to the diaphragm. The epithelium of the blind sac differs from the epithelium of the tubular part of the esophagus by being thicker. The content of the blind sac does not seem to differ from the rest of the esophagus. Immediately after passing through the transversal diaphragm, the esophagus enters the small stomach (Fig. 4d), the epithelium of which is folded and covered densely with cilia. The digestive gland (Figs. 3 and 4a, b, d) is the most voluminous organ situated in the visceral sac. The gland stretches from the diaphragm to the posterior end of the animal, and is divided by the median septum, so that two longitudinal main branches, which only anastomose anteriorly and posteriorly, are formed (Fig. 4b). The left branch joins the stomach on its posterior end ventrolaterally on the left, the right branch on the same level in a right, dorsolateral position. Both branches possess short side branches at regular intervals, leading to distinct lobes that extend alongside the integument to the dorsal side. In this way, the other major organs of the visceral sac (i.e. posterior genital glands on the right, hermaphrodite gonad on the left side) appear enclosed by the two branches of the digestive gland. Shortly anterior of the connections to the digestive gland, set on the right side of the stomach, the intestine (Fig. 4d) arises and forms a curve over the posterior genital glands. Shortly distal of the top point of the arch, the intestine unites with the nephroduct, and alongside a side branch of the kidney, it runs to the anus.
Central nervous system
The CNS (Figs. 5, 6 and 7) is euthyneurous and consists of a circumesophageal, postpharyngeal ring of paired cerebropleural and pedal ganglia, twice connected to each other, and a visceral loop with three ganglia of different size (Fig. 6). After the nomenclature of Haszprunar (1985) and Sommerfeld and Schrödl (2005), ganglia are named as left parietal, subintestinal/visceral and right parietal/supraintestinal ganglion, respectively. Alternatively, under a triganglionate hypothesis (see Dayrat and Tillier 2000), ganglia would refer to subintestinal, visceral/genital and supraintestinal ganglion, respectively. Additionally there are paired buccal ganglia within the circumesophageal ring, slightly anterodorsally of the pedal commissure.
a–f 3D reconstruction of the central nervous system (CNS) and main nerves of G. aprica. Optic and static nerves could not be detected. a Localization of the CNS in the specimen, right view. b Anterior view of the CNS, legend of pedal nerves omitted. c Left lateral view of complete CNS. d Right lateral view of complete CNS. e Posterior view of the complete CNS, connective of left parietal ganglion and fused subintestinal and visceral ganglion (*), connective of fused right parietal and supraintestinal ganglion and fused subintestinal and visceral ganglion (**). f CNS and encompassed digestive system right lateral view. bg buccal ganglion, bm buccal mass, cpc cerebropedal connective, cpg cerebropleural ganglion, dgl digestive gland, es esophagus, esd esophageal diverticulum, ey eye, ln labiotentacular nerve, ot oral tube, pan parietal nerve, pag parietal ganglion, pg pedal ganglion, pn1 pedal nerve 1, pn2 pedal nerve 2, pn3 pedal nerve 3, ppc pleuropedal connective rhg rhinophoral ganglion, rn rhinophoral nerve, sc statocyst, spn supraintestinal nerve, subg subintestinal ganglion, supg supraintestinal ganglion, vg visceral ganglion, vn1 visceral nerve 1, vn2 visceral nerve 2. Bars a, f 200 μm; b–e 100 μm
Schematic overview of the CNS of G. aprica, dorsal view. Connectives of the cerebropleural ganglia to the rest of the visceral chord not to scale. Optic and static nerve not found. bg Buccal ganglion, cpg cerebropleural ganglion, ey eye, ln labiotentacular nerve, pag parietal ganglion, pg pedal ganglion, rhg rhinophoral ganglion, rn rhinophoral nerve, sc statocyst, subg subintestinal ganglion, supg supraintestinal ganglion, vg visceral ganglion
a–c Semithin cross-sections showing aspect of the CNS and the eyes of G. aprica. a Cross-section through right eye showing layers. b Arrangement of several ganglia, buccal ganglia showing cortex and medulla, pedal ganglion with statocyst. c Left parietal ganglion with giant nerve cells, fibers of the pedal commissure. bg Buccal ganglion, ct connective tissue es esophagus, gnc giant nerve cell, le lens, pag parietal ganglion, pan parietal nerve, pco pedal commissure, pg pedal ganglion, pgl pedal gland, pgr layer of granular pigment, rs radula sheath, sc statocyst, sgd salivary gland duct, sgl salivary gland, spc connective of left parietal ganglion and fused subintestinal and visceral ganglion, ssc sensory cells, supg supraintestinal ganglion. Bars a 25 μm; b, c 50 μm
All ganglia are surrounded by a thick layer of connective tissue (Fig. 7c). They can be subdivided into an outer cortex that contains the cell bodies with dark blue stained nuclei, and an inner medulla that contains only nerve fibers. Therefore the medulla has the same histological appearance as nerves and connectives (i.e. light blue stained). Giant neurons are present in all major ganglia (Fig. 7c).
The paired, oval-shaped and totally fused cerebropleural ganglia (ca. 120 μm high, 130 μm wide and 110 μm long) are placed side by side, dorsally of the posterior end of the buccal mass. They are connected via a short and strong commissure (Fig. 5b). From each cerebropleural ganglion, there are two connectives to the pedal ganglion; the cerebropedal connective is placed more anterior than the pleuropedal connective (Fig. 5c). The short, double-rooted cerebrorhinophoral connectives emerge from the anterior side of each cerebropleural ganglion and lead to the small rhinophoral ganglion. The rhinophoral nerve ramifies into several branches. The labiotentacular nerve emerges from the anteroventral side of the cerebropleural ganglion, and also ramifies. Rhinophoral as well as labiotentacular nerve run anteroventrally.
Posteriorly, the cerebropleural ganglia connect to the visceral loop (Fig. 5e); beginning on the left, there is a short connective to the left parietal ganglion which is the smallest ganglion on the visceral loop. It is 45 μm high, 70 μm wide and 50 μm long, and situated adjacent to the posteroventral tip of the left cerebropleural ganglion. The left parietal nerve emerges from its posterior end and runs posteriorly along the left side of the esophagus and its diverticulum. A 40 μm long connective leads to a large, fused subintestinal/visceral ganglion, which is the biggest of the visceral loop (85 × 130 × 80 μm). It is located posterior of the left pedal ganglion, ventral of the esophagus. Two thick nerves emerge from the subintestinal/visceral ganglion and run posteriorly very close to each other, first on the ventral side of the esophagus, and then surrounded by esophagus, its diverticulum and the penial sheath gland (see Genital system). They penetrate the diaphragm dextral of the esophagus, and cling on the distal part of the oviduct. Shortly afterwards, they separate into a ventral and a dorsal branch, which run alongside the albumen gland, and the mucus gland, respectively (for gland nomenclature see Discussion).
The longest connective of the visceral loop (125 μm) connects the subintestinal/visceral ganglion and the right parietal/supraintestinal ganglion, running diagonally from ventral to lateral of the esophagus. The right parietal/supraintestinal ganglion is medium-sized (65 × 80 × 75 μm), and situated directly behind the right cerebropleural ganglion, to which it is connected via a very short connective. No other ganglia (i.e. genital or osphradial ganglia) connected to the right parietal/supraintestinal ganglion were detected, although there is a strong nerve pointing posterior and penetrating the diaphragm. Unfortunately, this nerve could not be followed further.
The pedal ganglia (105 × 130 × 105 μm) (Figs. 5b–f and 7b) are almost as large as the cerebropleural ganglia, but their commissure (Figs. 5e and 7c) is slightly longer and bridges over the posterior tip of the ascus that is located between them. Three nerves emerge from each ganglion. One (pn1) emerges on the anterior, ventral side and runs anteriorly into the foot. The second (pn2) emerges on the anterodorsal side, shortly posterior of the pleuropedal connective and lateral to the statocyst. It runs to the dorsal posterior area of the foot. The third nerve (pn3) emerges from the posteroventral end of the ganglion, passes partially through the pedal gland and runs posteriorly in the foot. On top of each pedal ganglion there is a spherical statocyst (Ø 20 μm) located mediodorsally. A single, spherical but dissolved structure may refer to remainders of a statolith. A static nerve could not be detected.
The paired buccal ganglia (52 × 65 × 50 μm) (Figs. 5b, e and 7b) are located at the posterodorsal end of the pharynx, flanking the emerging esophagus. They are connected to each other via a short commissure. The connection to the cerebropleural ganglia is short and emerges from the anterior tip of the buccal ganglia.
The eyes (Figs. 5b and 7a) are nearly spherical (Ø 65 μm), and are situated lateral to the anterior part of the pharynx (i.e. prepharyngeal) in the front of the head. They are structured in several layers. The outermost layer is a brownish colored, grainy pigment layer, which forms a retinal cup. It clasps around a convex, hyaline and acellular lens with a dark blue-stained border and a brownish interior that has grainy areas. Between the lens and the pigmented layer there is a light blue coloured layer that seems to be detached from the pigmented layer. Neither optical nerves nor accessory ganglia could be detected.
Circulatory system
Gascoignella aprica possesses a monotocardian heart consisting of a thin-walled posterior auricle and a thicker-walled, anterior ventricle (Fig. 8b). The heart, situated in the dorsoanterior, median part of the visceral sac, is surrounded by a pericardium (Fig. 8c). The auricle is fed by a single sinus that runs anteriorly, starting at the end of the visceral sac. It penetrates the pericardium on its posterior, dextral side and leads to the auricle via a short and narrow, venous vessel. This vessel appears closely attached to the right branch of the kidney, so does the auricle in its posterior area. The auricle (125 μm long and 200 μm wide) is very thin-walled, its light grey-stained epithelium is flimsy and highly folded.
a–f 3D reconstruction of the circulatory and excretory system, semithin cross sections of the excretory system of G. aprica. a Localization of the circulatory and excretory systems in the specimen, right view, note vertical branch of the kidney along the intestine (*). b Right lateral view of the circulatory system. c Circulatory and excretory systems, dorsal view, note H-shape of kidney. d Ventral view of circulatory and excretory systems, note vertical branch of the kidney (*), blind end of the nephroduct (**) and connection of nephroduct and intestine (arrow). e Nephroduct and adjacent structures. f Connection of nephroduct and intestine, arrows indicate the most distal portion of the nephroduct, characterized by the granular pigment. a Anus, alg albumen gland, ao aorta, au auricle, dgl digestive gland, it intestine, kd kidney, nd nephroduct, pc pericardium, vt ventricle. Bars a, c, d, 200 μm, b 100 μm, e 50 μm, f 25 μm
The auricle enters the bigger ventricle (260 μm long and 240 μm wide). Its content appears extremely homogenous, hyaline and of grey color. Dark blue stained, roundish cells are interspersed in the lumen of auricle and ventricle and attached to their epithelia. In phase contrast they look red.
The anteroventral tip of the ventricle leads to the aorta (Fig. 8b). It passes the arch of the intestine on the ventral side, penetrates the diaphragm, and runs further anterior on the dorsal side of the esophagus. At the anterior end of the esophagus it branches several times, the hemolymph seems to flow freely over the central nervous system.
Excretory system
The H-shaped kidney (Fig. 8c) is characterized by its spongy, highly vacuolated and light-stained tissue. It is located dorsally in the anterior two-thirds of the visceral sac, mostly directly under the integument. Both posterior branches are equally long (ca. 600 μm). The left and the right branches are connected posterior of the pericardium, their connection is 75 μm wide. The anterior branches encompass the pericardium laterally. The right anterior branch (length 540 μm) extends much further anterior than the left one (340 μm), sends a vertical branch alongside the intestine (Fig. 8a, d) and additionally encloses the pericardium anteriorly. A potential connection between the pericardium and the kidney (i.e. a renopericardial duct) shows no ciliation and therefore could not be definitively confirmed as such. The nephroduct emerges shortly posterior of the interconnection of the kidney branches. In its most proximal part, a small portion of the nephroduct is situated ventral of the left branch of the kidney, but does not connect to the latter (see Fig. 8d). The nephroduct runs alongside the ventrolateral right side of the kidney and unites with the intestine (Fig. 8f). Consequently, there is no discrete nephroporus visible from the outside. The cells of the nephroduct wall contain small granules of pigment (Fig. 8e, f).
Genital system
The androdiaulic genital system of G. aprica consists of a posterior arrangement of a hermaphroditic gonad, a bursa and three voluminous glandular structures of which one, the prostate, is connected by the vas deferens to an anterior, cephalic copulatory apparatus (Figs. 9, 10 and 11). The glandular structures are situated on the right side of the median septum. They are vicinal to each other, and twist slightly around their common axis (Fig. 10d)
Schematic overview of the genital system of G.aprica. Asterisk indicates opening of prostate into fertilization chamber. alg Albumen gland, amp ampulla, bs bursa stalk, bu bursa, fc fertilization chamber fgo female genital opening, gd postampullar gonoduct, gdp praeampullar gonoduct, go gonad, mgl mucus gland, mgo male genital opening, od oviduct, p penis, pr prostate, ps penial sheath, psg penial sheath gland, st hollow stylet, vd vas deferens
a–d 3D reconstruction of the genital system of G. aprica. a Localization of the genital system in the specimen, right view. b Left view of copulatory apparatus and adjacent ducts; arrows connections to the respective glands, asterisk connection of penial sheath and unknown penial sheath gland. c Ventral view of the genital system. d Dorsal view of the genital system. alg Albumen gland amp ampulla, bu bursa, bs bursa stalk, fc fertilization chamber, fgo female genital opening, gd postampullary gonoduct, gdp preampullary gonoduct, go gonad, mgl mucus gland mgo male genital opening, od oviduct, p penis, pr prostate, ps penial sheath, psg penial sheath gland, st hollow stylet, vd vas deferens. Bars a–d 200 μm
Semithin sections through the genital system of G. aprica. a, cross-section through the visceral sac and overview of the genital glands, arrow indicates connection of genital gland 2 and the oviduct; b, ampulla with tightly stored autosperm; c, putative prostate d, penial sheath gland; e, cross-section through the penial sheath and the penis, asterisk indicates a very common “type 1”-gland. alg Albumen gland, amp ampulla, au auricle, cm circular muscle layer, dgl digestive gland, es esophagus, esd esophageal diverticulum, go gonad, kd kidney, mgl mucus gland, od oviduct, p penis, pc pericardium, pgl pedal gland, pr prostate, prl lumen of prostate, ps penial sheath, psg penial sheath gland psl lumen of penial sheath, st hollow stylet, vd vas deferens, vn1 visceral nerve 1, vn2 visceral nerve 2. Bars a 250 μm; c 25 μm; b, d, e 50 μm
The gonad fills nearly the whole upper half of the left side of the visceral sac (Figs. 10d and 11a). Its total length is 1.64 mm. It is externally sac-like, but consists of several consecutive gonad lobes, which connect by their branching lumina. The gonad contains mature sperm and some yolky oocytes, as well as early stages of gametes. The oocytes contain granules of different size and color. There are small, blue or greenish colored yolk granules as well as big, optically empty (i.e. whitish) vesicles. The nucleus appears grayish, a nucleolus was not detected. Autosperm appear tightly packed in a parallel position, sometimes the heads are arranged around cells that could be nurse cells.
About one-third of its length from the anterior end, the gonad gives off a ciliated duct (Ø up to 55 μm), i.e. the proximal gonoduct that widens to the ampulla (ca. Ø125 μm), which is filled with autosperm (Fig. 10b). The ampulla runs anteriorly dorsal of the digestive gland, before it descends beside the longitudinal septum and narrows into a postampullary gonoduct. Ventrally along the anterior anastomosis of the digestive gland, the ciliated gonoduct ascends again and forms a cavity (Fig. 9) on the right side of the longitudinal septum. This cavity (herein called fertilisation chamber) separates the female and male genital systems. Four other ducts originate from the densely ciliated walls of the fertilisation chamber; the bursa stalk, the prostate, the proximal oviduct, and the vas deferens.
The bursa stalk (Ø approx 25 μm) (Fig. 10b–d) is a narrow, coiled and ciliated duct that connects to a roundish vesicle (260 × 190 × 170 μm; hereafter named bursa). It is located on the left side of the visceral sac, anterior to the gonad, and is enclosed by the left branch of the digestive gland, the right branch of the kidney and the intestine. It extends anterior to the diaphragm. Its voluminous, spherical lumen contains a grayish, hyaline mass with several blue-stained dots. The lumen itself is colored light pink. The wall is about 15 μm thick and partly vacuolated in a spongy way. No cilia are visible on the inside of this structure.
The prostate (Figs. 9, 10d and 11c) is 1.45 mm long, generally blue stained, sac like, with a central, circular and ciliated lumen that reaches to the very proximal end of the gland. The cells contain small grainy, dark blue stained vesicles as well as bigger aggregations of blue stained material. The nuclei tend to be found basally.
The ciliated oviduct (Ø ca. 45 μm) (Figs. 10a, b, d and 11a) runs posterior for approximately 200 μm, then extends into a conspicuous loop, and runs anteriorly again; 75 μm after the loop, the lumen of gland two enters the oviduct dorsomedially.
The albumen gland (Figs. 9 and 10a, d) is a multiple-lobed and flat gland. In its distal area there is only one, medium-sized, ciliated lumen that connects to the proximal oviduct. More proximally, the lumen branches several times. The cells surrounding the lumina contain numerous large, round, and very dark stained vesicles. The total length of the gland is approximately 1.3 mm. Following the oviduct for 135 μm further ahead, there is the connection to gland three (the distal oviduct, see below).
The mucus gland (Figs. 9, 10a, c and 11a) is a tubular folded gland, enclosing a broad, flat and ciliated lumen. Its most proximal part is connected to the oviduct and its distal end leads to the female genital opening, therefore it functionally constitutes the distal oviduct. The lumen is surrounded by elevated, columnar cells with a basal lying, blue stained nucleus. The cells are filled with small granular vesicles of a darker violet color. The gland per se is stained in different nuances from violet to pink. The dorsal part runs posteriorly and makes a U-turn. The ventral part runs a little further anterior almost to the diaphragm. At the distal end of the gland, the lumen opens to the ciliated female genital opening which is situated lateroventrally in the groove between foot and visceral sac.
The narrow coiled and densely ciliated vas deferens (Ø ca. 25 μm) connects the posterior genital system to the cephalic copulatory apparatus (Figs. 9 and 10b, d). Emerging most anteriorly on the ventral side of the fertilisation chamber, the vas deferens is muscular along its entire length. It penetrates the diaphragm, runs anteriorly alongside the dextral side of the esophagus and enters the penis sheath at its dorsal, anterior end. No glandular part of the vas deferens was detected. The copulatory apparatus (Figs. 10b and 11d, e) is located within the penis sheath and consists of a muscular penis (ca. 210 μm in length), armed with a straight and thin, hollow stylet (ca. 65 μm in length). The circular muscle layer within the penis is about 25 μm thick. The penis sheath opens on the right side of the animal into an extension of the cephalopedal groove (Fig. 10a). On its posterior end, the lumen of the penis sheath is connected to a conspicuous, nearly spherical structure (330 μm in length, 300 μm in diameter, “penial sheath gland” herein) situated on the ventral side of the esophagus. It is lined with thick and fringed epithelium of glandular cells (Fig. 11d). The elongate cells contain cytoplasm of blue color and grayish-white vesicles. There are no cilia and not a trace of sperm. The lumen of the penial sheath gland is connected to the exterior via the penis sheath. No other connected glandular structures or ducts could be detected.
Discussion
General morphology
In her description of Gascoignella aprica, Jensen (1985) examined numerous specimens that were found crawling on exposed algal mats in a tidal mudflat in Deep Bay, Hong Kong in 1983. Anatomical results came from dissection and serial sections.
We can confirm the general morphology information given by Jensen (1985), comprising a very flat body with no traces of rhinophores, cerata or parapodia, and the head-foot very distinctly set off from the visceral mass. A head-foot separated from the more or less freely projecting visceral sac occurs among plakobranchacean sacoglossans only in G. aprica, G. nukuli, and P. denudata, but is common state in shelled Oxynoacea. The special body shape of shell-less G. aprica and Platyhedyle otherwise resembles acochlidian panpulmonates, a fact that has lead to confusion regarding the systematic placement of Platyhedylidae (Salvini-Plawen 1973 versus Wawra 1988, 1991). Several differences between Platyhedylidae and acochlidians were summarised by Rückert et al. (2008). An additional difference not found in any known acochlidian is the very short but broad foot of Gascoignella, not reaching under the visceral sac. Jensen (1985) also stated the median position of the mouth in a cephalopedal groove, which forms two distinct notches lateroventrally on the anterior end of the head. The “penial opening” was reported to be situated in the notch shortly posteroventral of the right eye (Jensen 1985). This position can be confirmed herein. A female genital opening could not be detected in the relaxed specimens, only the dextral, lateroventrally located anus was mentioned to open to the groove between the foot and the visceral mass (see also Jensen 1991). No separate renal pore was observed. In this study, however, the female genital opening (oviductal opening) was found in the groove caused by the muscular, transverse diaphragm, and the anus was found to open posteriorly to this groove on the lateroventral surface of the visceral sac. Due to the fact that the nephroduct unites with the intestine inside the body the absence of an externally visible renal pore can be confirmed.
Body wall
Jensen (1985) described G. aprica to be entirely black on the dorsal surface, with a yellowish-white foot sole and a green-appearing ventral surface of the visceral sac, due to the green content of the digestive gland. The eyes were stated to be surrounded by a yellowish transparent area. In this study, we also explored the composition of the epidermis. A layer of 8 μm on average of pigment granules was found to cover the dorsal and lateral sides of the animal. Numerous glandular structures were found, opening to the exterior via apical pores. As the animals were described to crawl most often on algal mats in the open sunlight, we suggest that the thick dorsolateral epidermis with a pigmented layer and a large number of glands forms an adaptation to semiterrestrial life. Like all Plakobranchacea, G. aprica does not possess a shell, which means the epidermis constitutes a protective barrier against environmental stress. The semiterrestrial habitat of a tidal mudflat is characterized by continuously changing intensities concerning moisture, salinity, temperature and UV-radiation. Adaptations to this environment thus may have lead to the special external morphology (i.e. compact shape, lack of tentacles) in G. aprica, which is shared by some other amphibiously living plakobranchaceans as well [e.g. Alderia modesta (Loven, 1844)] or the amphibious, “bug-eating” acochlidian slug Aiteng ater Swennen and Buatip, 2009 (Neusser et al. 2011b: 332). Aiteng ater differs from other acochlidians in habitat and shape; in particular a thickened epidermis with underlying spongy tissue with large vacuoles obviously gives the soft notum some stability. We show that G. aprica also has a thick layer of notal tissue with spacious vacuolated appearance. From a histological point of view, the voluminous “type 1”-glands described in the results resemble the leftover cavities of spicules after decalcification (see, e.g. Brenzinger et al. 2011b). Spicules are characteristic for interstitial heterobranch gastropods like Acochlidia and Rhodopemorpha (Rieger and Sterrer 1975; Arnaud et al. 1986; Salvini-Plawen 1991), but have also been described for P. denudata (Rückert et al. 2008). However, leftover cavities found in G. aprica are probably not from spicules. Spicules usually leave cavities that are rather small and do not form aggregations that could be responsible for optically empty cavities found in G. aprica. More likely, holes are leftovers of big vacuoles in cells that build a resistant notal integument under the epidermis. Similar spongy layers of connective tissue are also known from the marine intertidal nudibranch Corambe lucea Marcus, 1959, where large vacuoles were confirmed by SEM examination (Schrödl and Wägele 2001). In the latter species, pairs of dorsoventral muscle bundles were suggested to enhance the sucking ability of the foot, but also keep the notum in shape and help the body wall musculature generating hemolymph pressure. We suggest that in Gascoignella aprica the dorsoventral longitudinal median septum in the visceral hump has a similar stabilising function.
Digestive system
The digestive system of G. aprica comprises a short oral tube, a well developed buccal apparatus with monostichous radula and the ascus, and an esophagus that widens to form two parallel swellings, only the right one penetrates the diaphragm to the stomach. The latter has a connection to the left and right ramus of the digestive gland, respectively, and a connection to the intestine that runs to the lateroventrally situated anus, shortly posterior of the muscular diaphragm on the right side of the animal. These findings largely agree with Jensen’s (1985) description. However, it is the right rather than the left part of the esophagus that unites with the stomach, and the anus opens on the lateroventral surface of the visceral sac rather than in the furrow between head-foot and the visceral sac built by the diaphragm. In this study, the “buccal glands” described by Jensen (1985) were found to be actually two paired and histologically different oral glands. Homology of these glands is unclear, and histological differences could occur due to different functional phases.
The bulge-like structures enclosing the entrance to the pharynx could have a function during feeding, for example as a kind of sphincter. As they are very small, they do not seem to be evertable. They might comprise a structure also referred to as “inner lips” in a schematic drawing in Jensen (1993a: fig. 1).
The digestive system redescribed for G. aprica herein is compared with the usual sacoglossan type as it has been examined in detail in several studies (Jensen 1980, 1981, 1991, 1993a, b, c, 1996, 1997). Generally, the suctorial pharynx of Sacoglossa is composed of four muscular units: (1) the dorsal septate muscle, (2) the odontophore, (3) the ventral, longitudinal ascus muscle, and (4) the pharyngeal pouch. The existence of muscles mentioned in point 1, 2 and 3 could be confirmed (see Fig. 4c), but a pharyngeal pouch is absent in G. aprica; the lack of a pharyngeal pouch corresponds to fig. 4F in Jensen (1991).
All known Sacoglossa have a uniseriate radula with an ascending and descending limb and an ascus where discarded teeth are stored. In this study, 11 teeth in the ascending limb, and 9–10 teeth in the descending limb were counted. This fits well with originally described 8–9 teeth in the ascending limb and 12 teeth in the descending limb. Jensen (1985) found at least 20 teeth stored as a heap in the ascus. This and other radula features that Jensen described (i.e. shape of teeth) cannot be efficiently studied from semithin serial histological sections and 3D-models.
The epithelium-lined ascus is a sacoglossan innovation. The ascus retains all radular teeth formed throughout the life of the animal (Jensen 1996). In G. aprica, the ascus and the descending limb are attached to the buccal mass (and not demarcated externally). In the reconstructed specimen, the ascus is positioned dorsal of the descending limb, connected to the latter at the posterior end, which is an unusual arrangement within the Sacoglossa. In most species the ascus is located outside the pharyngeal musculature (Jensen 1993a, b, c).
The buccal mass of G. nukuli differs markedly from that of G. aprica; Swennen (2001, fig. 1f) indicated large muscular swellings in the ventral part of the pharynx. The buccal mass of P. denudata possesses an identical, ventral muscular swelling (own observation) and thus seems to be more similar to G. nukuli than to G. aprica. There is thus a considerable variation in pharynx morphology among Platyhedylidae that might correspond to different food types. Gascoignella aprica is assumed to feed upon macroscopic intertidal filamentous chaetomorphal algae (Jensen 2003), while the only macroscopic algae available in the mesopsammic habitat of Platyhedyle denudata are stolons of Caulerpa spp. (M. Schrödl, own observation). In contrast, G. nukuli is assumed to prey upon subterranean branches of Derbesia marina or green micro-algae (Swennen 2001). Therefore, microalgae sucked in by the putative ventral sucking pump should be explored as a potential food source for Platyhedyle denudata.
The existence of salivary glands, corresponding paired ducts and reservoirs can be added to the description of G. aprica, as well as an enigmatic third, unpaired, duct that appears to emerge from the left salivary gland and connects to the pedal gland tube. A similar structure is missing in G. nukuli and P. denudata. The exact connectivity, homology and function of this duct are unclear but merits further research.
An esophageal pouch occurs in many sacoglossans [e.g. Elysia timida (Risso, 1818)] and differs in size as well as in muscular and glandular components (Jensen 1996). The esophageal diverticulum (= pouch) found in G. aprica has a thicker, more glandular epithelium than the esophagus, but is not lined by a remarkable muscular layer. A similar esophageal pouch is present in G. nukuli (own observations), but was not mentioned for P. denudata (see Rückert et al. 2008).
As in most sacoglossans, the stomach of G. aprica constitutes a rather small and simple part of the digestive system. While in many non-shelled Sacoglossa there appears to be a trend of enlargement of the surface area of the digestive gland (Jensen 1991), G. aprica only has short lobules on the long, wide, main ducts of the digestive gland. This character is shared with Limapontia and Platyhedyle, both of which do not have any cerata (Jensen 1996). The lobules of the digestive gland of G. nukuli and P. denudata seem more elaborated (own observations).
Since the intestine unites with the nephroduct at about two-thirds of its length, the distal part of the intestine functions as a joint rectum and nephroduct. The position of the anus was stated to be located in a groove on the right side of the head as in Bosellia and some Elysia spp. (Jensen 1996). As mentioned above, it is actually situated shortly posterior on the right ventrolateral side of the visceral sac.
The general arrangement of the digestive system of G. aprica is typical for Heterobranchia in its simplicity of e.g. the esophagus/intestine (Ponder and Lindberg 1997), but shows sacoglossan innovations that were modified in the different platyhedylid species to a variable extent, thus showing taxonomic and potential phylogenetic significance.
Central nervous system
The CNS of G.aprica consists of a postpharyngeal nerve ring of paired cerebropleural and pedal ganglia. The fused nature of the cerebral and pleural ganglia is shown by the fact that there are two connectives to the pedal ganglia per side. Paired rhinophoral and buccal ganglia are connected to the cerebropleural ganglia. This condition already stated by Jensen (1985) was also found in Platyhedyle denudata by Rückert et al. (2008) and is usual among sacoglossans. Precerebral accessory ganglia are present in mesopsammic P. denudata, many acochlidians (Schrödl and Neusser 2010), cephalaspidean Pluscula cuica Marcus, 1953 (Brenzinger et al. 2012) and rhodopemorphs (Brenzinger et al. 2011b), but were not found in intertidal G. aprica. This supports assumptions that accessory ganglia are an adaptation to mesopsammic habitats that independently evolved in many lineages. Giant neurons as found in G. aprica, however, are absent in P. denudata; they might have been lost due to body size reduction in mesopsammic species.
Rhinophores and other head tentacles are missing in G. aprica, but rhinophoral and labiotentacular nerves are present. Both major head nerves show multiple peripheral ramifications, thus indicating their sensory function in the head epithelium without forming distinct tentacles. Rhinophoral ganglia are connected with the cerebral ganglia by double nerves as found in many other panpulmonates (e.g. traditional pulmonates with procerebrum, but also several acochlidians; Neusser et al. 2007; Schrödl and Neusser 2010), possibly the mesopsammic cephalaspidean Pluscula cuica (Brenzinger et al. 2012), and rhodopemorphs (Brenzinger et al. 2011b).
Eyes in the examined specimens are well-developed and in an anterolateral position rather than situated in a more central position as in P. denudata. Similar laterally situated eyes were found in the amphibious acochlidians of the genus Aiteng (Swennen and Buatip 2009, Neusser et al. 2011b). Aiteng ater as well as G. aprica show clear areas above the eyes devoid of the pigment granules otherwise found in the dorsal integumental cells. Pigmentation can be explained as adaptation to semiterrestrial life and potential exposure to the sun.
The visceral loop of G. aprica is short and ganglionate as was observed by Jensen (1985); histology shows that there are three distinct ganglia of different sizes. The first ganglion on the left is smaller than the others, and bears a nerve leading to the body wall; it is thus identified as the left parietal ganglion. The second, most posteriorly situated ganglion is the largest; it can be identified as containing the subintestinal and visceral ganglia, since it bears two strong nerves running to the visceral mass. A genital ganglion as found on the visceral nerve of P. denudata by Rückert et al. (2008) was not detected in G. aprica. The right ganglion corresponds to the supraesophageal ganglion that connects to the putative osphradial ganglion in P. denudata (see Rückert et al. 2008). In G. aprica, the emerging nerve was detected, but no ganglion was found. There is no direct evidence on the existence and position of a right parietal ganglion in G. aprica. According to the Pentaganglionata hypothesis (Haszprunar 1985), euthyneurans show five separate visceral loop ganglia that may fuse during later ontogeny. No ontogenetic data is available on G. aprica, but interpreting the three ganglia according to their relative sizes as left parietal ganglion, fused subintestinal and visceral ganglion, and fused supraintestinal and right parietal ganglion fits with the Pentaganglionata idea. Nevertheless, this concept was criticised by Dayrat and Tillier (2000), and reliable pentaganglionate species, i.e. rhodopemorphs and acteonids, were shown to occur also outside of Euthyneura recently (Brenzinger et al. 2011b; Schrödl et al. 2011a). Thus, if there is a pentaganglionate condition involved, the concept may apply to a more inclusive group of heterobranchs rather than euthyneurans.
Circulatory and excretory system
Gascoignella aprica possesses a heart consisting of an auricle, a ventricle and a partly muscular aorta. This is in line with Jensen (1985), who stated the presence of a heart shaped as usual in sacoglossans. Some additional features can be added: the auricle is posteriorly connected to a single, dorsal hemolymph sinus. Dorsal vessels, as found in most Plakobranchacea are absent in Platyhedyle, Gascoignella and Plakobranchus (Jensen 1996). A similar heart is present in G. nukuli (see Swennen 2001), while absent in P. denudata (Rückert et al. 2008). In general, the molluscan heart is surrounded by the pericardial epithelium (epicardium) with an underlying extracellular matrix, representing the location of ultrafiltration (Fahrner and Haszprunar 2001). Though this cannot be confirmed by light-microscopy, it is likely that the pericardial complex of G. aprica is built the same way. The pericardium contains the primary urine, which is modified subsequently by the kidney. A general character in the Mollusca is the close ontogenetic and functional interrelation of the pericardial complex and the nephridia in excretion (e.g. Andrews 1988; Morse and Reynolds 1996; Baeumler et al. 2011, 2012). A nephrostome or a renopericardioduct (i.e. a ciliated connection of the pericard and the kidney) has been described for other sacoglossans with a similar renopericardial complex, e.g. Bosellia mimetica Trinchese, 1891 by Fahrner and Haszprunar (2001), but we failed to reliably detect such connection in G. aprica. The H-shaped kidney in G. aprica takes up a large part of the body (see Fig. 8); the large size could be due to an advanced osmoregulatory effort necessary in semiterrestrial habitats, and therefore constitute an adaptation to the latter. A similar way of adaptation is described by Neusser et al. (2011b) for the semiterrestrial acochlidian family Aitengidae. Their members possess a highly ramified system of dorsal vessels, which constitutes a modified part of the kidney and is connected to the pericardium at least in Aiteng mysticus. This system is assumed to enhance respiratory, secretory and excretory processes in this secondarily amphibious lineage.
The kidney of P. denudata was already recognised as such by Salvini-Plawen (1973). It is located in the right anterior section of the visceral hump, and is small, compared to the other members of the Platyhedylidae. The kidney secretes via a nephroduct that unites with the intestine quite distantly from the anus (Rückert et al. 2008) as it is the case in G. aprica as well.
A heart has apparently been lost secondarily in a few Sacoglossa [e.g. Alderia modesta, Placida viridis (Trinchese, 1873)]. Jensen (1996) stated the loss to be most likely as an adaptation to intertidal and/or estuarine living. Gosliner (1994) also presumed the reduction in Alderia modesta to be due to its semiterrestrial lifestyle rather than to its small body size. This trend cannot be confirmed for the Platyhedylidae, as the mesopsammic P. denudata is the only member lacking a heart. However, the reduction of the heart in P. denudata could be due to its small body size. Decreasing body sizes were long observed as an adaptation to mesopsammic life (Swedmark 1964), and microhedylacean acochlidians were generally assumed to lack a heart (Rankin 1979). This was, however, shown to be erroneous (e.g. Neusser et al. 2009b). The heart is present in all microhedylaceans reexamined by 3D reconstruction techniques, though hearts may be simplified and contain a detectable ventricle only. In absence of auricles the site of ultrafiltration is still unknown. Similarly, future ultrastructural investigations on P. denudata have to show whether or not there are at least some remainders of the heart, and whether or not there is an alternative site of ultrafiltration.
Genital system
G. aprica is a hermaphrodite with an androdiaulic genital system first described by Jensen (1985), but the identities and special arrangements of complex reproductive organs in small sacoglossans are very difficult to reveal even by sophisticated gross-morphological dissecting techniques (Jensen 2001). Therefore, the 3D model approach provides substantial additional and reliable data.
The hermaphroditic gonad is a rather compact structure located entirely on the left side of the longitudinal septum. It is not divided into follicles as in most other sacoglossans. Alderia modesta has a similar, compact gonad (Gascoigne 1976), which may be a convergent adaptation to semiterrestrial life. The ampulla is a widened section within the proximal hermaphroditic duct, where autosperm is stored prior to copulation.
The histology of the proximally situated receptacle named “bursa” in this study matches the definition (Wägele and Willan 2000) of an at least temporarily gametolytic gland in the Sacoglossa. Accordingly the grayish, hyaline content is presumably a bolus of partly dissolved surplus reproductive products (Jensen 1996). While in most shelled Sacoglossa the genital receptacle opens by a separate duct to the female genital papilla, in the non-shelled sacoglossans the connection of the genital receptacle with the reproductive system has moved from the genital opening to a more interior position.
The elongate, sac-like prostate was, despite of its size, not mentioned in the first description of G. aprica by Jensen (1985). The prostate is connected to the fertilisation chamber in G. aprica and situated close to the exit of the vas deferens. It is histologically almost identical to the proximal prostate described for P. denudata by Rückert et al. (2008), which, however, is an unbranched tubule that is connected directly to the vas deferens, and which does not reach as far posteriorly as in G. aprica.
The most proximal, sac-like gland of the female genital system constitutes the albumen gland. Jensen (1985) already described it as a flat, much-lobed gland that winds along the dorsal side of the “large oviduct” or mucus gland, but she did not give any information about its connection to the oviduct. In fact, the histology of the albumen gland in G. aprica remarkably resembles the albumen gland of Oxynoe viridis (Pease, 1861) illustrated by Klussmann-Kolb (2001). Jensen (1996) stated that the eggs do not traverse the albumen gland in sacoglossans. This is in line with the sac-like slender appearance of the albumen gland in G. aprica and suggests secretion into the proximal part of the oviduct.
The more distal portion of the female glands is tubular and can be referred to the mucus gland and distal oviduct (Klussmann-Kolb 2001). There is no distinct membrane gland in G. aprica, which is also the case in P. denudata (see Rückert et al. 2008), or the membrane gland at least is not clearly recognisable as such.
The vas deferens connects the fertilisation chamber with the copulatory organ (i.e. the penis); it is non-glandular, and therefore not prostatic. In the Oxynoacea, the prostate is a glandular part of the vas deferens, while in the non-shelled Plakobranchacea it is in general a separate gland that opens into the vas deferens (Sanders-Esser 1984). The latter is the case in G. aprica. An additional thickened and glandular region at the distal end of the vas deferens as described for P. denudata was not found in G. aprica.
The penial stylet of G. aprica has the shape of an injection needle. Hypodermal injection of sperm is known for several groups of slugs (e.g. the nudibranch Palio, Rivest 1984) and is achieved in a precise or an imprecise way. In the precise way the injection is made directly into a seminal receptacle with oriented sperm, as observed for Limapontia capitata (Müller, 1774) and Limapontia senestra (Quatrefages, 1844) by Gascoigne (1956). A precise hypodermic injection might be conceivable for G. aprica, as the stylet is about 70 μm long and therefore would reach the bursa which is lying directly under the integument. On the other hand the bursa described in this study seems to be a gametolytic sac (see above) and not a seminal receptacle. In addition, the bursa of G. aprica is very small compared to other species where a precise impregnation occurs. In the two specimens of G. aprica examined herein there is no evidence for imprecise injection, such as free sperm in the body cavity, nor would functional allosperm storage organs be expected under such sperm transfer scenario. On the other hand, imprecise sperm transfer appears common among plakobranchacean sacoglossans (Schmitt et al. 2007), despite the presence of allosperm receptacles (e.g. Elysia filicauda, see Jensen 1999). We cannot yet exclude the possibility that G. aprica copulates, transferring sperm and sperm liquid surplus that is stored and digested in the bursa. Copulation through the oviductal opening has not been observed in any non-shelled sacoglossan, though it has often been inferred, e.g. by Gascoigne (1976, 1979).
The penial sheath gland remains enigmatic. Jensen (1985) detected it and suspected that it would be a large seminal vesicle or prostate gland. Since the gland is not connected to the vas deferens or the female part of the genital system, it is unlikely that this gland could be used for the storage of sperm. However, due to its position it can be assumed that the gland is also functionally associated to the copulatory complex and plays a role during copulation, as it was suggested for the “paraprostatic” system of some hedylopsacean Acochlidia (Neusser et al. 2009a; Brenzinger et al. 2011a; Neusser et al. 2011a) or some gasteropterid cephalaspideans (e.g. Siphopteron, see Anthes and Michiels 2007).
Jensen (1985) assumed a pronounced protandry for G. aprica, since, in a serially sectioned, 1-mm-sized specimen she found the oviduct to be very small and not to connect to the oviductal opening. The hermaphrodite ampulla contained only spermatozoa. However, oocytes in different stages of maturity were found within the gonad of the two specimens examined herein.
Systematic remarks
The genus Gascoignella currently comprises three species: the herein redescribed type species G. aprica, the anatomically poorly known G. nukuli, and G. jabae, a somewhat Olea-like species tentatively placed into Gascoignella by Swennen (2001). In absence of detailed information on the others, we concentrate here on G. aprica and compare it with the similarly well-explored P. denudata, the sole described member of the genus Platyhedyle (Fig. 12). The first has a massive H-shaped, dorsal kidney, which is neither shared by Platyhedyle nor by any other sacoglossans known to us. Also, G. aprica has an esophageal diverticulum and a heart, which are sacoglossan symplesiomorphies. In contrast, in P. denudata an esophageal diverticulum and a heart are uniquely absent. While clearly separable, e.g. by the features given above, Jensen (1996) convincingly showed that the monotypic genus Platyhedyle is the sister group of Gascoignella; therefore the Gascoignellidae was stated to be a junior synonym of the Platyhedylidae. Morphological similarities between Platyhedyle and Gascoignella were also confirmed by Rückert et al. (2008), who regarded the presence of the “median septum”, a unique longitudinal muscular septum dividing large parts of the visceral sac into left and right hemispheres, as diagnostic for Platyhedylidae. Our results on G. aprica confirm this conclusion. Further unique features found in Gascoignella and Platyhedyle, and thus potential apomorphies for Platyhedylidae include: (1) a bipartite, posteriorly fused digestive gland, with (2) short peripheral tubules, (3) a spongy subepidermal tissue, concentrated in the dorsal head-foot, (4) reduction of pharyngeal pouches, (5) reduction of a receptaculum seminis, and (6) a proximal, single, sausage-like prostate. Further apomorphies of Platyhedylidae may exist, such as the reduction of rhinophores, the distinction of headfoot and visceral sac and the very short foot. However, these characters are homoplastic among Sacoglossa, and thus their polarity depends on sacoglossan topology and the exact origin of the family within the Sacoglossa.
Older morphology-based cladistic approaches (Jensen 1996; Mikkelsen 1996, 1998) recovered a basal sacoglossan dichotomy into non-shelled Plakobranchacea and shelled Oxynoacea; this was confirmed by molecular analyses that also show that Cylindrobulla is a member of Oxynoacea (Maeda et al. 2010; Göbbeler and Klussmann-Kolb 2011; Neusser et al 2011b). As a member of Plakobranchacea, the Platyhedylidae share the apomorphic reduction of the shell, mantle cavity, gill and glandular strips that are still present in Oxynoacea. Jensen (1996) recovered the Platyhedylidae as basal offshoot of the plakobranchacean superfamily Plakobranchoidea, and Mikkelsen’s (1998) reanalysis revealed Platyhedylidae as an inner branch of Plakobranchoidea. In contrast, a recent molecular analysis showed the Platyhedylidae as most basal branch of the Plakobranchacea, i.e. sister to a common clade of limapontioidean and plakobranchoidean members (Neusser et al. 2011b). Assuming this topology is stable against adding more taxa into molecular analyses, the absence of a fused headfoot and visceral sac, the still short foot and the absence of cerata and parapodia, in Platyhedylidae could be symplesiomorphies with Oxynoacea. The loss of rhinophores would be apomorphic (and convergent to losses among derived limapontioidean lineages).
Platyhedylidae thus could be a basal non-shelled sacoglossan offshoot that is specialised to extreme habitats, i.e. intertidal, semi-terrestrial benthic or infaunal (Gascoignella), and a subtidal mesopsammic life (Platyhedyle). Several ancestral features were retained in the family, such as a rather compact body shape, but modified to resist mechanical and other environmental forces. While many apomorphies support the common origin of Platyhedylidae, the internal topology and evolution of the three currently recognised members (Platyhedyle denudata, Gascoignella aprica, G. nukuli) is not yet well-resolved. Special reductions found in Platyhedyle denudata, such as that of a heart and a well-developed kidney may be correlated to size reduction during adaptation to mesopsammic life. If this adaptation to a mesopsammic habitat occurred within Gascoignella, this would render the genus paraphyletic. Microanatomical data on G. nukuli and molecular data on G. aprica are needed to reconstruct the inner phylogeny and evolution of the Platyhedylidae.
References
Andrews, E. B. (1988). Excretory systems of molluscs (pp. 381–448). London: Academic.
Anthes, N., & Michiels, N. K. (2007). Precopulatory stabbing, hypodermic injections and unilateral copulations in a hermaphroditic sea slug. Biology Letters, 3(2), 121–124.
Arnaud, P. M., Poizat, C., & Salvini-Plawen, L. V. (Eds.). (1986). Marine interstitial Gastropoda (including one freshwater species) Stygofauna Mundi (pp. 153–161). Leiden: Brill/Backhuys.
Baeumler, N., Haszprunar, G., & Ruthensteiner, B. (2011). Development of the excretory system in the polyplacophoran mollusc, Lepidochitona corrugata: the Protonephridium. Journal of Morphology, 272(8), 972–986.
Baeumler, N., Haszprunar, G., & Ruthensteiner, B. (2012). Development of the excretory system in a polyplacophoran mollusc: stages in metanephridial system development. Frontiers in Zoology, 9(1), 23. doi:10.1186/1742-9994-9-23.
Brenzinger, B., Neusser, T. P., Jörger, K. M., & Schrödl, M. (2011a). Integrating 3D microanatomy and molecules: natural history of the pacific freshwater slug Strubellia Odhner, 1937 (Heterobranchia: Acochlidia), with description of a new species. Journal of Molluscan Studies, 77, 351–374.
Brenzinger, B., Wilson, N. G., & Schrödl, M. (2011b). 3D microanatomy of a gastropod 'worm', Rhodope rousei n. Ssp. (HETEROBRANCHIA) from Southern Australia. Journal of Molluscan Studies, 77, 375–387.
Brenzinger, B., Padula, V., & Schrödl, M. (2012). Insemination by a kiss? Interactive 3D-microanatomy, biology and systematics of the mesopsammic cephalaspidean sea slug Pluscula cuica Marcus, 1953 from Brazil (Euopisthobranchia: Cephalaspidea: Philinoglossidae). Organisms, Diversity and Evolution. doi:10.1007/s13127-012-0093-3.
Clark, K. B., Jensen, K. R., & Stirts, H. M. (1990). Survey for functional kleptoplasty (Chloroplast symbiosis) among West Atlantic Ascoglossa (=Sacoglossa) (Mollusca: Opisthobranchia). Veliger, 33, 339–345.
DaCosta, S., Cunha, C. M., Simone, L. R. L., & Schrödl, M. (2007). Computer-based 3-dimensional reconstruction of major organ systems of a new aeolid nudibranch subspecies Flabellina engeli lucianae, from brazil. (Gastropoda: Opisthobranchia). Journal of Molluscan Studies, 73, 339–353.
Dayrat, B., & Tillier, S. (2000). Taxon sampling, character sampling and systematics: how gradist presuppositions created additional ganglia in gastropod euthyneuran taxa. Zoological Journal of the Linnean Society, 129, 403–418.
Dinapoli, A., & Klussmann-Kolb, A. (2010). The long way to diversity - Phylogeny and evolution of the Heterobranchia (Mollusca: Gastropoda). Molecular Phylogenetics and Evolution, 55(1), 60–76.
Fahrner, A., & Haszprunar, G. (2001). Anatomy and ultrastructure of the excretory system of a heart-bearing and a heart-less sacoglossan gastropod (Opisthobranchia, Sacoglossa). Zoomorphology, 121(2), 85–93.
Gascoigne, T. (1956). Feeding and reproduction in the Limapontiidae. Transactions of the Royal Society of Edinburgh, 63, 129–150.
Gascoigne, T. (1976). The reproductive system and classification of the Stiligeridae (Opisthobranchia: Sacoglossa). Journal of the Malacological Society of Australia, 3, 157–172.
Gascoigne, T. (1979). A redescription of Caliphylla mediterrana Costa, 1867 (Opisthobranchia: Ascoglossa). Journal of Molluscan Studies, 45(3), 300–311.
Göbbeler, K., & Klussmann-Kolb, A. (2011). Molecular phylogeny of the Euthyneura (Mollusca, Gastropoda) with special focus on Opisthobranchia as a framework for reconstruction of evolution of diet. Thalassas, 27, 121–154.
Golding, R. E. (2010). Anatomy in Toledonia warenella n. sp. (Gastropoda: Opisthobranchia: Diaphanidae) visualized by three-dimensional reconstruction. Invertebrate Biology, 129, 151–164.
Gosliner, T. M. (1981). Origins and relationships of primitive members of the Opisthobranchia (Mollusca, Gastropoda). Biological Journal of the Linnean Society, 16(3), 197–225.
Gosliner, T. M. (1994). Chapter 5. Gastropoda: Opisthobranchia. In F. W. Harrison & A. J. Kohn (Eds.), Macroscopic anatomy of Invertebrates: Mollusca I, volume 5 (pp. 253–355). New York: Wiley-Liss.
Gosliner, T., Behrens, D., & Valdes, A. (2008). Indo Pacific Nudibranchs and Sea Slugs: a field guide to the World’s most diverse fauna. San Francisco: California Academy of Sciences/Sea Challengers Natural History Books.
Händeler, K., Grzymbowski, Y. P., Krug, P. J., & Wägele, H. (2009). Functional chloroplasts in metazoan cells—a unique evolutionary strategy in animal life. Frontiers in Zoology, 6, 28. doi:10.1186/1742-9994-6-28.
Haszprunar, G. (1985). The Heterobranchia—a new concept of the phylogeny of the higher Gastropoda. Zeitschrift für zoologische Systematik und Evolutionsforschung, 23, 15–37.
Haszprunar, G., Speimann, E., et al. (2011). Interactive 3D anatomy and affinities of the Hyalogyrinidae, basal Heterobranchia (Gastropoda) with a rhipidoglossate radula. Organisms, Diversity and Evolution, 11(3), 201–236.
Henry, E. C. (1977). A method for obtaining ribbons of serial sections of plastic embedded specimens. Stain Technology, 52, 59–60.
Huber, G. (1993). On the cerebral nervous system of marine Heterobranchia (Gastropoda). Journal of Molluscan Studies, 59, 381–420.
Jensen, K. R. (1980). A review of sacoglossan diets with comparative notes on radular and buccal anatomy. Malacological Review, 13(1–2), 55–78.
Jensen, K. R. (1981). Observations on feeding methods in some Florida ascoglossans. Journal of Molluscan Studies, 47, 190–199.
Jensen, K. R. (1985). Annotated checklist of Hong-Kong Ascoglossa (Mollusca, Opisthobranchia), with descriptions of four new species. In B. Morton & D. Dudgeon (Eds.), Proceedings of the Second International Workshop on the Malacofauna of Hong Kong and Soutern China (pp. 77–107). Hong Kong: Hong Kong University Press.
Jensen, K. R. (1991). Comparison of alimentary systems in shelled and non-shelled Sacoglossa (Mollusca, Opisthobranchia). Acta Zoologica, 72, 143–150.
Jensen, K. R. (1993a). Evolution of buccal apparatus and diet radiation in the Sacoglossa (Opisthobranchia). Bollettino Malacologico, 29(5–8), 147–172.
Jensen, K. R. (1993b). Morphological adaptations and plasticity of radular teeth of the Sacoglossa (=Ascoglossa) (Mollusca: Opisthobranchia) in relation to their food plants. Biological Journal of the Linnean Society, 48, 135–155.
Jensen, K. R. (1993c). Sacoglossa (Mollusca: Opisthobranchia)—specialist herbivores and partial predators: Integrating ecological, physiological and morphological data. In B. Morton (Ed.), The marine biology of the South China Sea. Proceedings of the First International Conference on the Marine Biology of Hong Kong and the South China Sea. Hong Kong, 28 October–3 November 1990 (pp. 437–458). Hong Kong: Hong Kong University Press.
Jensen, K. R. (1995). The Diaphanidae as a possible sister group of the Sacoglossa (Gastropoda, Opisthobranchia). In J. Taylor (Ed.), Origin and evolutionary radiation of the Mollusca (pp. 231–247). Oxford: Oxford University Press.
Jensen, K. R. (1996). Phylogenetic systematics and classification of the Sacoglossa (Mollusca, Gastropoda, Opisthobranchia). Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 351(1335), 91–122.
Jensen, K. R. (1997). Evolution of Sacoglossa (Mollusca, Opisthobranchia) and the ecological associations with their food plants. Evolutionary Ecology, 11, 301–335.
Jensen, K. R. (1999). Copulatory behaviour in three shelled and five non-shelled sacoglossans (Mollusca, Opisthobranchia), with a discussion of the phylogenetic significance of copulatory behaviour. Ophelia, 51(2), 93–106.
Jensen, K. R. (2001). Review of reproduction in the Sacoglossa (Mollusca, Opisthobranchia). Bollettino Malacologico, 37(5–8), 81–98.
Jensen, K. R. (2003). Distributions, diets and reproduction of Hong Kong Sacoglossa (Mollusca: Opisthobranchia): A summary of data, 1980–2001. In B. Morton (Ed.), Perspectives on marine environmental change in Hong Kong and Southern China, 1977–2001: Proceedings of an International Workshop Reunion Conference, Hong Kong 2001 (pp. 347–365). Hong Kong: Hong Kong University Press.
Jörger, K. M., Stöger, I., et al. (2010). On the origin of Acochlidia and other enigmatic euthyneuran gastropods, with implications for the systematics of Heterobranchia. BMC Evolutionary Biology, 10, 323. doi:10.1186/1471-2148-10-323.
Klussmann-Kolb, A. (2001). Comparative investigation of the genital systems in the Opisthobranchia (Mollusca, Gastropoda) with special emphasis on the nidamental glandular system. Zoomorphology, 120, 215–235.
Klussmann-Kolb, A., Dinapoli, A., et al. (2008). From sea to land and beyond - New insights into the evolution of euthyneuran Gastropoda (Mollusca). BMC Evolutionary Biology, 8, 57. doi:10.1186/1471-2148-8-57.
Maeda, T., Kajita, T., et al. (2010). Molecular phylogeny of the Sacoglossa, with a discussion of gain and loss of kleptoplasty in the evolution of the group. The Biological Bulletin, 219(1), 17–26.
Malaquias, M. A. E., Mackenzie-Dodds, J., Bouchet, P., Gosliner, T., & Reid, D. G. (2009). A molecular phylogeny of the Cephalaspidea sensu lato (Gastropoda: Euthyneura): architectibranchia redefined and Runcinacea reinstated. Zoologica Scripta, 38, 23–41.
Martynov, A., Brenzinger, B., et al. (2011). 3D-anatomy of a new tropical peruvian nudibranch gastropod species, Corambe Mancorensis, and novel hypotheses on dorid gill ontogeny and evolution. Journal of Molluscan Studies, 77, 129–141.
Medina, M., Lal, S., Vallès, Y., Takaoka, T. L., Dayrat, B. A., Boore, J. L., et al. (2011). Crawling through time: transition of snails to slugs dating back to the Paleozoic, based on mitochondrial phylogenomics. Marine Genomics, 4, 51–59.
Mikkelsen, P. M. (1996). The evolutionary relationships of cephalaspidea SL (Gastropoda: Opisthobranchia): a phylogenetic analysis. Malacologia, 37(2), 375–442.
Mikkelsen, P. M. (1998). Cylindrobulla and Ascobulla in the western Atlantic (Gastropoda, Opisthobranchia, Sacoglossa): systematic review, description of a new species, and phylogenetic reanalysis. Zoologica Scripta, 27(1), 49–71.
Morse, P. M., & Reynolds, P. D. (1996). Ultrastructure of the heart-kidney complex in smaller classes supports symplesiomorphy of molluscan coelomic characters. In J. Taylor (Ed.), Origin and evolutionary radiation of the Mollusca (pp. 89–97). Oxford: Oxford University Press.
Neusser, T. P., & Schrödl, M. (2007). Tantulum elegans reloaded: a computer-based 3D-visualization of the anatomy of the Caribbean freshwater acochlidian gastropod. Invertebrate Biology, 126(1), 18–39.
Neusser, T. P., Jörger, K. M., & Schrödl, M. (2007). Exploring cerebral features in Acochlidia (Gastropoda: Opisthobranchia). Bonner zoologische Beiträge, 55, 301–310.
Neusser, T. P., & Schrödl, M. (2009). Between Vanuatu tides: 3D anatomical reconstruction of a new brackish water acochlidian gastropod from Espiritu Santo. Zoosystema, 31(3), 453–469.
Neusser, T. P., Heß, M., Haszprunar, G., & Schrödl, M. (2006). Computer-based three-dimensional reconstruction of the anatomy of Microhedyle remanei (Marcus, 1953), an interstitial acochlidian gastropod from Bermuda. Journal of Morphology, 267, 231–247.
Neusser, T. P., Heß, M. & Schrödl, M. (2009a). Tiny but complex – interactive 3D visualization of the interstitial acochlidian gastropod Pseudunela cornuta (Challis, 1970). Frontiers in Zoology, 6(20):17.
Neusser, T. P., Martynov, A. V., & Schrödl, M. (2009b). Heartless and primitive? 3D reconstruction of the polar acochlidian gastropod Asperspina murmanica. Acta Zoologica, 90, 228–245.
Neusser, T. P., Jörger, K. M., & Schrödl, M. (2011a). Cryptic speciation in tropic sands—interactive 3D anatomy, molecular phylogeny and evolution of meiofaunal Pseudunelidae (Gastropoda, Acochlidia). PLoS One. doi:10.1371/journal.pone.0023313.
Neusser, T. P., Fukuda, H., et al. (2011b). Sacoglossa or Acochlidia? 3D reconstruction, molecular phylogeny and evolution of the Aitengidae (Gastropoda: Heterobranchia). Journal of Molluscan Studies, 77, 332–350.
Ponder, W. F., & Lindberg, D. R. (1997). Towards a phylogeny of gastropod molluscs: an analysis using morphological characters. Zoological Journal of the Linnean Society, 119, 83–265.
Rankin, J. J. (1979). A fresh water shell-less mollusc Tantulum elegans new-genus new-species from the Caribbean. Structure biotics and contribution to a new understanding of the Acochlidioidea. Royal Ontario Museum Life Sciences Contributions, 116, 1–123.
Richardson, K. C., Jarett, L., & Finke, E. H. (1960). Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technology, 35(6), 313–323.
Rieger, R. M., & Sterrer, W. (1975). New spicular skeletons in Turbellaria, and the occurrence of spicules in marine meiofauna (Part 1). Zeitschrift für zoologische Systematik und Evolutionsforschung, 13, 249–278.
Rivest, B. R. (1984). Copulation by hypodermic injection in the nudibranchs Palio zosterae and P. dubia (Gastropoda, Opisthobranchia). The Biological Bulletin, 167, 543–554.
Romeis, B. (1989). Mikroskopische Technik. Munich: Urban and Schwarzenberg.
Rückert, I. M., Altnöder, A., et al. (2008). Computer-based 3D anatomical reconstruction and systematic placement of the mesopsammic gastropod Platyhedyle denudata Salvini-Plawen, 1973 (Opisthobranchia, Sacoglossa). Organisms, Diversity and Evolution, 8(5), 358–367.
Ruthensteiner, B. (2008). Soft part 3D visualization by serial sectioning and computer reconstruction. Zoosymposia, 1, 63–100.
Ruthensteiner, B., & Heß, M. (2008). Embedding 3D models of biological specimens in pdf publications. Microscopy Research and Technique, 71, 778–786.
Salvini-Plawen, L. (1973). Contribution to the knowledge of the Philinoglossacea and the Acochlidiacea with Platyhedylidae new family (Gastropoda, Cephalaspidea). Zeitschrift für Zoologische Systematik und Evolutionsforschung, 11(2), 110–133.
Salvini-Plawen, L. V. (1991). The status of Rhodopidae (Gastropoda: Euthyneura). Malacologia, 32, 301–311.
Sanders-Esser, B. (1984). Vergleichende Untersuchungen zur Anatomie und Histologie der vorderen Genitalorgane der Ascoglossa (Gastropoda, Euthyneura). Zoologische Jahrbücher, Abteilung für Anatomie und Ontogenie der Tiere., 111, 195–243.
Schmitt, V., Anthes, N., & Michiels, N. K. (2007). Mating behaviour in the sea slug Elysia timida (Opisthobranchia, Sacoglossa): hypodermic injection, sperm transfer and balanced reciprocity. Frontiers in Zoology, 4(17). doi:10.1186/1742-9994-4-17.
Schrödl, M., & Neusser, T. P. (2010). Towards a phylogeny and evolution of Acochlidia (Mollusca: Gastropoda: Opisthobranchia). Zoological Journal of the Linnean Society, 158, 124–154.
Schrödl, M., & Wägele, H. (2001). Anatomy and histology of Corambe lucea Marcus, 1959 (Gastropoda, Nudibranchia, Doridoidea), with a discussion of the systematic position of Corambidae. Organisms, Diversity and Evolution, 1, 3–16.
Schrödl, M., Jörger, K. M., et al. (2011a). Bye bye "Opisthobranchia"! A review on the contribution of mesopsammic sea slugs to euthyneuran systematics. Thalassas, 27(2), 101–112.
Schrödl, M., Jörger, K. M., et al. (2011b). A reply to Medina, et al. (2011b): crawling through time: transition of snails to slugs dating back to the Paleozoic based on mitochondrial phylogenomics. Marine Genomics, 4(4), 301–303.
Sommerfeld, N., & Schrödl, M. (2005). Microanatomy of Hedylopsis ballatinei, a new interstitial acochlidian Gastropod from the Red Sea, and its significance for Phylogeny. Journal of Molluscan Studies, 71, 153–165.
Spurr, A. R. (1969). A low-viscosity resin embedding medium for electron microscopy. Journal of Ultrastructural Research, 26, 31–43.
Stöger, I. & Schrödl, M. (2012). Mitogenomics does not resolve deep molluscan relationships (yet?). Molecular Phylogenetics and Evolution. 10.1016/j.ympev.2012.11.017.
Swedmark, B. (1964). The interstitial fauna of marine sand. Biological Reviews, 39, 1–42.
Swennen, C. (2001). Two new sacoglossans (Gastropoda: Opisthobranchia) from Thailand. Beaufortia, 51(3), 75–81.
Swennen, C., & Buatip, S. (2009). Aiteng ater, new genus, new species, an amphibious and insectivorous sea slug that is difficult to classify (Mollusca: Gastropoda: Opisthobranchia: Sacoglossa(?): Aitengidae, new family. Raffles Bulletin of Zoology, 57(2), 495–500.
Wägele, H., & Klussmann-Kolb, A. (2005). Opisthobranchia (Mollusca, Gastropoda)—more than just slimy slugs. Shell reduction and its implications on defence and foraging. Frontiers in Zoology. doi:10.1186/1742-9994-2-3.
Wägele, H., & Willan, R. C. (2000). On the phylogeny of the Nudibranchia. Zoological Journal of the Linnean Society, 130, 83–181.
Wägele, H., Klussmann-Kolb, A., Vonnemann, V., & Medina, M. (2008). Heterobranchia I, the Opisthobranchia. In W. F. Ponder & D. Lindberg (Eds.), Phylogeny and evolution of the Mollusca (pp. 385–408). Berkeley: University of California Press.
Wawra, E. (1988). Beitrag zur Kenntnis des Zentralnervensystems von Platyhedyle denudata Salvini-Plawen 1973 (Ascoglossa, Gastropoda). Annalen des Naturhistorischen Museums in Wien Serie B Botanik und Zoologie B, 90, 269–275.
Wawra, E. (1991). Beitrag zur Kenntnis des Genitaltraktes von Platyhedyle denudata Salvini-Plawen, 1973 (Mollusca: Gastropoda: Ascoglossa). Annalen des Naturhistorischen Museums in Wien B, 92, 269–275.
Wilson, N. G., Rouse, G. W., & Giribet, G. (2010). Assessing the molluscan hypothesis Serialia (Monoplacophora + Polyplacophora) using novel molecular data. Molecular Phylogenetics and Evolution, 54, 187–193.
Acknowledgements
Katharina Jörger and Timea Neusser (both LMU) are thanked for embedding the examined specimen. Dr. Benjamin Franklin (Port Blair) is thanked for photographing the slices during a guest stay at the ZSM (financed by a Schering research stipend). Martin Heß (LMU) is thanked for his help compiling the interactive 3D model. Barbara Eder and Eva Lodde (both ZSM) are thanked for their help during the project. Two reviewers provided constructive and helpful comments on the manuscript. This study was financed by a grant of the German Research Foundation (DFG SCHR667/4), three dimensional reconstruction was supported by the GeoBioCenter/LMU München.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Suppl Fig. 1
(PDF 3900 kb)
Rights and permissions
About this article
Cite this article
Kohnert, P., Brenzinger, B., Jensen, K.R. et al. 3D- microanatomy of the semiterrestrial slug Gascoignella aprica Jensen, 1985—a basal plakobranchacean sacoglossan (Gastropoda, Panpulmonata). Org Divers Evol 13, 583–603 (2013). https://doi.org/10.1007/s13127-013-0142-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-013-0142-6