Abstract
The libellulid dragonfly genus Sympetrum has been recognized since 1833, but lacks any morphological synapomorphies to unite the taxon. Previous researchers have disagreed over which species belong in Sympetrum, bringing the monophyly of the genus into question. We use DNA sequence data from 6 genetic loci (16S, tRNA-valine, 12S, elongation factor 1 alpha, cytochrome oxidase subunit I, and the second internal transcribed spacer region) and 25 morphological characters (mainly genitalic) to test the monophyly of Sympetrum with Bayesian inference and maximum likelihood analyses. Under Bayesian inference, all Sympetrum species included in this study form a clade, which also contains the Hawaiian monotypic genus Nesogonia, often considered a close relative of Sympetrum. Phylogenetic analyses also reveal at least six strongly supported clades (treated as species groups) within Sympetrum, but relationships between these species groups remain unresolved or unsupported. Although the relationships between Sympetrum species groups remain unresolved, several species groups include taxa from multiple biogeographic regions/continents, and the species group sister to the rest of Sympetrum contains migratory species from the New World and Africa. This pattern suggests a complex biogeographic history in Sympetrum shaped by vicariance and dispersal. Preliminary estimates of the divergence dates of Sympetrum species groups outline a rapid radiation of the groups approximately 32-38 million years ago, possibly influenced by cooling and drying climates of the late Eocene and early Oligocene.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sympetrum Newman 1833 (Libellulidae) contains over 60 species and occurs on every continent except Australia (Needham et al. 2000; Walker and Corbet 1975). The genus has few tropical species (7 species endemic to Central and South America and 1 species in sub-Saharan Africa) and is most species-rich in the Holarctic (∼13 species in North America and nearly 40 species in Europe or Asia). Most species of Sympetrum are predominantly red and are relatively small for dragonflies (most <40 mm long). Sympetrum species are found most often in habitats with slow or stationary water such as bogs, fens, or marshes where they are most typically observed perched on reeds or other vegetation.
The taxonomic limits of Sympetrum are problematic because the description of the genus appears to include no synapomorphies. The original generic description (Newman 1833) is vague at best. More recent re-descriptions (Needham et al. 2000; Walker and Corbet 1975) of the genus outline the taxon as a unique combination of non-unique characters. The most striking characteristic of the genus is an expanded, bilobed prothorax fringed with long setae; this character, however, is shared with several other libellulid genera such as Celithemis Hagen, Erythemis Hagen, Erythrodiplax Brauer, Leucorrhinia Brittinger, Nesogonia Kirby, and Pachydiplax Brauer (Walker and Corbet 1975). Some other characteristics of Sympetrum (e.g., slender legs, rounded head, slightly compressed pterothorax; Walker and Corbet 1975) are descriptive, but also are present in many other libellulid genera.
The lack of synapomorphies for Sympetrum could explain the long-standing debate over which taxa should be included in the genus. In 1936, Needham and Fisher erected Tarnetrum to contain two species previously placed in Sympetrum, S. corruptum (Hagen) 1861 and S. illotum (Hagen) 1861. Tarnetrum was successively expanded to include other species such as S. nigrocreatum (Calvert) and S. gilvum (Hagen) of South America (Borror 1945), S. madidum (Hagen) of western North America (Walker and Corbet 1975), S. fonscolombii (Selys), a migratory species from Africa (Schmidt 1987), and S. roriamae DeMarmels and S. villosum Ris, also of South America (Carle 1993). The status of Tarnetrum as a valid genus is not without controversy, as some authors later treated it as a subgenus of Sympetrum (Cannings 1981; Dunkle 2000; Needham et al. 2000; Walker and Corbet 1975) or considered it invalid (Gloyd and Wright 1959; Kormondy 1958, 1960; Paulson 2009). Further questions concerning the limits of Sympetrum involve the monotypic Hawaiian endemic Nesogonia Kirby, which also has been suggested as a possible sister to Sympetrum or may even belong within the genus (Carle 1993; Kiauta 1969).
The relationships within Sympetrum are even more unsettled than the validity of Tarnetrum or Nesogonia, because no phylogenetic work on Sympetrum has been done. Recent taxonomic work has focused on alpha taxonomy, with several new species described in the last 20 years (Cannings and Garrison 1991; Carle 1993; DeMarmels 1994, 2001; Han and Zhu 1997), and others invalidated (Pilgrim and von Dohlen 2007). North American species have been arranged into three ‘sections’ based on similarity of their genitalia (Walker and Corbet 1975), and some have been placed into a subgenus Kalosympetrum (Carle 1993). These North American groups are likely artificial, however, because the morphological similarities between Palearctic species and Nearctic species have never been addressed with respect to relationships. The Nearctic species have been regarded as a single lineage even though some North American species have greater morphological similarities to some Palearctic species than to the other North American groups. To date, there has been no phylogenetic analysis to test the monophyly of Sympetrum or to delineate species-groups based on clades instead of geographic range.
Apart from the taxonomic issues surrounding the genus, Sympetrum constitutes an interesting subject from a biogeographic perspective. The nearly global distribution of the genus could suggest that it is an ancient lineage whose distribution could be due to global vicariance events. Putative fossils of Sympetrum, however, are known only from a few species and from European specimens, and only as far back as the Upper Miocene (Gentilini 1988) when the positions of continents were very similar to the present. The presence of multiple fossil species from the Miocene would suggest that the genus is older, possibly dating to earlier periods when the positions of landmasses, and connections between them, were very different. Regardless of the age of Sympetrum, the historical biogeography of the genus is likely complex. The strong flight capabilities of Sympetrum species imply that dispersal could also play a major role in the current distribution of the genus. Sympetrum currently contains two migratory species (S. corruptum and S. fonscolombii), but the possibility that these two species belong in another genus (see above) complicates the potential role of migratory ancestors as dispersal agents for Sympetrum. Previous biogeographic work in Calopteryx damselflies, which are predominately Holarctic like Sympetrum, found that the genus arose in the Palearctic with the Nearctic taxa resulting from a single dispersal event to North America (Misof et al. 2000). The center of diversity for Sympetrum is the Palearctic, but the center of diversity for a lineage may not necessarily be the center of origin for that group (Lomolino et al. 2005). The morphological affinities between different groups of North American species and Eurasian species hint at the possibility of a complex biogeographic history with multiple dispersal events.
The goal of this study was to develop a robust phylogeny of Sympetrum to test the monophyly of the genus and address the historical biogeography of the group. Our taxon sample included 40 species, including the monotypic genus Nesogonia, and outgroup taxa from several related Libellulidae genera. Data were collected from morphological characters and DNA sequences of the nuclear genes elongation factor-1α (EF1) and the second ribosomal internal transcribed spacer (ITS2), and the mitochondrial genes 16S/tRNA-val/12S, and cytochrome oxidase I (COI).
Materials and methods
Specimen collection, vouchers, and taxon selection
We sampled recently collected specimens from museum loan as well as freshly collected material. Fresh material was stored in acetone for approximately 24 h to remove moisture and lipids. Specimens were then stored dry in polypropylene envelopes. Loan material included both acetone-dried specimens as well as specimens collected into 95-100 % ethanol. Voucher specimens were deposited into the Department of Biology Insect Collection, Utah State University, Logan, UT, or returned to the loaning institution or individual.
The 35 Sympetrum species included in the study represented all previously designated species groups and the full geographic range of the genus (Table 1). Two populations of Sympetrum danae were included because this widely distributed species actually may represent two cryptic species. The Hawaiian, monotypic genus Nesogonia was included as a possible synonym of Sympetrum, as well as five of the eight species formerly included in Tarnetrum; we follow Garrison et al. (2006) in regarding Tarnetrum as a junior synonym of Sympetrum. Three outgroup genera (Celithemis, Leucorrhinia, and Rhyothemis) were also represented: Celithemis and Leucorrhinia are the closest relatives of Sympetrum, and Rhyothemis variegata (Linnaeus) is a more distant outgroup (Pilgrim and von Dohlen 2008).
Morphological characters
Twenty-five morphological characters were coded for all Sympetrum taxa and outgroups Celithemis and Leucorrhinia (see Appendix for character descriptions and data matrix). All characters were treated as unordered and equally weighted. Although the entire morphology of these taxa was investigated, the most informative characters derived predominantly from male and female genitalia. Penises were dissected from ethanol-softened specimens for examination.
Molecular techniques
DNA was isolated from fresh specimens stored in 95 % ethanol or from relatively recent, dried museum specimens. To extract DNA, a middle and hind leg of each specimen was removed, leaving the remainder of the specimen as voucher. DNA was extracted with the High Pure PCR Template Preparation Kit (Roche Pharmaceuticals, Indianapolis, IN).
Sequence data were collected from the nuclear genes EF-1α and ITS2, and the mitochondrial genes 16S, tRNA-valine, 12S, and COI. We also obtained sequences for the ITS1 region, but this locus was too variable to be aligned with confidence in homology; thus, we elected not to include it. ITS1 should be informative, however, for more focused analyses within species groups or closely related species. For EF-1α, 16S, tRNA-valine, 12S, and COI, the PCR conditions, primers, and thermal cycler programs followed those described in Pilgrim and von Dohlen (2008). For ITS2, the PCR conditions, primers, and thermal cycler programs followed those described in Pilgrim and von Dohlen (2007). PCR products were visualized on agarose gels stained with ethidium bromide, and successful amplifications were cleaned using standard isopropanol purification. PCR amplifications of the 16S through 12S gene of Celithemis elisa consistently failed, as did amplifications of the EF-1α gene of C. eponina; therefore, the 16S/12S sequence of C. eponina and the EF-1α sequence of C. elisa were pooled for the combined genetic analysis and are hereafter referred to as Celithemis sp.
DNA sequencing reactions were performed using either the ABI Big Dye Terminator Cycle Sequencing Ready Reaction Kit (PE Applied Biosystems, Foster City, CA) or the DYEnamic ET Terminator Cycle Sequencing Kit (Amersham Biosciences, Piscataway, NJ) following the manufacturer’s protocols. Purified products were analyzed with either an ABI Prism 377 Genetic Analyzer or ABI 3730xl DNA Analyzer. All sequence data have been deposited in GenBank (see Table 1 for accession numbers).
All PCR products were sequenced in both directions and were assembled into complete contigs using Sequencher v4.1-4.8 (Gene Code Corp., Ann Arbor, MI). COI and EF-1α data sets were aligned in Sequencher. Introns in the EF-1α data set were removed prior to alignment. The 16S-tRNA-valine-12S and ITS2 data sets each were aligned in MUSCLE (Edgar 2004) with minor editing post-alignment. Most indels in the alignments were only 1-2 bp in length, and we did not remove any portions of the data sets because of unreliable alignment.
Phylogenetic analyses
For model-based inference, the appropriate models of evolution for individual genes were determined with MrModeltest v2.2 (Nylander 2004) (GTR + I + G in all cases). Model-based analyses were performed on the combined-gene data set. Bayesian inference (BI) was performed with MrBayes v3.1.2 (Ronquist and Huelsenbeck 2003). Data were structured into six partitions: 16S + tRNA-val + 12S, EF-1α codon positions 1 + 2, EF-1α codon position 3, COI positions 1 + 2, COI position 3, and ITS2 (six partitions). The partition containing EF-1α codon positions 1 + 2 was modeled as nst = 2 and nucleotide frequencies as equal; all other partitions were modeled as nst = 6 and base frequencies estimated. Analyses included two independent runs with three heated and one cold chain in each run. Trees were sampled every 1,000 generations for 10 million generations. Convergence, ESS values, and burn-in were assessed with Tracer v1.5 (Rambaut and Drummond 2007). Maximum-likelihood (ML) analyses were performed with Garli v2.0 at molecularevolution.org. The combined-gene data set was analyzed with partitions modeled as in strategy (ii) above, except with no invariant rate category; base frequencies and proportion of invariant sites were estimated. Two analyses of 500 bootstrap replicates were performed.
Analysis of combined molecular and morphological data sets was performed under BI. Morphological data were treated as a seventh, unlinked data partition with the model set to the standard Markov k model used for morphological characters in MrBayes.
Clock-constrained Bayesian inference and divergence time estimation
Clock-constrained phylogenetics and nodal age estimates on the combined-gene data set were performed with BEAST v1.6.2 (Drummond and Rambaut 2007), after preparing the input xml file with BEAUti v1.6.2 (in the BEAST package). Data were partitioned as in strategy (ii) above. Substitution models were set to GTR + G [following the recommendation of Stamatakis (2006) to omit the invariant category] for the mitochondrial RNA and nuclear ITS2 partitions. Models for protein-coding COI and EF-1α were set to SRD06 (Shapiro et al. 2006), which partitions the data into 1st + 2nd vs. 3rd codon positions and applies the HKY model with rate variation. All model parameters were unlinked across partitions, the clock model set to uncorrelated lognormal, and tree prior set to Yule process. Putative fossils of Sympetrum have been described from Late Miocene deposits (Gentilini 1988). However, there are two issues regarding their utility as calibrations for the dating analysis. First, in our opinion, these fossils (consisting solely of wings) are only dubiously assigned to Sympetrum. While they are definitely Libellulidae, we strongly suspect they are not Sympetrum. Second, they bear no affinity to any particular extant Sympetrum species, and thus cannot be assigned to an internal node in the phylogeny. At best, they place an absolute minimum date for the origin of the genus, which is almost certainly much older. Therefore, to calibrate the molecular clock, we used a divergence date estimated in Ware et al. (2008). These authors dated a phylogeny of Libelluloidea from mitochondrial and nuclear rRNA data, calibrated with multiple fossils applied to family-level and older nodes. The age of the node subtending Sympetrum, Leucorrhinia, and Celithemis was estimated at 65 Mya; thus, we set the treeModel.rootHeight in our analysis to a mean of 65.0 and standard deviation of 2.0. Priors were left at default values except for .mu priors and yule.birthRate, which were set to normal distributions based on results from preliminary runs. Two final runs of 50 million generations were performed, with auto-optimize operators enabled and trees and parameters sampled every 10,000 generations. In addition, the analysis was run twice from an empty alignment to assess the influence of the priors on the posterior distribution. Tree files were combined with LogCombiner 1.6.2, and the consensus tree was calculated with TreeAnnotator 1.6.2 (both programs included in the BEAST package).
Results
Phylogenetic analyses
As the 16S, tRNA-val, and 12S loci were all amplified and sequenced together, these loci were also aligned together to produce an alignment of 1,680 characters with 483 variable sites. The COI alignment consisted of 752 bases with 254 variable sites. For nuclear loci, the EF-1α alignment of 705 bp had 78 variable characters, and the ITS2 alignment of 383 bp had 153 variable characters.
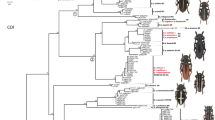
BI and ML analyses of the genetic data set recovered similar topologies (Fig. 1) showing a monophyletic Sympetrum with six strongly supported clades (PP > 95 %), each containing four to eight species (Fig. 1). We have designated each of these clades as species groups named for the most senior species in each group: danae group, fonscolombii group, flaveolum group, infuscatum group, pedemontanum group, and vulgatum group (Table 2). Our designated fonscolombii group includes species sometimes placed in the genus Tarnetrum, but does not include all the Sympetrum species that have been attributed to Tarnetrum, such as S. illotum and S. madidum. Bayesian and ML analyses lacked either support or resolution for relationships among the danae, flaveolum, infuscatum, pedemontanum, and vulgatum species groups (Fig. 1). These model-based analyses nested Nesogonia within Sympetrum (between the fonscolombii group and the rest of Sympetrum) with high posterior probability. BI analysis of the combined molecular and morphological data set produced an identical topology and similar PPs as with genetic data alone.
Sample post-burnin tree from Bayesian analysis of the combined-gene (EF-1α, ITS2, 16S, tRNA-valine, 12S, COI) data set. Numbers preceding the nodes represent BI posterior probabilities and ML bootstraps, respectively. Unsupported or unresolved nodes are marked with a hyphen. Gray circle 1 denotes Sympetrum sensu lato. Gray circle 2 denotes Sympetrum sensu stricto
Divergence estimates and historical biogeography
Relaxed-clock BI produced a consensus topology with fully resolved relationships between species groups, although PP values for these relationships were below 0.9 (Fig. 2). This analysis also placed Nesogonia as sister to Sympetrum (but without support). Divergence time estimates suggest that Sympetrum arose nearly 50 million years ago (MYA), with the fonscolombii group being the oldest species group in the genus. These date estimates also indicate that the remaining five Sympetrum species groups arose ∼30 to 40 MYA, with the ancestor of the infuscatum group diverging ∼37.8 MYA, the flaveolum group diverging ∼34.9 MYA, the danae group diverging ∼32.9 MYA, and the pedemontanum and vulgatum groups diverging ∼31.8 MYA.
The lack of well-supported relationships between species groups presents some difficulty for inferring the historical biogeography of Sympetrum, but the strongly supported species groups and the geographic ranges of their extant taxa provide some information. The fonscolombii group has extant taxa that are migratory (S. corruptum and S. fonscolombii) and that cover a broad geographic range (S. corruptum: most of North America through Central America into northern South America; S. villosum: much of South America; S. fonscolombii: most of Africa and Europe). The infuscatum and pedemontanum groups have Palearctic taxa only, but the danae, flaveolum, and vulgatum groups have Nearctic and Palearctic extant species. Within the danae group, S. costiferum and S. semicinctum are exclusively Nearctic, S. croceolum, S. depressiusculum, S. speciosum, and S. uniforme are exclusively Palearctic, and S. danae encompasses a Nearctic lineage and a Eurasian-Beringian lineage. The vulgatum group contains Palearctic-restricted taxa such as S. meridionale, S. sanguineum, S. striolatum, and S. vulgatum, but if the Nearctic species S. signiferum and S. vicinum are included in this species group (strongly supported in BI), then the species group would be considered to have a Holarctic distribution. In the flaveolum group, only S. flaveolum has a Palearctic distribution, while the rest of the taxa in the species group are entirely Nearctic. None of the analyses show the Nearctic taxa as a single clade or the Palearctic taxa as a single clade.
Discussion
Our analyses provide strong evidence for circumscription of Sympetrum as a monophyletic taxon, although delineating exactly which species should be placed within the genus could require more study. Conforming to the ideal of classification based on robust monophyletic groups, two possible options emerge from our study. Option one would encompass all species currently considered as Sympetrum, plus Nesogonia and all species previously placed in Tarnetrum (e.g., Needham and Fisher 1936; Schmidt 1987; Carle 1993) (Fig. 1). Option two would restrict Sympetrum to the well-supported lineage of S. illotum and its sister clade of five species groups (Fig. 1). This option would preserve Nesogonia and reinstate Tarnetrum as represented by the fonscolombii group. We think it is premature to choose between these options at this time, because we were unable to acquire specimens of the other, rare South American Sympetrum species that could belong to the fonscolombii group. Until such material can be included in a future analysis, we suggest referring to the clade in option one as Sympetrum sensu lato and the clade in option two as Sympetrum sensu stricto. Our results do show, however, that if Tarnetrum is considered a valid genus in the future, S. illotum and S. madidum should not be included because they did not cluster with the fonscolombii species group members formerly assigned to that genus. Our results also show that the subgenus Kalosympetrum (see Table 1) (Carle 1993) does constitute a clade, but is nested within the flaveolum species group, and therefore its utility as a taxon is reduced as it is now a subgenus nested within a species group. Future molecular studies should be combined with more extensive morphological studies to describe synapomorphies (if they exist) to support the choice of option one or two, above.
Lack of resolution and support along the backbone of the tree topologies suggest that that Sympetrum may have undergone a rapid radiation during the divergence of its species groups. All analyses produced topologies with short internode branches with low PP support between highly supported species group; such short internode branch lengths have been suggested as a hallmark of a rapid radiation (e.g., Whitfield and Kjer 2008). An argument could be made that the genetic loci chosen in this work simply lack the information necessary to resolve species group relationships; however, the nuclear and mitochondrial genetic loci used herein produced over 900 variable characters for determining relationships between ∼40 taxa, strongly suggesting that locus choice likely has not hindered generation of a resolved phylogeny. Our divergence time estimates corroborate a period of rapid radiation in Sympetrum, indicating that five of the six species groups arose within a narrow interval of approximately 6 million years, as compared to the 50 million year history of the entire group (see below).
The species groups recovered in our study are supported by various morphological characters. Taxa within each species group have similar penis morphology (Fig. 3), such as a lack of large cornua (horn-like projections on the apical penis segment) in the fonscolombii group, flattened, down-curved cornua in the flaveolum group, and tusk-like, up-curved cornua in the danae and vulgatum groups. The flaveolum group is also united by the shapes of the male hamules and the female vulvar laminae (both are external accessory structures used in species recognition for mating). Outlining morphological characters to define Sympetrum species groups is beyond the scope of this work, but further research could test the use of genitalia characters for placing Sympetrum species not included herein to the proper species group.
Given the unresolved nature of the relationships between most of the Sympetrum species groups, the divergence estimates of the species groups should be considered preliminary. Unfortunately, the fossil record for Sympetrum (Gentilini 1988) is questionable, and the use of a secondary calibration from another study was necessary (Ware et al. 2008). Those caveats being considered, however, the divergences between many of the Sympetrum species groups do coincide with known changes in global climate. Our results suggest five of the six species groups arose during the late Eocene to early Oligocene when the global climate became cooler and drier, and increases in the volume of polar ice caps led to drops in sea level (Sanmartin et al. 2001; Miller et al. 2008). These changes in climate could have fragmented ancestral Sympetrum populations leading to divergence and speciation.
Although this phylogenetic work did not recover highly supported relationships between many of the Sympetrum species groups, we may still draw several conclusions about the historical biogeography of this genus. Neither the Nearctic nor Palearctic Sympetrum species forms exclusive clades, and several of the species groups include taxa from both regions. This implies that both dispersal and vicariance have shaped the biogeographic history of the genus, including several dispersal events between the Nearctic and Palearctic. Our divergence date estimates and tree topology also imply the importance of dispersal, as it places most cladogenesis in the last ∼30 million years, which is well after major vicariance events induced by continental drift. This period in the late Tertiary, however, was marked by continued climate change, appearance, and submergence of Nearctic-Palaearctic land bridges, mid-continental orogeny, and erosion—all contributing to the possibility of multiple disruptions in gene flow, or facilitation of dispersal (Sanmartin et al. 2001; Denk et al. 2010). As the earliest branching lineages in the phylogeny, the fonscolombii group, Nesogonia, and S. illotum could provide insight into the geographic origins for Sympetrum. The current broad geographic ranges encompassed by these lineages (the fonscolombii group extends through North America, Central America, South America, Europe, and Africa; Nesogonia occurs in Hawaii; S. illotum occurs in North and Central America), however, confound our efforts to place a geographic origin for Sympetrum. Their distributions could even suggest a point of origin in the Southern Hemisphere, well outside the current center of diversity for Sympetrum (Asia). Future systematic studies of Sympetrum should attempt to include the other South American species to help address the geographic origin of this genus.
References
Allioni, C. (1766). Manipulus Insectorum Taurinensium. Melanges de la Societe de Turin, 3(7), 185–198.
Bartenev, A. K. (1913). Contributions à la connaissance des Odonates de l’Asie paléarctique du Musée Zoologique de l’Académie Impériale des Sciences de St. Pétersbourg, 2. Annuaire du Musee Zoologique de l’Academie des Sciences de St. Petersbourg, 17(3/4), 289–310.
Bartenev, A. K. (1914). Matériaux pour l’étude de la faune des Libellules de la Sibérie. 16. Odonata de la province d’Oussouri. Horae Societatis Entomological Rossica, 41(2), 1–21.
Borror, D. J. (1945). A key to the New World genera of Libellulidae (Odonata). Annals of the Entomological Society of America, 38, 168–194.
Calvert, P. P. (1890). In Hagen, H.A: A synopsis of the odonata genus Leucorrhinia Britt. Transactions of the American Entomological Society, 17, 229–236.
Cannings, R. A. (1981). The larva of Sympetrum madidum (Hagen) (Odonata: Libellulidae). Pan-Pacific Entomologist, 57, 341–346.
Cannings, R. A., & Garrison, R. W. (1991). Sympetrum signiferum, a new species of dragonfly (Odonata: Libellulidae) from western Mexico and Arizona. Annals of the Entomological Society of America, 84, 474–479.
Carle, F. L. (1993). Sympetrum janeae spec. nov. from eastern North America, with a key to Nearctic Sympetrum (Anisoptera: Libellulidae). Odonatologica, 22, 1–16.
Charpentier, T. (1840). Libellinae Europaeae descriptae ac depictae. Voss: Lipsiae.
DeMarmels, J. (1994). Sympetrum chaconi spec. nov. from Auyan-Tepui, Venezuela, with notes on a pantepuyan form of Tramea binotata (Rambur) (Anisoptera: Libellulidae). Odonatologica, 23, 405–412.
DeMarmels, J. (2001). Sympetrum paramo sp. n. (Odonata: Libellulidae) from the Venezuelan high Andes, with a key to the species of Sympetrum Newman, 1833 found in Venezuela. Entomotropica, 16, 15–19.
Denk, T., Grimsson, F., & Zetter, R. (2010). Episodic migration of oaks to Iceland: Evidence for a North Atlantic "land bridge" in the latest Miocene. American Journal of Botany, 97, 276–287.
Drummond, A. J., & Rambaut, A. (2007). BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evolutionary Biology, 7, 214.
Drury, D. (1773). Illustrations of Natural History. London, England: Simpkin.
Dunkle, S. D. (2000). Dragonflies through Binoculars. New York: Oxford University Press.
Edgar, R. C. (2004). MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research, 32(5), 1792–1797.
Garrison, R. W., von Ellenrieder, N., & Louton, J. A. (2006). Dragonfly genera of the New World. Baltimore, Maryland: Johns Hopkins University Press.
Gentilini, G. (1988). The Upper Miocene dragonflies of Monte Castellaro (Marche, Central Italy) (Odonata: Libellulidae). Memoire della Societa Entomologica Italiana, 67, 251–272.
Gloyd, L. K., & Wright, M. (1959). Odonata. In W. T. Edmondson (Ed.), Freshwater biology (pp. 917–940). New York: J. Wiley.
Hagen, H. A. (1861). Synopsis of the Neuroptera of North America. Washington, D.C.: Smithsonian Institute.
Hagen, H. A. (1867). Revision der von Herrn Uhler beschriebenen Odonaten. Stettiner Entomologische Zeitung, 28, 87–95.
Hagen, H. A. (1874). Report on the Pseudo-Neuroptera and Neuroptera collected by Lieut. W. L. Carpenter in 1873 in Colorado. Report for the U.S. Geological Survey, 7, 571–606.
Hagen, H. A. (1890). A synopsis of the odonata genus Leucorrhinia Britt. Transactions of the American Entomological Society, 17, 229–236.
Han, F.-Y., & Zhu, H.-Q. (1997). Sympetrum xiaoi spec. nov., a new dragonfly from Shanxi, China (Anisoptera: Libellulidae). Odonatologica, 26, 343–345.
Kiauta, B. (1969). The chromosomes of the Hawaiian endemic dragonflies Megalagrion oahuense (Coenagrionidae: Pseudoagrioninae) and Nesogonia blackburni (Libellulidae: Sympetrinae) with a note on the cytotaxonomic affinities between the genera Nesogonia and Sympetrum (Odonata). Proceedings of the Hawaiian Entomology Society, 20, 429–433.
Kormondy, E. J. (1958). Catalogue of the Odonata of Michigan. Miscellaneous Publications of the Museum of Zoology of the University of Michigan, 104, 43.
Kormondy, E. J. (1960). New North American records of anisopterous Odonata. Entomology News, 71, 121–130.
Linnaeus, C. (1758). Systema naturae (10th ed.). Laurentii Salvii: Holmiae.
Linnaeus, C. (1763). Centuria Insectorum. Proposuit Boas Johansson. Amoenitates Acadamicae, 6, 384–415.
Lomolino, M. V., Riddle, B. R., & Brown, J. H. (2005). Biogeography (3rd ed.). Sinauer Associates: Sunderland, Massachusetts.
McLachlan, R. (1883). Neuroptera of the Hawaiian Islands. Part I. Pseudo-Neuroptera. Annals and Magazine of Natural History, 5(12), 226–240.
Miller, K. G., Browning, J. V., Aubry, M.-P., Wade, B. S., Katz, M. E., Kulpecz, A. A., et al. (2008). Eocene-Oligocene global climate and sea-level changes: St, Stephens Quarry, Alabama. Geological Society of America Bulletin, 120(1/2), 34–53.
Misof, B., Anderson, C. T., & Hadrys, H. (2000). A phylogeny of the damselfly genus Calopteryx (Odonata) using mitochondrial 16S rDNA markers. Molecular Phylogenetics and Evolution, 15(1), 5–14.
Montgomery, B. E. (1943). Sympetrum internum, new name for Sympetrum decisum Auct., nec Hagen (Odonata, Libellulidae). Canadian Entomologist, 75(3), 57–58.
Müller, R. A. (1764). Fauna Insectorum Fridrichsdalina. Gleditsch: Hafniae et Lipsiae.
Needham, J. G., & Fisher, E. (1936). The nymphs of North American Libelluline dragonflies (Odonata). Transactions of the American Entomological Society, 62, 107–116.
Needham, J. G., Westfall, M. J., Jr., & May, M. L. (2000). Dragonflies of North America. Gainesville, Florida: Scientific Publishers.
Newman, E. (1833). Entomological Notes. Entomological Magazine, 1, 505–514.
Nylander, J. A. A. (2004). MrModeltest v2. Program distributed by the author. Uppsala: Evolutionary Biology Centre, Uppsala University.
Oguma, K. (1915). A list of dragonflies collected by Mr. Oguma from Kiushiu and Loo-Choo. Entomology Magazine Kyoto, 1, 141–148.
Oguma, K. (1922). The Japanese Dragonfly-Fauna of the Family Libellulidae. Deutsche Entomologische Zeitschrift, 1922, 96–112.
Paulson, D. (2009). Dragonflies and Damselflies of the West. Princeton, New Jersey: Princeton University Press.
Pilgrim, E. M., & von Dohlen, C. D. (2007). Molecular and morphological study of species-level questions within the dragonfly genus Sympetrum (Odonata: Libellulidae). Annals of the Entomological Society of America, 100(5), 688–702.
Pilgrim, E. M., & von Dohlen, C. D. (2008). Phylogeny of the Sympetrinae (Odonata: Libellulidae): further evidence of the homoplasious nature of wing venation. Systematic Entomology, 33(1), 159–174.
Rambaut, A., & Drummond, A. J. (2007). Tracer v1.4. Published by authors: http://beast.bio.ed.ac.uk/Tracer.
Rambur, J. P. (1842). Histoire naturelle des insectes. Névroptères. Paris, France: Roret.
Ris, F. (1911). Ueber einige Gomphinen von Südbraisilien und Argentina. Mémoires: Société entomologique de Belgique, 19, 101–119.
Ronquist, F., & Huelsenbeck, J. P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Sanmartin, I., Enghoff, H., & Ronquist, F. (2001). Patterns of animal dispersal, vicariance and diversification in the Holarctic. Biological Journal of the Linnean Society, 73, 345–390.
Say, T. (1839). Descriptions of new North American Neuropterous insects, and observations on some already described. Journal of the Academy of Natural Science of Philadelphia, 8(1), 9–46.
Schmidt, E. (1987). Generic reclassification of some West Palearctic Odonata taxa in view of their Nearctic affinities (Anisoptera: Gomphidae, Libellulidae). Advances in Odonatology, 3, 135–145.
Selys, E. (1840). Monographie des Libellulidées d’Europe. Paris, France: Roret.
Selys, E. (1841). Nouvelles Libellulidées d’Europe. Revue Zoologique, 4, 243–246.
Selys, E. (1850). Revue des odonates; ou libellules d’Europe. Paris, France: Chez Roret.
Selys, E. (1883). Les Odonates du Japon. Annales de la Société entomologique belge, 27, 82–143.
Selys, E. (1884). Révision des Diplax paléarctiques. Annales de la Société entomologique belge, 28, 29–45.
Shapiro, B., Rambaut, A., & Drummond, A. J. (2006). Choosing appropriate substitution models for the phylogenetic analysis of protein-coding sequences. Molecular Biology and Evolution, 23, 7–9.
Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22, 2688–2690.
Sulzer, J. H. (1776). Abgekürtze Geschichte der Insekten nach dem Linnaeischen System. 1. Germany: Winterthur, H. Steiner & County.
Walker, E. M., & Corbet, P. S. (1975). The Odonata of Canada and Alaska vol. 3. Toronto: Toronto University Press.
Ware, J. L., Simon, Y. W., Ho, & Kjer, K. (2008). Divergence dates of libelluloid dragonflies (Odonata: Anisoptera) estimated from rRNA using paired-site substitution models. Molecular Phylogenetics and Evolution, 47(1), 426–432.
Whitfield, J. B., & Kjer, K. M. (2008). Ancient rapid radiations of insects: Challenges for phylogenetic analysis. Annual Review of Entomology, 53, 449–472.
Acknowledgements
We wish to thank all the individuals that provided specimens for this study: K.D. Dijkstra, Kiyoshi Inoue, Margi Chriscinske, Jerrell Diagle, Jim Johnson, Mike May, Blair Nikula, Heath Ogden, Bill Radke, Graham Reels, Todd Sformo, and Steve Valley. We would also like to thank James Pitts, Paul Wolf, Terry Griswold, and Carol Dehler for reviews of previous versions of this research, and Carrie Drake and Usha Spaulding for laboratory assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
This is a contribution to the Festschrift for Michael L. May
Appendix
Appendix
Descriptions of the morphological characters used in the phylogenetic analyses.
-
1.
Cornua of penis: (0) absent; (1) two cornua present; (2) four cornua present.
Cornua are structures that are believed to aid in the removal of sperm from previous matings. They do not occur on all male odonate genitalia, but are found in pairs when they do occur. All Sympetrum species had at least one pair of cornua. As the cornua were not found in the outgroup species, these taxa were coded as missing for characters 2, 3, 4, 9, 10, and 17.
-
2.
Cornua curve: (0) anterior pointing; (1) posterior pointing.
Only members of the flaveolum group had posterior-directed cornua (Fig. 4a). All other clades of Sympetrum exhibited anterior pointing cornua (Fig. 4b, c).
-
3.
Cornua thickness: (0) flattened; (1) thin/cylindrical.
The S. flaveolum and S. infuscatum clades had flattened cornua (Fig. 4a), along with S. illotum and N. blackburni, whose affiliations to species-clades were unclear. The basal clade (fonscolombii group) had cylindrical, but shortened cornua. The other species clades (danae, pedemontanum, and vulgatum groups) all exhibited long cylindrical cornua (Fig. 4b, c). Sympetrum signiferum, a member of the danae group, had flattened cornua.
-
4.
Cornua tips: (0) tapering smoothly to point; (1) hooked at point; (2) rounded tips.
The distal ends of the cornua tapered smoothly to a point for all the members of the danae, flaveolum, and vulgatum species groups. Members of the fonscolombii and pedemontanum clades along with N. blackburni had cornua that were distinctly hooked at their distal ends (Fig. 4). The exception for this second state was S. pedemontanum. Sympetrum illotum, only, had cornua that were rounded into paddle-like shapes at the tip.
-
5.
Setal patches of S4 of penis: (0) absent; (1) present.
These tufts of wide setae (Fig. 4a) were found only in members of the flaveolum group, but not in S. flaveolum or S. madidum.
-
6.
Basal medial nodule on S4 sclerite of penis: (0) absent; (1) present.
For most Sympetrum, the distal edge of the S4 sclerite was without ornamentation. The flaveolum and infuscatum species groups, however, had a distinct median nodule along this distal margin (Fig. 4). Sympetrum sanguineum of the vulgatum group also exhibited this nodule.
-
7.
Width of penile S4: (0) widest at base; (1) widest apically where cornua originate; (2) subequal width throughout.
For Sympetrum species, the fourth segment of the penis was either widest at its base at the distal margin of the third segment, widest at its distal margin, or nearly the same width throughout its length. Members of the pedemontanum group were the only taxa to exhibit a wider base than distal margin for S4. The flaveolum and vulgatum groups along with S. illotum and N. blackburni had S4 sclerites that were widest at their distal margins. Members of the danae, fonscolombii, and infuscatum groups had S4 sclerites of nearly equal widths throughout. Within these clades, however, several species (S. danae, S. darwinianum, S. depressiusculum, S. frequens, and S. maculatum) had S4 sclerites that were widest at their distal margins. This character certainly varied in its utility for distinguishing species groups.
-
8.
Ligula notch of S2 of penis: (0) prominent/hooked but less than 1/3 width of S2; (1) larger than 1/3 width of S2.
The ligula is a hooked structure responsible for helping hold the penis internally when not in use. All Sympetrum species, except S. villosum, had ligulae that were less than a third of the width of the second penis segment. The outgroup Leucorrhinia species had larger ligulae than those found in Sympetrum.
-
9.
Cornua origin: (0) in plane of lateral lobes of S4; (1) above plane of lateral/central lobes of S4.
For most Sympetrum species, the cornua originated from the same area of S4 as the lateral lobes. For N. blackburni, S. illotum, and the flaveolum group, the cornua arose from the S4 margin above where the lateral and central lobes originated.
-
10.
Distance between cornua: (0) subparallel along length; (1) diverging apically; (2) converging apically.
For the flaveolum and infuscatum groups, and also for S. illotum, the cornua were basically parallel along their length. When viewed ventrally, the cornua of the danae, fonscolombii, pedemontanum, and vulgatum groups along with N. blackburni were distinctly divergent along their length. Converging cornua was an autapomorphy for S. vicinum.
-
11.
Penis vesicle (S1): (0) smooth at base; (1) basal margin projecting outward.
-
12.
Lateral margin of S1 of penis: (0) smoothly curved; (1) angular projection at widest point.
Characters 11 and 12 showed variability within the Sympetrum clades and therefore were not phylogenetically informative with respect to relationships among species groups. These characters could be useful as a key character, but not as a synapomorphy for a species group.
-
13.
Lateral margin of S4 sclerite of penis: (0) weak/absent; (1) expanded ventrally beyond cornua; (2) expanded dorsally behind cornua; (3) expanded dorsally and ventrally beyond cornua.
The lateral margin of the S4 sclerite had multiple states. Sympetrum gracile, S. infuscatum, and S. risi had S4 sclerites that were not expanded on their lateral margins. Members of the flaveolum, fonscolombii, and pedemontanum groups had S4 sclerites that were expanded only ventrally beyond the cornua. The S4 sclerites were expanded dorsally for N. blackburni, S. darwinianum, S. illotum, S. maculatum, and the danae group. The vulgatum group had S4 sclerites that were expanded dorsally and ventrally, except for S. signiferum and S. vicinum, which had sclerites that were only ventrally expanded.
-
14.
S4 sclerite of penis surface: (0) smooth; (1) heavily contoured with bumps and ridges.
A heavily contoured S4 sclerite was a synapomorphy for the flaveolum group and did not occur in any other taxa.
-
15.
Central unexpanded lobe of S4 of penis: (0) in line w/ lateral lobes; (1) above plane of lateral lobes; (2) below plane of lateral lobes.
For many taxa, the lateral and central lobes of the last segment of the penis were small and arranged in the small plane (state 0) as in the infuscatum group. Some species of the pedemontanum and vulgatum groups had a central lobe that was above the plane of the lateral lobes (state 1). Many taxa, including the outgroups Celithemis and Leucorrhinia, S. illotum, N. blackburni, and the fonscolombii and danae groups, had a central lobe that was well below the plane of the lateral lobes (state 2).
-
16.
Width of S1 of penis: (0) more than ½ S1 length; (1) less than ½ S1 length.
For most species, including outgroups, the width of the 1st penal segment was much greater than half its length. Sympetrum fonscolombii and S. villosum of the fonscolombii group were the only species that had a thin 1st penal segment.
-
17.
Visibility of cornua: (0) obscured by central/lateral lobes of S4; (1) visible along nearly entire length.
For species in the fonscolombii group, the cornua are so short that their visibility is obscured by the central and lateral lobes of the last penis segment. This character state was a synapomorphy for this species clade.
-
18.
Ventral tooth of cerci: (0) prominent; (1) weak; (2) absent.
In many libellulid taxa, the cerci (superior anal appendages) of the male have a prominent tooth-like projection on the ventral edge prior to the cerci tapering to a point. This tooth was found in outgroups, and all species of the flaveolum, infuscatum, and pedemontanum groups. This tooth was completely absent in the fonscolombii group, but varied between weak and prominent within the danae and vulgatum groups.
-
19.
Curve of dorsal side of cerci: (0) straight; (1) curved dorsally; (2) curved ventrally.
In libellulids, the cerci can remain uncurved along their length, or can curve dorsally or ventrally as they taper to a point. In this study, ventral curving was rare and only occurred in the outgroup taxa Celithemis, and Leucorrhinia hudsonica, and in the ingroup taxa N. blackburni and S. illotum. All the species of the danae, fonscolombii, infuscatum, and vulgatum groups had uncurved cerci. The species of the pedemontanum group consistently had dorsal curving cerci, and the flaveolum group varied between uncurved and dorsally curved cerci. The utility of this character may, however, be somewhat doubtful due to individual variation. As the adult emerges from the exuviae, the cerci may be distorted and would then harden, possibly with curving that may not occur for all members of that species.
-
20.
Epiproct length: (0) subequal to ventral projection of cerci; (1) longer than the ventral projection of cerci; (2) shorter than the ventral projection of cerci.
In Anisoptera, the epiproct is the inferior anal appendage of the male and is typically shorter than the full length of the cerci. The epiproct may extend beyond the ventral projection of the cerci (see character 19), be subequal in length to this projection, or not reach the projection. The danae and fonscolombii groups were consistent with all species having an epiproct subequal to the ventral projection of the cerci. The species of the infuscatum and pedemontanum groups had epiprocts that extended beyond the ventral projection. Members of the flaveolum and vulgatum groups were variable with some species having a subequal length, and some species having a much shorter epiproct.
-
21.
Dorsal apical teeth of epiproct: (0) absent; (1) present.
The distal edge of the dorsum of the epiproct often had several tooth-shaped projections. After examining all the taxa here, only Nesogonia blackburni was found to be without these tooth-like projections.
-
22.
Genital lobe shape: (0) pointed; (1) rounded; (2) quadrate.
The genital lobe of libellulids projects from the posterior margin of the genital fossa in males. This lobe may taper to a point, have a rounded margin, or have a quadrate shape. The species of the danae group all had rounded genital lobes. The only taxon to have a quadrate lobe was S. corruptum. The other species clades varied between pointed and rounded genital lobes. The utility of this character was doubtful, partly due to within-clade variation, but also because of difficulty coding individuals as either pointed or rounded.
-
23.
Genital lobe angle: (0) ventral; (1) ventro-posterior; (2) posterior.
The genital lobe may point directly ventral, ventro-posterior, or directly posterior. All species of the infuscatum group had ventro-posterior angled genital lobes, but the other species clades varied in the states within these clades. Coding this character was difficult, because consistently determining the angle of the lobe was problematic. Intraspecific variation occurs for some species.
-
24.
Carina of abdominal segment 4: (0) present; (1) absent.
The presence of a transverse carina on the 4th abdominal segment was used as a key character in Needham et al. (2000), and was described as a possible character for validating Tarnetrum. Further examination of this character showed that it may vary intraspecifically, and that it was found in many other taxa that were never considered to belong in Tarnetrum.
-
25.
Lateral ridges of occiput: (0) absent; (1) present.
Lateral ridges were found only on species of the flaveolum group, except for S. flaveolum and S. madidum.

Rights and permissions
About this article
Cite this article
Pilgrim, E.M., von Dohlen, C.D. Phylogeny of the dragonfly genus Sympetrum (Odonata: Libellulidae). Org Divers Evol 12, 281–295 (2012). https://doi.org/10.1007/s13127-012-0081-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13127-012-0081-7