Abstract
Long non-coding RNAs (lncRNAs) and microRNAs (miRNAs) play important roles in the pathogenesis of spinal cord injury (SCI). This study investigated the effects of lncRNA Mirt2 and miR-429 on lipopolysaccharide (LPS)-induced injuries in PC12 cells. Serum samples were collected from 36 patients with SCI and the healthy controls. The expression of lncRNA Mirt2 in serum samples was measured by qRT-PCR. The in vitro model of SCI was established by treating PC12 cells with LPS. The effects of lncRNA Mirt2 and miR-429 on the cell model were evaluated by CCK-8 assay, flow cytometry, western blot, qRT-PCR, and ELISA. Further, the activation of NF-κB and p38MAPK pathways was tested by western blot. LPS induced obvious cell injuries in PC12 cells, as cell viability was reduced, apoptosis rate was increased, caspase-3 and -9 were cleaved, and the release of TNF-α and IL-6 was induced. lncRNA Mirt2 was up-regulated in LPS-stimulated PC12 cells and serum samples derived from SCI patients. Overexpression of lncRNA Mirt2 protected PC12 cells against LPS-induced injuries. Further studies found that lncRNA Mirt2 acted as the molecular sponge of miR-429 and miR-34a-5p. lncRNA Mirt2 did not protect PC12 cells when miR-429 was overexpressed. Moreover, the inhibitory effects of lncRNA Mirt2 on NF-κB and p38MAPK pathways were abolished when miR-429 was overexpressed. lncRNA Mirt2 exerts protective effects in an in vitro model of SCI by down-regulating miR-429. This study shed light on the treatment of SCI by using the lncRNA-miRNA regulation network.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Spinal cord injury (SCI) is a kind of central nervous system (CNS) trauma with high disability rate. According to statistics, the incidence of SCI in USA is approximately 24–77 per million people [3, 5]. Despite that the pathogenesis of SCI has not been known clearly, it is generally believed that oxidative damage, neuron apoptosis, and necrosis caused by SCI [7] can result in sensation loss and muscle dysfunction which significantly reduced the quality of life of patients [23]. Moreover, inflammation induced by SCI can exaggerate the neuronal dysfunction and neuron death [10]. Thus, suppressing SCI-induced inflammation and the following cell death will be effective for preventing further damage of SCI.
Long non-coding RNAs (lncRNAs) are defined as a group of non-coding RNA transcripts with length more of than 200 nucleotides. Current studies have demonstrated that lncRNAs can regulate the expression of downstream genes and thus involved in the pathogenesis of many human diseases. In regard of SCI, lncRNA ZNF667-AS expression has been reported to be gradually down-regulated, and restoring its expression inhibited SCI-induced inflammatory response and was able to promote SCI recovery [17]. However, the effect of lncRNAs in SCI has not been widely explored [9]. Myocardial infarction–associated transcript 2 (Mirt2) is one of the recently recognized lncRNAs. Its expression is sensitive to lipopolysaccharide (LPS), and it was regarded as a negative regulator of inflammation [11]. Based on this reason, we are interested in investigating the role of lncRNA Mirt2 in SCI-induced inflammatory injury.
MicroRNAs (miRNAs) are a class of endogenous, small non-coding, single-stranded RNAs with a length of about 22 nucleotides. miRNAs can regulate gene expression through binding with the 3′-untranslated region (3′-UTR) of target genes at the post-transcriptional level [2]. At present, many miRNAs were found to be closely associated with the pathological responses after SCI [15]. For example, overexpression of miR-136-5p promoted the generation of pro-inflammatory cytokines in rat SCI and further damaged the spinal cord [8]. Likewise, miR-429 was reported to be critical in repressing neuroinflammation via inhibiting the release of pro-inflammatory cytokines [25], indicating the potential of miR-429 in regulating SCI-induced inflammatory injury. However, since miR-429 was found to be involved in lung inflammatory injury [24] and neuron death [12], the effects of miR-429 on SCI have not been studied.
PC12 is a cell line that is derived from rat adrenal gland pheochromocytoma that can differentiate into neuron-like cells. Recently, PC12 cells have been considered as important experiment materials for investigating neuronal dysfunction–related diseases including SCI [16]. In this study, PC12 cells were utilized and stimulated by lipopolysaccharide (LPS) to mimic the inflammatory injury following SCI. The effects of lncRNA Mirt2 on the established in vitro SCI model were then investigated. Besides that, the regulatory relationship between lncRNA Mirt2 and miR-429 was studied to reveal the possible underlying mechanisms of which lncRNA Mirt2 exerted its function. The findings of this study will enlarge our understanding of lncRNA-miRNA cross talk in SCI, and suggest the novel target of SCI treatment.
Materials and methods
Patient serum samples
Serum samples were collected from thirty-six patients with SCI in the Affiliated Hospital of Hangzhou Normal University from December, 2018 to March, 2019. Normal serum samples were collected from the age- and gender-matched non-SCI patients and referred as healthy control. Written consent was obtained from each adult volunteer before serum collection. The Ethics Committee of Affiliated Hospital of Hangzhou Normal University granted approval of this study.
Cell culture and treatment
PC12 and HEK 293T cells respectively purchased from Kunming Institute of Zoology (Kunming, China) and American Type Culture Collection (Manassas, VA) were cultured in DMEM (Gibco, Grand Island, NY) with 10% (v/v) fetal bovine serum (Gibco), 100 U/mL penicillin, and 100 μg/mL streptomycin (both from Sigma-Aldrich, St. Louis, MO) at 37 °C in humidified 5% CO2.
PC12 cells were treated by LPS (Sigma-Aldrich) at 1, 2, 5, and 10 μg/mL for 12 h.
Cell Counting Kit-8 assay
Cells were seeded in a 96-well plate at a density of 5000 cells/well, and cell viability was assessed by a Cell Counting Kit-8 (CCK-8) kit (Dojindo Molecular Technologies, Kyushu, Japan). After stimulation of the indicated concentration of LPS for 12 h, the cell cultures were incubated with CCK-8 solution for 1 h in the dark. The absorbance was measured at 450 nm using a Microplate Reader (Bio-Rad, Hercules, CA).
Apoptosis assay
Cell apoptosis analysis was conducted by using an Annexin V FITC/PI Apoptosis Detection Kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instruction. Briefly, collected cells (1 × 105 cells per sample) were washed in phosphate-buffered saline (PBS) and fixed in 5 μL Annexin V-FITC and 5 μL PI for 30 min in the dark at 37 °C. Cell apoptosis was detected on a FACScan (Beckman Coulter, Fullerton, CA), and the cell apoptosis rate was analyzed by using FlowJo software.
Transfection
pc-Mirt2 and sh-Mirt2 plasmids for expression of the full legend of lncRNA Mirt2 and the lncRNA Mirt2–specific shRNA were constructed by GenePharma Co. (Shanghai, China). The control plasmids carrying nothing or a non-targeting sequence were considered as negative controls (pcDNA3.1 and sh-NC, respectively). miR-429 mimic, miR-34a-5p mimic, and the scrambled controls (NC mimic) were synthesized by Invitrogen (Carlsbad, CA). Cell transfection was conducted in 6-well plates (1 × 105 cells/well) by using the Lipofectamine 3000 reagent (Invitrogen) according to the manufacturer’s instructions. The final concentration of vectors and oligonucleotides was 2 μg/mL and 50 nM, respectively.
qRT-PCR
Total RNAs in PC12 cells (1 × 105 cells in 6-well plates) and serum samples were extracted by using TRIzol reagent (Invitrogen). Five micrograms of total RNA was reverse transcribed to cDNA by using PrimeScript™ RT Master Mix (Takara, Dalian, China). The amplification of cDNA was performed with an ABI PRISM 7900 Sequence Detector system (Applied Biosystems, Foster City, CA) using the TB Green Premix Ex Taq II kit (Takara). For the test of miRNAs, the following two kits were used for reverse transcriptional and PCR: Mir-X™ miRNA First Strand Synthesis Kit and Mir-X™ miRNA qRT-PCR TB Green™ Kit (both from Takara). The results were calculated using the 2−△△CT method. U6 and β-actin were used as the internal control for miRNA and mRNA expression, respectively. The primers were designed by GenePharma.
Western blot
The proteins in PC12 cells (1 × 105 cells in 6-well plates) were extracted using RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China). After being quantified using the BCA™ Protein Assay Kit (Pierce, Appleton, WI), 40 μg protein samples was separated by 12% SDS-PAGE, transferred to PVDF, blocked, and incubated with primary antibodies (anti-pro/cleaved Caspase-3, anti-pro/cleaved Caspase-9, anti-IL-6, anti-TNF-α, anti-t/p-IκBα, anti-t/p-p65, and anti-t/p-p38 MAPK, all from Cell Signaling Technology, Danvers, MA) at 4 °C overnight. After being rinsed and incubated with corresponding secondary antibody for 1 h at room temperature, the blots in the PVDF membrane were developed with ECL solution (Pierce) and visualized by using Image Lab™ Software (Bio-Rad).
Enzyme-linked immunosorbent assay
PC12 cells (5 × 104) in 24-well plates were stimulated with LPS as indicated, and then the culture supernatant was collected. The concentrations of inflammatory cytokines (TNF-α and IL-6) were measured by using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Abingdon, UK) according to the manufacturer’s instruction.
Luciferase reporter assay
The fragment from lncRNA Mirt2 containing the predicted miR-429-binding site was amplified by PCR and then cloned into a pmirGLO Dual-luciferase miRNA Target Expression Vector (Promega, Madison, WI) to form the reporter vector Mirt2-wild type (Mirt2-wt). The full length of Mirt2 cDNA (971 bp) containing the miR-429-binding site was amplified by PCR with the following primers: forward 5′-TCAACACTTTCCATAGGT-3′, reverse 5′-ATTGTGAGGTCCAGATAG-3′. To mutate the putative binding site of miR-429 in the lncRNA Mirt2, the sequence of the putative binding site was replaced by using TaKaRa MutanBEST Kit (Takara) and was named as Mirt2-mutated-type (Mirt2-mut). Then, the vectors and miR-429 mimics were co-transfected into HEK 293T cells. The Dual-Luciferase Reporter Assay System (Promega) was used for testing the luciferase activity. The same procedure was performed to test the binding effects of lncRNA Mirt2 on miR-34a-5p. The NCBI BLAST website (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to predict the target sites.
Statistical analysis
All experiments were repeated at least three times independently. The results of multiple experiments are presented as the mean ± SD. Statistical analyses were performed using SPSS 13.0 (SPSS Inc., Chicago, IL). The differences between two groups were analyzed by Student’s t test, and multiple comparisons were conducted by one-way or two-way analysis of variance (ANOVA) followed by a post hoc Student–Newman–Keuls test. Statistical significance was specified as P < 0.05.
Results
LPS induced obvious inflammatory injury in PC12 cells
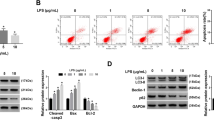
Firstly, PC12 cells were stimulated with 1, 2, 5, and 10 μg/mL of LPS for 12 h. Cell viability was measured using CCK-8 assay. We found that LPS significantly decreased cell viability in a dose-dependent manner (Fig. 1a). Cell apoptosis was next assessed through flow cytometry and western blot. The results in Fig. 1b showed that apoptosis rates were increased by LPS also in a dose-dependent manner. Considering that the viability of PC12 cells has been cut in half and apoptosis has been significantly induced by treating with 5 μg/mL LPS, 5 μg/mL was selected as a LPS-treating condition for use in the subsequent experiments. We found that caspase-3 and -9 were both clearly cleaved in cells treated with 5 μg/mL LPS (P < 0.001 and P < 0.01, Fig. 1c, d). Then, qRT-PCR and ELISA were conducted to examine the effect of LPS on the release of pro-inflammatory cytokines (TNF-α and IL-6). The results in Fig. 1e, f showed that the mRNA levels of TNF-α and IL-6 (P < 0.01 and P < 0.001), as well as their protein concentrations in cell supernatant (P < 0.01 and P < 0.001), were significantly increased by LPS compared with the control. These results above indicated that LPS induced obvious cell injuries in PC12 cells.
LPS induced obvious inflammatory injury in PC12 cells. PC12 cells were stimulated with 1, 2, 5, and 10 μg/mL of LPS for 12 h. a Cell viability was measured using CCK-8 assay. b Apoptosis rate was valued through flow cytometry. PC12 cells were suffered from 5 μg/mL LPS for 12 h, after which c, d the cleavage of caspases, e the mRNA levels of TNF-α and IL-6, and the f the concentrations of TNF-α and IL-6 in culture supernatant were respectively assessed by western blot, qRT-PCR, and ELISA. n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control (CTRL) group
lncRNA Mirt2 was up-regulated during SCI
The expression of lncRNA Mirt2 in PC12 cells following LPS stimulation was detected by using qRT-PCR. As shown in Fig. 2a, lncRNA Mirt2 level was elevated in the LPS group as compared with the control (P < 0.05). The same trend was observed in Fig. 2b, where the high level of lncRNA Mirt2 in the serum sample derived from SCI patients is displayed (P < 0.01).
lncRNA Mirt2 was up-regulated during SCI. a PC12 cells were treated with 5 μg/mL LPS for 12 h. The expression of lncRNA Mirt2 was detected through qRT-PCR. n = 3. b The expression of lncRNA Mirt2 in serum samples derived from SCI patients was detected through qRT-PCR. Normal serum samples collected from non-SCI patients were used as healthy controls. n = 36. *P < 0.05 compared with the control (CTRL) group. **P < 0.01 compared with the healthy control group
Overexpression of lncRNA Mirt2 alleviated LPS-induced injury
To evaluate the effect of lncRNA Mirt2 on LPS-induced inflammatory injury, PC12 cells were transfected with pc-Mirt2 or sh-Mirt2. Transfection efficiency was tested by qRT-PCR, and results showed that the expression of lncRNA Mirt2 was dramatically elevated by pc-Mirt2 transfection while it was repressed by sh-Mirt2 transfection (both P < 0.01, Fig. 3a, b). Further studies found that LPS-induced cell viability loss was weakened by lncRNA Mirt2 overexpression while it was aggravated by lncRNA Mirt2 silence (both P < 0.01, Fig. 3c). Meanwhile, LPS-induced apoptosis was prevented by lncRNA Mirt2 overexpression and was aggravated by lncRNA Mirt2 silence, as seen in the alterations of apoptosis rate and caspase cleavage (P < 0.05 or P < 0.01, Fig. 3d–f). Not surprisingly, the release of TNF-α and IL-6 induced by LPS was also attenuated by lncRNA Mirt2 overexpression and was enhanced by lncRNA Mirt2 silence (P < 0.05 or P < 0.01, Fig. 3g, h). These results demonstrated that overexpression of lncRNA Mirt2 alleviated LPS-induced injury through decreasing pro-inflammatory cytokine release and suppressing cell apoptosis.
Overexpression of lncRNA Mirt2 alleviated LPS-induced injury. PC12 cells were transfected with pc-Mirt2 or sh-Mirt2. pcDNA3.1 or sh-NC was transfected as the negative control. a, b Transfection efficiency was tested by qRT-PCR. Then, the transfected cells were incubated with 5 μg/mL LPS for 12 h to induce injury as indicated. c Cell viability, d apoptosis rate, e, f caspase activation, g the mRNA levels of TNF-α and IL-6, and h the concentrations of TNF-α and IL-6 in culture supernatant were respectively detected through CCK-8 assay, flow cytometry, western blot, qRT-PCR, and ELISA. n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the indicated group
miR-429 was demonstrated to be a downstream effector of lncRNA Mirt2
miR-429 was previously identified as an LPS-sensitive miRNA [24]. Next, we studied whether lncRNA Mirt2 prevented LPS-evoked injury via regulating LPS-sensitive miRNAs. Figure 4a shows that the expression of miR-429 was increased by LPS (P < 0.01). Overexpression of lncRNA Mirt2 abolished LPS-elevated miR-429 expression, while lncRNA Mirt2 silence promoted the expression (both P < 0.01). Besides that, the expression of lncRNA Mirt2 was repressed by transfection of cells with miR-429 mimic (P < 0.05, Fig. 4b). The result demonstrated the negative regulation between lncRNA Mirt2 and miR-429. Luciferase reporter assay results indicated that miR-429 could directly bind with lncRNA Mirt2, as the luciferase activity was significantly reduced by transfection with miR-429 mimic and Mirt2-wt (P < 0.01, Fig. 4c). Apart from miR-429, miR-34a-5p was also found to be down-regulated by lncRNA Mirt2. As shown in Fig. 3d, e, negative regulation between lncRNA Mirt2 and miR-34a-5p was observed (P < 0.01). Relative luciferase activity was significantly declined by transfection with miR-34a-5p mimic and Mirt2-wt (P < 0.05, Fig. 4f).
miR-429 and miR-34a-5p were demonstrated to be down-regulated by lncRNA Mirt2. a PC12 cells were transfected with pc-Mirt2 or sh-Mirt2. pcDNA3.1 or sh-NC was transfected as the negative control. The transfected cells were suffered from 5 μg/mL LPS for 12 h. The expression of miR-429 was tested by qRT-PCR. b PC12 cells were transfected with miR-429 mimic. NC mimic was transfected as the negative control. The expression of lncRNA Mirt2 was tested by qRT-PCR. c Luciferase activity assay was conducted to value the binding effects between lncRNA Mirt2 and miR-429. d PC12 cells were transfected with pc-Mirt2 or sh-Mirt2. pcDNA3.1 or sh-NC was transfected as the negative control. The expression of miR-34a-5p was tested by qRT-PCR. e PC12 cells were transfected with miR-34a-5p mimic. NC mimic was transfected as the negative control. The expression of lncRNA Mirt2 was tested by qRT-PCR. f Luciferase activity assay was conducted to value the binding effects between lncRNA Mirt2 and miR-34a-5p. n = 3. *P < 0.05, **P < 0.01 compared with the indicated group
Next, the functional effects of miR-429 in PC12 cells were studied. Results in Fig. 5a displayed that miR-429 expression significantly declined in patients with SCI as compared with the healthy control (P < 0.01). The expression of miR-429 in PC12 cells was overexpressed by transfection with its specific mimic (P < 0.01, Fig. 5b). As a result, viability of PC12 cells was decreased (P < 0.05, Fig. 5c), apoptosis rate was increased (P < 0.05, Fig. 5d), and cleavage of caspases was promoted (P < 0.001, Fig. 5e, f) by miR-429 mimic, indicating the pro-apoptotic function of miR-429 towards PC12 cells.
miR-429 overexpression induced cell apoptosis death of PC12 cells. a The expression of miR-429 in serum samples derived from SCI patients was detected through qRT-PCR. Normal serum samples collected from non-SCI patients were used as healthy controls. n = 36. PC12 cells were transfected with miR-429 mimic. NC mimic was transfected as the negative control. Thereafter, b miR-429 expression, c cell viability, d apoptosis rate, and e, f caspase activation were respectively measured by qRT-PCR, CCK-8, flow cytometry, and western blot. n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the indicated group
Next, whether lncRNA Mirt2 prevented LPS-evoked damage through binding with miR-429 was studied. Results in Fig. 6a–d showed that, lncRNA Mirt2 overexpression could not significantly attenuate LPS-induced viability loss, apoptosis, and caspase activation when miR-429 was overexpressed (all P < 0.01). Meanwhile, lncRNA Mirt2 overexpression did not repress TNF-α and IL-6 release made by LPS when miR-429 was overexpressed (P < 0.05 or P < 0.01, Fig. 6e, f).
lncRNA Mirt2 alleviated LPS-induced injury through down-regulating miR-429. PC12 cells were co-transfected with pc-Mirt2 and miR-429 mimic. pcDNA3.1 or NC mimic was transfected as the negative control. Then, the transfected cells were incubated with 5 μg/mL LPS for 12 h to induce injury as indicated. a Cell viability, b apoptosis rate, c, d caspase activation, e the mRNA levels of TNF-α and IL-6, and f the concentrations of TNF-α and IL-6 in culture supernatant were respectively measured by CCK-8, flow cytometry, western blot, qRT-PCR, and ELISA. n = 3. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the indicated group
lncRNA Mirt2 inactivated the NF-κB and p38MAPK signal pathways through miR-429
To explore the associated signal pathways through which the lncRNA Mirt2/miR-429 axis exerted its function, we assessed the expression of core proteins in NF-κB and p38MAPK signal pathways. The results in Fig. 7a–d showed that the phosphorylation of IκBα, p65, and p38MAPK was induced by LPS (all P < 0.001). Overexpression of lncRNA Mirt2 attenuated LPS-induced phosphorylation of these proteins, while this effect of lncRNA Mirt2 was abolished when miR-429 was overexpressed (all P < 0.01). Thus, the above results revealed that lncRNA Mirt2 inactivated the NF-κB and p38MAPK signal pathways through down-regulating miR-429.
lncRNA Mirt2 inactivated the NF-κB and p38MAPK signal pathways through down-regulating miR-429. PC12 cells were co-transfected pc-Mirt2 and miR-429 mimic. pcDNA3.1 or NC mimic was transfected as the negative control. Then, the transfected cells were incubated with 5 μg/mL LPS for 12 h to induce injury. Protein levels of core factors related with a, b NF-κB and c, d JAK/STAT signal pathways were measured by western blot. n = 3. **P < 0.01, ***P < 0.001 compared with the indicated group
Discussion
Neuroinflammation is one of the key components of the secondary injury mechanisms of SCI [1]. The levels of inflammatory cytokines, like TNF-α and IL-6, can reach the highest level in a few hours after SCI [30]. Inhibition of TNF-α reduced the development of inflammation, apoptosis, and tissue injury in SCI [13]. Likewise, suppression of IL-6 promoted axonal sprouting and functional recovery following SCI [26]. Apart from neuroinflammation, cell death is another major event in the secondary injury mechanisms that can occur when inflammation is not suppressed. In general, neurons and glial cells die through apoptosis, and thereby preventing and reversing apoptosis will be beneficial for SCI management [1]. At present, a small fraction of studies have used neuron-like PC12 cells and LPS to make an in vitro model of SCI [16, 18], since LPS is an excellent stimulant for inducing inflammatory response and apoptosis. Consistent with these previous studies, the SCI model was established in this study and the effects of lncRNA Mirt2 were explored. Results found that overexpression of lncRNA Mirt2 could protect PC12 cells against LPS-induced damage. The neuroprotective impacts of lncRNA Mirt2 may be due to the suppressed expression of miR-429 and the deactivated NF-κB and p38MAPK signal pathways.
At present, an increasing literature has recognized that lncRNAs are key regulators of gene expression in many cell life processes. However, the function of lncRNAs in SCI is not well-characterized. Herein, we aimed to explore the effect of lncRNA Mirt2 on LPS-induced injury in PC12 cells, hoping to get a better understanding about the pathomechanism of SCI and find available therapeutic targets and directions. We found that lncRNA Mirt2 was highly expressed in LPS-stimulated PC12 cells, which was consistence with a previous study [11] indicating lncRNA Mirt2 as a LPS-sensitive lncRNA. Besides that, lncRNA Mirt2 was found to be highly expressed in a serum sample of SCI patients. Further studies found that LPS induced the release of TNF-α and IL-6 and cell apoptosis in PC12 cells was attenuated when lncRNA Mirt2 was overexpressed. Based on these results, it seems that lncRNA Mirt2 acted as a neuroprotective gene in SCI.
There is increasing evidence showing that lncRNAs exert important roles in multiple neurological diseases through regulating the expression of miRNAs [14, 29]. Although the regulation between lncRNAs and miRNAs has not been well-established, it is widely believed that lncRNAs can function as competing endogenous RNAs or molecular sponges in the course of the interaction between miRNAs [6]. For the selected example, lncRNA MALAT1 knockdown was expected to attenuate SCI through working as miR-199b sponge [31]. lncRNA Map2k4 promoted spinal cord neuron growth through sequestering miR-199a [19]. Herein, miR-429 was found to be negatively regulated by lncRNA Mirt2 and they can bind with each other. It seems that lncRNA Mirt2 acted as a molecular sponge for miR-429. Further study revealed that lncRNA Mirt2 exerted its neuroprotective effects possibly via suppressing the expression of miR-429. Beside miR-429, we found that miR-34a-5p may be another downstream effector of lncRNA Mirt2, which confirmed the findings elsewhere described [27]. Thus, it seems that lncRNA Mirt2 will induce a complex response in the regulation of the miRNA pattern.
NF-κB is a classical family of transcription factor that is critical in regulating multiple damage responses including tissue oxidative stress injury and cell apoptosis. During SCI, the NF-κB signal pathway is activated and induces the release of a series of pro-inflammatory factors, thereby inhibiting the neuroprotective procedure in CNS. Inhibiting the activation of NF-κB is one of the key nodes in SCI treatment [4]. For example, curcumin administration markedly down-regulated the levels of pro-inflammatory cytokines through suppressing the TLR4/NF-κB signal pathway in the injured rat spinal cord following SCI [20]. In our study, we found that the NF-κB signal pathway induced by LPS was suppressed by lncRNA Mirt2 through miR-429.
The MAPK signal pathway plays an important role in cell differentiation and proliferation of CNS. ERK, p38MAPK, and JNK are three-tiered classical MAPK pathways [28]. Among which, p38MAPK is activated after cell injury stress and then triggers a series of downstream inflammation-related signal pathways [21]. The expression of p38MAPK is often found to be increased in injured spinal cord neurons, astrocytes, and macrophages, and the suppression of p38MAPK expression following SCI can promote functional recovery of SCI [22]. In the current study, we found that lncRNA Mirt2 blocked the MAPK signal pathway by regulating miR-429.
Taken together, our present studies demonstrated that lncRNA Mirt2 protected PC12 cells against LPS-induced inflammatory injury. The neuroprotective effects of lncRNA Mirt2 in the in vitro model of SCI may be via down-regulating miR-429 and thus inactivating the NF-κB and p38MAPK signal pathways. It seems that, by transferring lncRNA Mirt2 into the diseased cells, the expression pattern of miRNAs is altered and the following pathological reaction is induced which ultimately prevents SCI and reduces functional deficits. This study shed light on the treatment of SCI by using the lncRNA-miRNA regulation network. However, further studies are required to illustrate the clinical value of lncRNA Mirt2 in SCI.
Change history
30 September 2020
An editorial Expression of Concern on this article has now been published: https://doi.org/10.1007/s13105-021-00793-1
01 March 2021
An Editorial Expression of Concern to this paper has been published: https://doi.org/10.1007/s13105-021-00793-1
References
Alizadeh A, Dyck SM, Karimi-Abdolrezaee S (2019) Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol 10:282. https://doi.org/10.3389/fneur.2019.00282
Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116:281–297
Bernhard M, Gries A, Kremer P, Bottiger BW (2005) Spinal cord injury (SCI)--prehospital management. Resuscitation 66:127–139. https://doi.org/10.1016/j.resuscitation.2005.03.005
Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD, Yezierski RP (1998) Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci 18:3251–3260
Burke DA, Linden RD, Zhang YP, Maiste AC, Shields CB (2001) Incidence rates and populations at risk for spinal cord injury: a regional study. Spinal Cord 39:274–278. https://doi.org/10.1038/sj.sc.3101158
Cao MX, Jiang YP, Tang YL, Liang XH (2017) The crosstalk between lncRNA and microRNA in cancer metastasis: orchestrating the epithelial-mesenchymal plasticity. Oncotarget 8:12472–12483. https://doi.org/10.18632/oncotarget.13957
David S, Kroner A (2011) Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci 12:388–399. https://doi.org/10.1038/nrn3053
Deng G, Gao Y, Cen Z, He J, Cao B, Zeng G, Zong S (2018) miR-136-5p regulates the inflammatory response by targeting the IKKbeta/NF-kappaB/A20 pathway after spinal cord injury. Cell Physiol Biochem 50:512–524. https://doi.org/10.1159/000494165
Di Gesualdo F, Capaccioli S, Lulli M (2014) A pathophysiological view of the long non-coding RNA world. Oncotarget 5:10976–10996. https://doi.org/10.18632/oncotarget.2770
Donnelly DJ, Popovich PG (2008) Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol 209:378–388. https://doi.org/10.1016/j.expneurol.2007.06.009
Du M, Yuan L, Tan X, Huang D, Wang X, Zheng Z, Mao X, Li X, Yang L, Huang K, Zhang F, Wang Y, Luo X, Huang D, Huang K (2017) The LPS-inducible lncRNA Mirt2 is a negative regulator of inflammation. Nat Commun 8:2049. https://doi.org/10.1038/s41467-017-02229-1
Fu S, Zhang J (2018) Knockdown of miR-429 attenuates abeta-induced neuronal damage by targeting SOX2 and BCL2 in mouse cortical neurons. 43:2240–2251. https://doi.org/10.1007/s11064-018-2643-3
Genovese T, Mazzon E, Crisafulli C, Di Paola R, Muia C, Esposito E, Bramanti P, Cuzzocrea S (2008) TNF-alpha blockage in a mouse model of SCI: evidence for improved outcome. Shock (Augusta, Ga) 29:32–41. https://doi.org/10.1097/shk.0b013e318059053a
Kaur H, Sarmah D, Saraf J, Vats K, Kalia K, Borah A, Yavagal DR, Dave KR, Ghosh Z, Bhattacharya P (2018) Noncoding RNAs in ischemic stroke: time to translate. Ann N Y Acad Sci 1421:19–36. https://doi.org/10.1111/nyas.13612
Li F, Zhou MW (2019) MicroRNAs in contusion spinal cord injury: pathophysiology and clinical utility. https://doi.org/10.1007/s13760-019-01076-9
Li G, Chen T, Zhu Y, Xiao X, Bu J, Huang Z (2018) MiR-103 alleviates autophagy and apoptosis by regulating SOX2 in LPS-injured PC12 cells and SCI rats. Iran J Basic Med Sci 21:292–300. https://doi.org/10.22038/ijbms.2018.25980.6392
Li JW, Kuang Y, Chen L, Wang JF (2018) LncRNA ZNF667-AS1 inhibits inflammatory response and promotes recovery of spinal cord injury via suppressing JAK-STAT pathway. Eur Rev Med Pharmacol Sci 22:7614–7620. https://doi.org/10.26355/eurrev_201811_16375
Li R, Yin F, Guo Y, Ruan Q, Zhu Q (2018) Angelica polysaccharide protects PC-12 cells from lipopolysaccharide-induced injury via down-regulating microRNA-223. Biomed Pharmacother 108:1320–1327. https://doi.org/10.1016/j.biopha.2018.09.147
Lv HR (2017) lncRNA-Map2k4 sequesters miR-199a to promote FGF1 expression and spinal cord neuron growth. Biochem Biophys Res Commun 490:948–954. https://doi.org/10.1016/j.bbrc.2017.06.145
Ni H, Jin W, Zhu T, Wang J, Yuan B, Jiang J, Liang W, Ma Z (2015) Curcumin modulates TLR4/NF-kappaB inflammatory signaling pathway following traumatic spinal cord injury in rats. J Spinal Cord Med 38:199–206. https://doi.org/10.1179/2045772313y.0000000179
Saklatvala J (2004) The p38 MAP kinase pathway as a therapeutic target in inflammatory disease. Curr Opin Pharmacol 4:372–377. https://doi.org/10.1016/j.coph.2004.03.009
Wang J, Su B, Zhu H, Chen C, Zhao G (2016) Protective effect of geraniol inhibits inflammatory response, oxidative stress and apoptosis in traumatic injury of the spinal cord through modulation of NF-kappaB and p38 MAPK. Exp Ther Med 12:3607–3613. https://doi.org/10.3892/etm.2016.3850
Wang Y, Zhao X, Xie H (2019) Quality of life and its predictors in people with traumatic spinal cord injury in mainland China. Spinal Cord. https://doi.org/10.1038/s41393-019-0279-z
Xiao J, Tang J, Chen Q, Tang D, Liu M, Luo M, Wang Y, Wang J, Zhao Z, Tang C, Wang D, Mo Z (2015) miR-429 regulates alveolar macrophage inflammatory cytokine production and is involved in LPS-induced acute lung injury. Biochem J 471:281–291. https://doi.org/10.1042/bj20131510
Yan XT, Zhao Y, Cheng XL, He XH, Wang Y, Zheng WZ, Chen H, Wang YL (2018) Inhibition of miR-200b/miR-429 contributes to neuropathic pain development through targeting zinc finger E box binding protein-1. J Cell Physiol 233:4815–4824. https://doi.org/10.1002/jcp.26284
Yang G, Tang WY (2017) Resistance of interleukin-6 to the extracellular inhibitory environment promotes axonal regeneration and functional recovery following spinal cord injury. Int J Mol Med 39:437–445. https://doi.org/10.3892/ijmm.2017.2848
Zhang B, Li H, Li D, Sun H, Li M, Hu H (2019) Long noncoding RNA Mirt2 upregulates USP10 expression to suppress hepatic steatosis by sponging miR-34a-5p. Gene 700:139–148. https://doi.org/10.1016/j.gene.2019.02.096
Zhang H, Wang Y (2016) Identification of molecular pathway changes after spinal cord injury by microarray analysis. J Orthop Surg Res 11:101. https://doi.org/10.1186/s13018-016-0437-3
Zhang Y, Cruickshanks N, Pahuski M, Yuan F, Dutta A, Schiff D, Purow B, Abounader R (2017) Noncoding RNAs in glioblastoma. In: De Vleeschouwer S (ed) Glioblastoma. Codon Publications Copyright. The Authors, Brisbane (AU). https://doi.org/10.15586/codon.glioblastoma.2017.ch6
Zhao J, Wang L, Li Y (2017) Electroacupuncture alleviates the inflammatory response via effects on M1 and M2 macrophages after spinal cord injury. Acupunct Med 35:224–230. https://doi.org/10.1136/acupmed-2016-011107
Zhou HJ, Wang LQ, Wang DB, Yu JB, Zhu Y, Xu QS, Zheng XJ, Zhan RY (2018) Long noncoding RNA MALAT1 contributes to inflammatory response of microglia following spinal cord injury via the modulation of a miR-199b/IKKbeta/NF-kappaB signaling pathway. Am J Physiol Cell Physiol 315:C52–c61. https://doi.org/10.1152/ajpcell.00278.2017
Author information
Authors and Affiliations
Contributions
Haibo Li and Yu Xu are co-first authors.
Corresponding author
Ethics declarations
Written consent was obtained from each adult volunteer before serum collection. The Ethics Committee of Affiliated Hospital of Hangzhou Normal University granted approval of this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Haibo Li and Yu Xu co-first authors
Statement
Shaoxing People’s Hospital (Shaoxing Hospital of Zhejiang University), Shenzhen University General Hospital, and The Affiliated Hospital of Hangzhou Normal University did not provide our authors the institutional email addresses. So we can only use our personal email address to submit the paper. We confirm that they are not fake email addresses. The authors can be reached at their email addresses.
Rights and permissions
About this article
Cite this article
Li, H., Xu, Y., Wang, G. et al. Long non-coding RNA Mirt2 relieves lipopolysaccharide-induced injury in PC12 cells by suppressing miR-429. J Physiol Biochem 75, 403–413 (2019). https://doi.org/10.1007/s13105-019-00691-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-019-00691-7