Abstract
Glycosylation of cell surface proteins regulates critical cellular functions, including invasion and metastasis in cancer cells. Emerging evidence has shown that microRNAs (miRNAs) are involved in regulating both the glycosylation modifications on cell surface and the progression of cancer. In this study, we investigated the role of miR-9 in α-2,6-linked sialylation and the metastasis of mouse hepatocellular carcinoma (HCC). According to array-based miRNA expression profiling data of HCC cell lines Hepa1–6, Hca-P, and Hca-F with different lymphatic metastatic capacities, reverse correlation was found between miR-9 expression levels and the metastatic potential in these HCC cells. Additionally, β-galactoside α-2,6-sialyltransferase 1 (St6gal1) expression level is associated negatively with miR-9 and positively with metastatic potential. Bioinformatics analysis indicated that miR-9 could target St6gal1, which was verified by luciferase reporter assays. miR-9 overexpression reduced expression of St6gal1, which subsequently suppressed HCC cells metastatic potential. Moreover, upregulation of miR-9 could inhibit integrin-β1/FAK-mediated cell motility and migration signaling in mouse HCC cells. Together, our results suggest that miR-9 could act as a tumor suppressor and regulate mouse HCC cells migration and invasion by inhibiting the α-2,6-linked sialylation. This finding may provide insight into the relationship between abnormal miRNA expression and aberrant cell surface glycosylation during tumor lymphatic metastasis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Glycosylation has been shown to be one of the most important post-translational modifications of proteins, and approximately 50% of all cellular proteins are glycosylated. Glycosylation acts as a key regulatory mechanism involving in several physiological and pathological processes, including cell proliferation, differentiation migration, and tumor metastasis. Glycosylation requires the coordinated activity of various glycosyltransferases. As a result, aberrant glycosylation, due to the impaired glycosyltransferase activity, has been highly correlated with carcinogenesis [5]. Sialic acid is a nine-carbon acidic monosaccharide that is involved in various biological functions, such as tumor metastasis and invasion. More than 20 different sialyltransferase genes were found in human genome. The β-galactoside α-2,6-sialyltransferase 1 (St6gal1) synthesizes terminal α-2,6-sialic acid linkages on complex N-glycans. St6gal1 is overexpressed in various types of cancer, including oral, pancreatic, ovarian, and hepatic, and its expression is positively correlated with aggressiveness and metastatic potential of these tumors [15, 17, 23, 26]. However, the regulation of St6gal1 expression is not completely clear.
We and others have reported that alterations in microRNA (miRNA) expression might result in promoting oncogenesis by direct suppression of glycosyltransferases [9, 10, 12]. miRNAs are short non-coding RNAs that are complementary to the 3′-untranslated regions (UTRs) of the target genes and act as a potential regulator by silencing target gene expression. Through binding to perfect or nearly perfect complementary sequences in the 3′-UTRs of target mRNAs, miRNAs can silence genes by either mRNA degradation or translational repression [1]. As a result, miRNAs are involved in multifarious cellular processes, including cell differentiation, proliferation, and apoptosis, and function as either oncogenes or tumor suppressors in several human malignancies [6]. Accumulating evidence suggests that miRNAs prove crucial in regulating tumor metastasis. For example, miR-30a and miR-26a suppress tumor metastasis in hepatocellular carcinoma (HCC) [7, 8], while miR-203 inhibits cell migration and invasion in colorectal cancer [4]. In our previous research, it was found that both Let-7c and miR-34a inhibit the lymphatic metastatic potential of mouse HCC cells [11, 12]. Despite the abundant evidence on the important roles of miRNAs and aberrant glycosylation in tumor metastasis, the molecular mechanisms of miRNAs in sialylation-related metastasis are poorly understood.
The purpose of this study was to investigate the role of a cancer-related miRNA, miR-9, in suppressing mouse HCC cell metastasis and explore its mechanisms. The miR-9 expression level was found to be significantly downregulated in HCC cells with high lymphatic metastatic potential and St6gal1 expression levels. Further analyses revealed that miR-9 was an important factor in the process of sialylation-induced metastasis by targeting St6gal1 in mouse HCC cells. Herein, we also found that miR-9 could attenuate metastasis in HCC cells by inhibiting α-2,6-linked sialylation and integrin-β1/FAK signaling pathway.
Materials and methods
Cell culture
The non-metastatic mouse HCC Hepa1–6 cell line was obtained from the Cell Bank of Peking Union Medical University (Beijing, China). Hca-P and Hca-F cell lines were obtained from the Department of Pathology, Dalian Medical University (Dalian, China). Human HCC cell lines HepG2 (low metastasis), SMMC-7721 (high metastasis), and human invasive breast cancer cell line MDA-MB-231 were obtained from Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cell lines were used within 6 months of initial culturing. Cell lines were cultured in RPMI-1640 (Gibco, USA) supplemented with 10% fetal bovine serum (HyClone, Logan, UT, USA) and 1% penicillin/streptomycin antibiotics (Gibco, USA). All cells were maintained in a humidified incubator at 37 °C with 5% CO2.
miRNA microarrays
MicroRNA array analysis was performed as previously described [12]. Briefly, the microarray assay included labeling, hybridization, scanning, normalization, and data analysis. All of the processes were performed by KangChen Bio-Tech (Shanghai, China). RNA samples were isolated from Hepa1–6, Hca-P, and Hca-F and then analyzed by Exiqon A/S using a miRCURY™ Array Power Labeling kit.
Quantitative RT-PCR
Quantitative RT-PCR (qRT-PCR) was performed as previously described [12]. RT-PCR primers were as follows: for St6gal1, 5′-AGCCCTTTTACATCCTCAAG-3′ (forward) and 5′-ATGATGATACCAAGCATCCC-3′ (reverse); for GAPDH, 5′-CACCCTGTTGCTGTAGCCAAATTC-3′ (forward) and 5′-GACATCAAGAAGGTGGTGAAGCAG-3′ (reverse). RT-specific primers for miRNAs qRT-PCR were designed RiboBio Co, Ltd. (Guangzhou, China).
Transient transfection
2.5 × 105 Hepa1–6, Hca-P, and Hca-F cells were seeded in culture plates before transfection. Scrambled miRNA (miRNA-Scr), miR-9 mimic and miR-9 inhibitor (antisense oligonucleotide) were purchased from RiboBio Co, Ltd. (Guangzhou, China) based on the sequence of miR-9 in miRBase database. Each cell line was transfected with these oligonucleotides at working concentrations of 50 nM using riboFECT™ CP transfection reagent (RiboBio, Guangzhou, China) according to the manufacturer’s instruction. For St6gal1 over expression, 2.5 × 105 Hepa1–6 cells were transfected with 3 μg of pcDNA-St6gal1 [3] using Lipofectamine 2000 reagent (Invitrogen, MA, USA) according to the manufacturer’s instruction.
Lectin blot, Western blot, and immunoprecipitation
Cells were lysed with RIPA lysis buffer (Beyotime, China) containing a protease inhibitor (Roche, Switzerland). Lectin blot, Western blot, and immunoprecipitation were performed as previously described [11]. The following antibodies and lectin were used: St6gal1, GAPDH, integrin-β1, EGFR, and caveolin-1 were from Abcam (MA, USA); FAK and phosphorylated FAK (p-FAK, Tyr-397) were from Cell Signaling Technology (MA, USA). Biotinylated Sambucus nigra agglutinin (SNA) lectin was from Vector Laboratories (CA, USA).
Flow cytometry assay
In total, 1 × 106 cells were incubated in the dark with FITC-SNA (S. nigra agglutinin, Vector Laboratories, USA) for 0.5 h at 4 °C in a final concentration of 20 μg/mL. Before flow cytometry assays, the cells were washed twice with PBS and fixed with 50 μL of 1% paraformaldehyde. BD FACSCalibur flow cytometer (CA, USA) was used for flow cytometry analysis.
Luciferase reporter assay
Mouse HCC cell lines (5 × 105 cells per well) were seeded in six-well culture plates before transfection. Mouse ST6gal1 3′-UTR sequence (4000 to 4508 bp) was inserted into the pGL3 control reporter vector. pGL3-St6gal1–3′-UTR vector or control luciferase plasmid plus miR-9 mimics or inhibitor was transfected into HCC cells using Lipofectamine 2000 reagent. For negative control, pGL3-Mgat3a-3′-UTR vector was used. Culture medium was replaced after 6 h. Cells lysis was obtained 48 h after transfection using the Dual-Luciferase Reporter Assay System (Promega, CA, USA).
Cell proliferation assay
Mouse HCC cells were transfected with oligonucleotides. Cell Counting kit 8 (CCK8, Beyotime, Nanjing, China) were used to assessed cell proliferation after transfection, and the OD (optical density) values at 450 nm were measured.
Cell adhesion, migration, and invasion assays
For the cell adhesion assay, culture plates were coated with fibronectin (FN, Abcam, USA), collagen I (COL, Sigma, USA), or laminin (LN, Sigma, USA) at a concentration of 10 nM. Bovine serum albumin (BSA) was used as negative control and poly-L-lysine (PL) was used as positive control. HCC cell lines (4 × 104 cells/mL) were pre-treated with 5-μM mitomycin-C for 2 h to inhibit proliferation. Cells were suspended in serum-free medium containing 0.1% BSA and then seeded into the coated 96-well culture plates. Cells were incubated at 37 °C for 1 h. The attached cells were labeled with 0.3% crystal violet (Sigma, MA, USA) and measured using a VersaFluor Fluorometer (Bio-Rad, CA, USA) at 570 nm.
A transwell plate (Corning, New York, USA) was used to assess cell migration abilities. Cells (2 × 104) were transfected with oligonucleotides. Cell proliferation was inhibited by 5-μM mitomycin-C. Cells were seeded in the upper chamber filled with serum-free medium, while the lower chamber contained complete culture medium supplemented with 10% FBS. Four hours later, the cells that had passed through the upper chamber was counted. The experiments were repeated three times.
For the cell invasion assay, ECMatrix gel (BD, MA, USA) was thawed at 4 °C overnight and then used to coat the transwell plate chambers. A 5-μM mitomycin-C was used to inhibit cell proliferation. Cells (4 × 104) were seeded in the coated upper chamber. Twelve hours later, the cells that had invaded through the ECMatrix gel were counted using a microscope. Three independent experiments were performed.
SNA precipitation assay
Cell lysate was incubated with 150 μg of SNA-agarose (Vector laboratories, CA, USA). Samples were incubated at 4 °C overnight on a rotator. α-2,6-Linked sialylated proteins were then precipitated by centrifugation and washed three times with ice-cold PBS. Precipitates were resolved by SDS-PAGE and immunoblotted with indicated antibody.
Results
miR-9 expression is inversely correlated with metastatic ability in mouse HCC cell lines
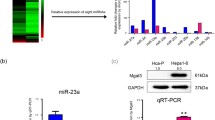
Hepa1–6, Hca-P, and Hca-F are three sister mouse HCC cell lines isolating from the same HCC ascites cell line. Therefore, Hca-F with ~ 80%, Hca-P with ~ 30%, and Hepa1–6 with no lymph node metastatic potentials have similar genetic backgrounds [12]. This characteristic makes these three cell lines a suitable model to study metastasis. The results of miRNA microarray profiling revealed that the expression levels of various miRNAs were different across these three cell lines (Fig. 1a). Among St6gal1 candidate miRNAs developed by bioinformatic analysis, the well-known tumor-related miR-9 showed significant fold changes and negative correlation of expression levels in mouse HCC cells. miR-9 expression levels were lower in cells with high metastatic potential than those with low metastatic potential, indicating that miR-9 might play a role in malignant metastasis (Fig. 1b). We then verified miR-9 levels in mouse HCC cells by hairpin RT-PCR. Consistent with the microRNA profiling, we detect a greater than twofold and fourfold higher level of miR-9 in Hepa1–6 cells compared to those of Hca-P and Hca-F cells, respectively. These data implied that miR-9 might participate in antagonizing St6gal1-induced malignant metastasis in mouse HCC cells.
High levels of miR-9 were associated with low metastatic potential in mouse HCC cell lines. a Expression levels of miRNAs in Hepa1–6, Hca-F, and Hca-P cells were determined by microRNA profiling analysis. b Relative expression levels of predicted miRNAs with a binding site in the 3′-UTR of St6gal1 in a microarray are displayed. c miR-9 expression levels in Hepa1–6, Hca-P, and Hca-F cells were assayed by qRT-PCR. The data were normalized to U6. Data are presented as the mean ± SEM (* p < 0.05, ** p < 0.01)
miR-9 targets St6gal1 in HCC cells directly
We previously reported that modification of α-2,6-linked sialic acid mediates the metastatic properties of human HCC cells [29]. Similar to this observation, examination of mouse HCC cell lines with distinct lymphatic metastatic capacities showed that St6gal1 protein and mRNA levels were significantly downregulated in Hepa1–6 cells with no lymphatic metastatic potential compared with Hca-P cells, while Hca-F cells expressed the highest St6gal1 level (Fig. 2a, b).
St6gal1 is a target gene of miR-9. a–b Western blotting and qRT-PCR analysis showed St6gal1 protein and mRNA levels in mouse HCC cell lines. c Schematic of bioinformatics analysis of predicted binding sites showed that miR-9 bound to the 3′-UTR of St6gal1 mRNA across mammalian species. The predicted consequential paring between the target region (position 4004–4026 of St6gal1 3′-UTR) and the seed sequence of miR-9 is shown. d–e St6gal1 was a direct target of miR-9. Hepa1–6 and Hca-F cells were transfected with a reporter vector consisting of a luciferase cDNA fused to the 3′-UTR of St6gal1 for 48 h. The pGL3 vector and pGL3-Mgat3–3′-UTR were used as controls. Data are presented as the mean ± SEM (* p < 0.05, ** p < 0.01)
Then, the publicly available databases TargetScan, PicTar, and miRDB were used to analyze the potential interaction between miR-9 and St6gal1. We found that St6gal1 have conserved miR-9 binding site in its 3′-UTR sequence in multiple mammalian species mRNAs (Fig. 2c). To investigate whether St6gal1 is an authentic and direct target of miR-9, St6gal1 wild-type 3′-UTR containing the putative miR-9-binding site was cloned into pGL-3 luciferase reporter vector downstream of the luciferase reporter gene. This luciferase reporter vector was co-transfected with a miR-9 mimic or inhibitor in HCC cells. miR-9 inhibitor significantly stimulated relative luciferase activity compared with scrambled miRNA in Hepa1–6 cells with an endogenous high miR-9 level, whereas luciferase activity did not decrease in the presence of the Mgat3a (monoacylglycerol acyltransferase 3a, Mgat3a, used as a control) 3′-UTR reporter, indicating that functionality depends on the miR-9-binding seed region. Alternatively, miR-9 significantly suppressed pGL3-St6gal1–3′-UTR activity in Hca-F cells with an endogenous low miR-9 level. The results suggest that miR-9 could target St6gal1 in HCC cell lines and might influence cell metastatic properties.
miR-9-mediated St6gal1 silencing downregulates α-2,6-linked sialic acid levels in HCC cell lines
To further verify miR-9-mediated silencing of endogenous St6gal1 at the transcription and protein levels, HCC cells were transient transfected with miR-9 oligonucleotides. Lower St6gal1 mRNA and protein levels were detected in Hepa1–6 cells than metastatic HCC cells. qRT-PCR showed an observable reduction of St6gal1 mRNA in Hepa1–6 cells transfected miR-9 inhibitor compared with the scrambled miRNA group. miR-9 upregulation with the miR-9 mimic significantly reduced St6gal1 expression in Hca-P and Hca-F cells (Fig. 3a). Moreover, miR-23a mimic significantly decreased St6gal1 expression at protein levels compared with the controls in both metastatic mouse HCC cell lines. In contrast, miR-9 downregulation with the miR-9 inhibitor significantly increased St6gal1 expression (Fig. 3b). Similar results were obtained in human HCC cell lines HepG2 and SMMC-7721 with different metastatic potentials (Fig. S1), indicating that a similar miR-9-mediated mechanism also exists in human HCC cells.
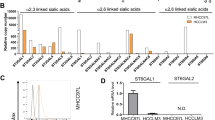
miR-9 suppresses the expression of St6gal1 and α-2,6-linked sialic acid in mouse HCC cell lines. a–b Hepa1–6, Hca-P, and Hca-F cells were transfected with scrambled miRNA, miR-9 mimic, or miR-9 inhibitor for 48 h and then assessed using qRT-PCR and western blotting. miR-9 inhibitor caused an upregulation of St6gal1 mRNA and protein levels in Hepa1–6 cells, whereas miR-9 mimic caused a down-regulation of St6gal1 in Hca-P and Hca-F cells. c The α-2,6-linked sialic acid levels were determined by SNA lectin staining. d α-2,6-Linked sialic acid expression levels in Hepa1–6, Hca-P, and Hca-F cells were analyzed by flow cytometry assay. Data are presented as the mean ± SEM (* p < 0.05, ** p < 0.01)
Because lectin S. nigra agglutinin (SNA) was used to specifically recognize α-2,6-linked sialic acid, the expression levels of α-2,6-linked sialic acid were analyzed in HCC cells using a lectin blot. The metastatic HCC cells were much more heavily sialylated than Hepa1–6 cells (Fig. 3c). Interestingly, in Hepa1–6 cells transfected with the miR-9 inhibitor, the syntheses of α-2,6-linked sialic acid were increased. Conversely, the miR-9 mimic transfection decreased α-2,6-linked sialic acid in metastatic Hca-P and Hca-F cells. Flow cytometry analysis by labeling α-2,6-linked sialic acid with fluorescein isothiocyanate SNA lectin was also performed. miR-9 overexpression suppressed the levels of α-2,6-linked sialic acid, and conversely, miR-9 downregulation increased the levels of these structures (Fig. 3d). Therefore, miR-9 might repress mouse HCC metastasis by inhibiting aberrant sialylation.
miR-9 suppressed cell adhesive, metastatic, and invasive ability in HCC cell lines
To ascertain whether miR-9-mediated St6gal1 and sialylation downregulation affect HCC metastatic properties, we explored the effect of miR-9 on cell migration and invasion in vitro using transwell chambers with or without Matrigel. First, HCC cell proliferation rates were significantly reduced by miR-9, indicating the tumor suppressive function of this miRNA. Further, we examined cell migration and invasive ability using a transwell assay (Fig. 4b, c). The miR-9 inhibitor-transfected Hepa1–6 cells had significantly increased invasion and migration ability compared with the control groups, while the miR-9 mimic decreased Hca-P and Hca-F migration and invasion ability. Consistent mechanism was observed in a highly metastatic human breast cancer cell line MDA-MB-231 (Fig. S2). These findings suggest that miR-9 could inhibit cell migration and invasion in different types of cancer cells. We then investigated the effect of miR-9 on HCC cell adhesion to FN, LN or COL. As shown in Fig. 4d, Hepa1–6 cells revealed an enhanced cell adhesion to FN, LN, or COL by transfecting with miR-9 inhibitor. However, adhesion of Hca-P and Hca-F cells to FN, LN, or COL was decreased significantly when α-2,6-linked sialic acid was reduced by the miR-9 mimic. These results suggest that miR-9 could inhibit the metastatic potential of HCC cells at least partially via repressing St6gal1 expression.
miR-9 modulates cell growth, migration, invasion and adhesion in mouse HCC cell lines. a Hepa1–6, Hca-P, and Hca-F were transfected with scrambled miRNA, miR-9 mimic, or miR-9 inhibitor for 48 h and then assessed at the indicated time by CCK8. b–c The miR-9 mimic attenuated the migration and invasion ability of Hepa1–6 and Hca-F cells. Transwell migration and invasion assays demonstrated the invasion ability of Hepa1–6, Hca-P, and Hca-F cells transiently transfected with scrambled miRNA, miR-9 mimic, or miR-9 inhibitor for 48 h. A total of 5-mM mitomycin-C was used to inhibit proliferation. d A cell adhesion assay using Hepa1–6, Hca-P, and Hca-F on FN, LN, and COL at a concentration of 10 nM after transfection with scrambled miRNA, miR-9 mimic, or miR-9 inhibitor for 48 h. Data are presented as the mean ± SEM (* p < 0.05, ** p < 0.01)
miR-9 decreases α-2,6-linked sialylation of integrin-β1 and activate FAK signaling pathway
To elucidate the mechanisms by which St6gal1-mediated protein sialylation is involved in HCC metastasis, St6gal1-regulated proteins were studied. To this end, cell lysates were incubated with agarose-conjugated SNA lectin to precipitate α-2,6-linked sialylated proteins. These proteins were resolved by SDS-PAGE and immunoblotted for HCC metastasis related protein EGFR [2, 22], caveolin-1 [16], and integrin-β1 [25] (Fig. 5a). Invisible difference between the EGFR protein levels was observed in mouse HCC cells, whereas no caveolin-1 bands were detected in SNA precipitate, suggesting that EGFR and CAVEOLIN-1 did not contribute to miR-9 regulated HCC metastasis. Integrin-β1, a known substrate of St6gal1, together with associated adaptor molecules, such as p130CAS, plays a role in promoting the migration of cancer cells. Here, integrin-β1 was found α-2,6-sialylated significantly higher in high-metastatic HCC cells than low-metastatic cells (Fig. 5a, b). Concurrently, downstream of integrin-β1, the cancer cell motility-, and migration-related protein focal adhesion kinase (FAK) was highly activated in Hca-P and Hca-F cells, suggesting that miR-9 may regulate HCC metastatic potential through sialylation and activation of integrin-β1/FAK signaling pathway.
miR-9 regulates the activation of integrin-β1/FAK signaling pathway by modulating sialylation. a To measure levels of sialylated metastasis related proteins in HCC, cell lysates were incubated with SNA-agarose. Sialylated proteins were precipitated and then immunoblotted for indicated antibody. b Integrin-β1 immunoprecipitations were performed in indicated cells, and immunoprecipitated fractions were analyzed by lectin blot for sialylation. Western blot was also performed to analyze expression levels of other indicated proteins. c Hepa1–6, Hca-P, and Hca-F were transfected with scrambled miRNA, miR-9 mimic, or miR-9 inhibitor for 48 h. Integrin-β1 immunoprecipitations were performed, and immunoprecipitated fractions were analyzed by lectin blot for sialylation. Western blot was also performed to analyze expression levels of other indicated proteins. d–e Transwell migration assays showed the migration ability of indicated cells transiently transfected with St6gal1 (St) for 48 h, or treated with 1 μM of PF-562,271 (PF) for 48 h. A total of 5 mM of mitomycin-C was used for inhibiting proliferation. Protein levels were examined by immunoprecipitations and Western blotting. Data are presented as the mean ± SEM (* p < 0.05, ** p < 0.01)
As FAK has been identified as a key component of the signal transduction pathways triggered by integrin-β1 [25], the functions of miR-9-regulted integrin-β1 sialylation in FAK signaling were demonstrated. As shown in Fig. 5c, St6gal1 protein level was upregulated in Hepa1–6 cells transfected with the miR-9 inhibitor, the levels of α-2,6-sialylated integrin-β1 and phosphorylated FAK were significantly increased. Sialylated integrin-β1 and phosphorylated FAK were significantly reduced by miR-9 compared to control groups in Hca-P and Hca-F cells, indicating that miR-9 could regulate the activation of integrin-β1/FAK signaling by modulating α-2,6-linked sialylation. Likewise, St6gal1 overexpression in Hepa1–6 cells increased the sialylation level of integrin-β1 and activation of FAK accompanying with a significant increase in cell metastatic capacity. Then, we examined whether FAK signaling inhibition could attenuate HCC metastatic capacity (Fig. 5d). PF-562,271 (PF, a small-molecule inhibitor of FAK) downregulated phosphorylation of FAK without affecting the sialylation of integrin-β1 in St6gal1 overexpressed Hepa1–6 cells [27]. PF also prevented the upregulation of metastatic capacity by St6gal1 overexpression. Similarly, the inhibition of FAK signaling could reduce cell metastatic capacity in Hca-P and Hca-F cells, suggesting an important role of FAK signaling in St6gal1 induced HCC metastasis (Fig. 5e). These data demonstrated that miR-9 could regulate the activation of integrin-β1/FAK signaling pathway by modulating sialylation, and this would attenuate the metastasis in mouse HCC cells.
Discussion
We and others have previously reported that miRNAs play an important role in tumor progression by targeting specific glycosyltransferases that catalyze the formation of specific glycan structures [9, 10, 12]. Although many studies have indicated that increasing the α-2,6-linked sialyation of glycoproteins is a crucial event in the both processes of oncogenic transformation and metastasis [15, 17], the data we obtained in this study suggest a more important role of miRNAs in regulating α-2,6-linked sialyation-mediated HCC metastasis. To the best of our knowledge, the present study has revealed a previously unknown mechanism by which St6gal1 and α-2,6-linked sialyation levels are regulated by miR-9. miR-9 targeted St6gal1 directly and reversed HCC cells metastasis. Moreover, using a miR-9 mimic and inhibitor confirmed the α-2,6-linked sialyation attenuating activity of miR-9. Finally, cell metastatic, invasive, and adhesion ability could be reduced by miR-9, and the sialyation and activation of integrin-β1/FAK signaling pathway were also decreased, resulting in the suppression of mouse HCC cell metastasis. Thus, these data proposed a potential biological mechanism by which miR-9 influences St6gal1 expression and results in tumor metastasis suppression consequences.
Metastatic spreading is a complex, multi-step process and acts as the main cause of death in patients diagnosed with cancer. This process requires that cancer cells display an aberrant phenotypic plasticity, including glycosylation. Although protein-related pathways involved in this complex phenomenon have been characterized deeply, we are not currently able to account for the accurate mechanisms of metastasis.
Hca-F and Hca-P cells, lymphatic metastasizing clones isolated from the H22 cell line, form lymphatic metastasis in 615 mice upon subcutaneous injection into the foot pad, while Hepa1–6 cells do not cause lymphatic metastasis. miRNA microarray was performed to analyze the miRNA profiles in these HCC cells. miR-9 levels were found to be negatively correlated with cell metastatic potential. The results of bioinformatics analysis suggested that miR-9 may regulate St6gal1, which is a key enzyme of cancer metastasis-related sialyation. Consistently, a strong correlation between the expression of St6gal1 mRNA and metastatic potential of HCC cells was detected, indicating an inverse relationship between miR-9 and St6gal1 in HCC cell metastasis.
Although the first miRNA was identified decades ago, researchers have only just begun to understand the scope and diversity of these non-coding regulatory RNAs in recent years [19]. Growing evidence has shown that miRNAs represent a variety of crucial regulatory functions in biological processes, including cell growth, differentiation, and apoptosis, and that they are associated with a wide variety of human diseases [21]. Unfortunately, limited research has linked miRNAs to aberrant glycosylation-mediated cancer metastasis. miR-9 is a cancer-suppressor miRNA [14]. It was reported that miR-9 was downregulated in various cancer cell lines and tumor samples. miR-9 was also identified as one of the most downregulated miRNAs in recurrent tumors and participated in the determination of neural fates in embryonic stem cell differentiation [18]. In early breast cancer development, miR-9 was transcriptionally downregulated by methylation [20]. It is becoming increasingly evident that miR-9 act as a tumor-suppressor in HCC by affecting cell proliferation and metastasis [13, 28]. By coincidence, our data indicated that the miR-9 level was upregulated by 4-fold and 2-fold in Hepa1–6 (with no metastatic lymphatic potential) than in Hca-F (with higher lymphatic metastatic potential) and Hca-P (with lower lymphatic metastatic potential) cells. Furthermore, an inverse correlation between miR-9 level and St6gal1/α-2,6-linked sialic acid modification was found in mouse HCC cells. Given the metastatic potential of tumor cells was correlated with the expression of α-2,6-linked sialic acid structures which recognized specifically by SNA lectin, we speculate that miR-9 may act as a metastasis-suppressor via repressing α-2,6-linked sialyation.
To confirm that St6gal1 is a target of miR-9, luciferase reporter assays were performed. Further experiments demonstrated that miR-9 overexpression decreased α-2,6-linked sialic acid expression on the cell surface and reduced adhesive and metastatic capacity of Hca-P and Hca-F cells. Meanwhile, a miR-9 downregulation increased α-2,6-linked sialic acid expression and stimulates the adhesive and invasive ability of Hepa1–6 cells. Therefore, miR-9 could inhibit mouse HCC cells metastatic activity by, at least in part, repressing St6gal1 activity in sialylation pathway in.
As a regulator of sialyation, miR-9 could also modulate signaling pathways downstream of St6gal1 indirectly. Among them, integrin-β1 is known as one of integrin family of cell adhesion receptors, which are involved in cell-matrix adhesion in cancer cells [25]. It was reported that St6gal1could regulate the cell migration and invasion partly by increasing the α-2,6-linked sialyation of integrin-β1 [24]. Coincidentally, our results showed that miR-9 could modulate the sialyation of integrin-β1 by suppressing St6gal1 expression. Moreover, FAK signaling pathway, which controls cell motility [25], was found to be activated by sialyation of integrin-β1. These findings suggested that integrin-β1/FAK signaling takes part in miR-9-induced suppression of mouse HCC cells adhesion and metastasis. However, the exact mechanism underlying miRNA, sialyation, and metastasis requires further investigation.
In summary, our findings identify, as a cancer-suppressor, that miR-9 could repress metastasis by targeting St6gal1, which regulates the α-2,6-linked sialyation on mouse HCC cells. This study provides novel insights into the mechanisms by which miR-9 inhibits metastasis and reveals an inverse correlation between miR-9 and α-2,6-linked sialic acid structure levels. This novel evidence may contribute to understand the potential roles of miR-9 in HCC metastasis and supports the possibility of targeting St6gal1 by miR-9 as a novel therapeutic strategy for cancer metastasis.
References
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Britain CM, Holdbrooks AT, Anderson JC, Willey CD, Bellis SL (2018) Sialylation of EGFR by the ST6Gal-I sialyltransferase promotes EGFR activation and resistance to gefitinib-mediated cell death. J Ovarian Res 11:12
Chen X, Wang L, Zhao Y, Yuan S, Qiang W, Zhu X, Niang B, Wang S, Zhang J (2016) ST6Gal-I modulates docetaxel sensitivity in human hepatocarcinoma cells via the p38 MAPK/caspase pathway. Oncotarget 7:51955–51964
Deng B, Wang B, Fang J, Zhu X, Cao Z, Lin Q, Zhou L, Sun X (2016) MiRNA-203 suppresses cell proliferation, migration and invasion in colorectal cancer via targeting of EIF5A2. Sci Rep 6:28301. https://doi.org/10.1038/srep28301
Dennis JW, Granovsky M, Warren CE (1999) Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta Gen Subj 1473:21–34
Esquela-Kerscher A, Slack FJ (2006) Oncomirs—microRNAs with a role in cancer. Nat Rev Cancer 6:259–269
Fu X, Meng Z, Liang W et al (2014) miR-26a enhances miRNA biogenesis by targeting Lin28B and Zcchc11 to suppress tumor growth and metastasis. Oncogene 33:4296–4306
Fu XT, Shi YH, Zhou J, Peng YF, Liu WR, Shi GM, Gao Q, Wang XY, Song K, Fan J, Ding ZB (2017) MicroRNA-30a suppresses autophagy-mediated anoikis resistance and metastasis in hepatocellular carcinoma. Cancer Lett 412:108–117. https://doi.org/10.1016/j.canlet.2017.10.012
Gao Y, Liu T, Huang Y (2015) MicroRNA-134 suppresses endometrial cancer stem cells by targeting POGLUT1 and Notch pathway proteins. FEBS Lett 589:207–214
González-Vallinas M, Molina S, Vicente G, Zarza V, Martín-Hernández R, García-Risco MR, Fornari T, Reglero G, De Molina AR (2014) Expression of microRNA-15b and the glycosyltransferase GCNT3 correlates with antitumor efficacy of Rosemary diterpenes in colon and pancreatic cancer. PLoS One 9:e98556
Guo Y, Li S, Qu J, Wang S, Dang Y, Fan J, Yu S, Zhang J (2011) MiR-34a inhibits lymphatic metastasis potential of mouse hepatoma cells. Mol Cell Biochem 354:275–282
Guo Y, Li S, Qu J, Ye L, Wang S, Fan J, Wang Q, Zhang J (2014) Let-7c inhibits metastatic ability of mouse hepatocarcinoma cells via targeting mannoside acetylglucosaminyltransferase 4 isoenzyme A. Int J Biochem Cell Biol 53:1–8
Higashi T, Hayashi H, Ishimoto T, Takeyama H, Kaida T, Arima K, Taki K, Sakamoto K, Kuroki H, Okabe H (2015) miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in hepatocellular carcinoma cells. Br J Cancer 113:252–258
Huang Y, Shen XJ, Zou Q, Wang SP, Tang SM, Zhang GZ (2011) Biological functions of microRNAs: a review. J Physiol Biochem 67:129–139. https://doi.org/10.1007/s13105-010-0050-6
Hung WC, Chiang CH (2017) Up-regulation of sialyltransferases increases lymphatic metastasis in pancreatic cancer via the integrin-mediated pathway. Pancreatology 17:S19
Jia L, Wang S, Zhou H, Cao J, Hu Y, Zhang J (2006) Caveolin-1 up-regulates CD147 glycosylation and the invasive capability of murine hepatocarcinoma cell lines. Int J Biochem Cell Biol 38:1584–1593
Jungjin Park ML (2013) Increasing the α-2,6-sialylation of glycoproteins may contribute to metastatic spread and therapeutic resistance in colorectal cancer. Gut Liver 7:629–641
Krichevsky AM, Sonntag KC, Isacson O, Kosik KS (2006) Specific microRNAs modulate embryonic stem cell-derived neurogenesis. Stem Cells 24:857–864
Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW (2004) Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117:69–81
Lehmann U, Hasemeier B, Christgen M, Müller M, Römermann D, Länger F, Kreipe H (2008) Epigenetic inactivation of microRNA gene hsa-mir-9-1 in human breast cancer. J Pathol 214:17–24
Li Y, Qiu C, Tu J, Geng B, Yang J, Jiang T, Cui Q (2013) HMDD v2. 0: a database for experimentally supported human microRNA and disease associations. Nucleic Acids Res 42(D1):D1070–D1074
Li T, Dong ZR, Guo ZY, Wang CH, Zhi XT, Zhou JW, Li DK, Chen ZT, Chen ZQ, Hu SY (2015) Mannose-mediated inhibitory effects of PA-MSHA on invasion and metastasis of hepatocellular carcinoma via EGFR/Akt/IκBβ/NF-κB pathway. Liver Int 35:1416–1429
Schultz MJ, Swindall AF, Wright JW, Sztul ES, Landen CN, Bellis SL (2013) ST6Gal-I sialyltransferase confers cisplatin resistance in ovarian tumor cells. J Ovarian Res 6:25
Seales EC, Jurado GA, Brunson BA, Wakefield JK, Frost AR, Bellis SL (2005) Hypersialylation of beta1 integrins, observed in colon adenocarcinoma, may contribute to cancer progression by up-regulating cell motility. Cancer Res 65:4645
Seguin L, Desgrosellier JS, Weis SM, Cheresh DA (2015) Integrins and cancer: regulators of cancer stemness, metastasis, and drug resistance. Trends Cell Biol 25:234
Shah MH, Telang SD, Shah PM, Patel PS (2008) Tissue and serum α2-3- and α2-6-linkage specific sialylation changes in oral carcinogenesis. Glycoconj J 25:279–290
Stokes JB, Adair SJ, Slackdavis JK, Walters DM, Tilghman RW, Hershey ED, Lowrey B, Thomas KS, Bouton AH, Hwang RF (2011) Inhibition of focal adhesion kinase by PF-562,271 inhibits the growth and metastasis of pancreatic cancer concomitant with altering the tumor microenvironment. Mol Cancer Ther 10:2135–2145
Tan HX, Qian W, Chen LZ, Huang XH, Chen JS, Fu XH, Cao LQ, Chen XL, Wen L, Zhang L (2010) MicroRNA-9 reduces cell invasion and E-cadherin secretion in SK-Hep-1 cell. Med Oncol 27:654
Zhao Y, Li Y, Ma H, Dong W, Zhou H, Song X, Zhang J, Jia L (2014) Modification of sialylation mediates the invasive properties and chemosensitivity of human hepatocellular carcinoma. Mol Cell Proteomics 13:520–536
Funding
The authors would like to gratefully acknowledge support from the Natural Science Foundation of China (21502015, 31570802) and the Fundamental Research Funds for the Central Universities (DUT17JC21, DUT18ZD208, DUT18LAB09).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there are no conflicts of interest.
Electronic supplementary material
ESM 1
(DOCX 957 kb)
Rights and permissions
About this article
Cite this article
Han, Y., Liu, Y., Fu, X. et al. miR-9 inhibits the metastatic ability of hepatocellular carcinoma via targeting beta galactoside alpha-2,6-sialyltransferase 1. J Physiol Biochem 74, 491–501 (2018). https://doi.org/10.1007/s13105-018-0642-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-018-0642-0