Abstract
Exposure to fine particulate matter (PM2.5) air pollution is a risk factor for type 2 diabetes (T2DM). We argue whether the potentiating effect of PM2.5 over the development of T2DM in high-fat diet (HFD)-fed mice would be related to modification in cell stress response, particularly in antioxidant defenses and 70-kDa heat shock proteins (HSP70) status. Male mice were fed standard chow or HFD for 12 weeks and then randomly exposed to daily nasotropic instillation of PM2.5 for additional 12 weeks under the same diet schedule, divided into four groups (n = 14–15 each): Control, PM2.5, HFD, and HFD + PM2.5 were evaluated biometric and metabolic profiles of mice, and cellular stress response (antioxidant defense and HSP70 status) of metabolic tissues. Extracellular to intracellular HSP70 ratio ([eHSP72]/[iHSP70]), viz. H-index, was then calculated. HFD + PM2.5 mice presented a positive correlation between adiposity, increased body weight and glucose intolerance, and increased glucose and triacylglycerol plasma levels. Pancreas exhibited lower iHSP70 expression, accompanied by 3.7-fold increase in the plasma to pancreas [eHSP72]/[iHSP70] ratio. Exposure to PM2.5 markedly potentiated metabolic dysfunction in HFD-treated mice and promoted relevant alteration in cell stress response assessed by [eHSP72]/[iHSP70], a relevant biomarker of chronic low-grade inflammatory state and T2DM risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fine particulate matter of 2.5 μm or smaller sizes (PM2.5) contributes to air pollution being a consequence of urbanization process and an important risk factor for the development of diseases in a global scale [7, 9]. Epidemiological studies show important links between the exposure to PM2.5 and the risk for the development of chronic diseases, such as type 2 diabetes mellitus (T2DM) [4, 28, 30]. Inhalation of PM2.5 induces systemic effects, including injuries to several tissues, such as the metabolic ones. PM2.5 can promote tissue injury by direct effects of particle components leading to oxidative and cellular stress, and, also, by indirect effects, triggering the production of pro-inflammatory mediators, with consequent systemic low-grade inflammation [11, 26].
The link between PM2.5 exposure and T2DM may be mediated by enhanced insulin resistance and visceral inflammation [29], this being increasingly remarkable when associated with other risk factors, such as consumption of high-fat diet [29, 34]. These two factors (HFD and PM2.5) seem to be of particular relevance, once changes in lifestyle and environment are consequences of urbanization, reaching global dimensions on public health.
HFD promotes changes in stress response, preceding major metabolic defects associated with T2DM, and may be an important event in the pathogenesis of the disease, while this harmful combination (HFD and PM2.5) can also make the individual susceptible to a series of different additional risk factors [12]. PM2.5 per se triggered metabolic tissues and may increase this pathophysiological effects on the development of metabolic disorders [25]. Here, we wonder how the metabolic tissues are defending themselves through classical stress responses, in the context of the risks posed by urbanization, such as the consumption of HFD and exposure to air pollution by PM2.5.
Under stressful conditions (e.g., environmental and metabolic challenges), cells respond by synthesizing a particular set of stress response proteins, which play a fundamental role in metabolic disorders, the 70-kDa family of heat shock proteins (HSP70) [12]. Inside the cells, both the 73-kDa constitutive and the 72-kDa inducible forms of HSP70 (taken as a whole as iHSP70) exert several chaperone and anti-inflammatory effects while decreased iHSP70 levels are related to insulin resistance [6, 18]. On the other hand, the inducible form released toward the extracellular space (eHSP72) activates pro-inflammatory pathways and is associated to oxidative stress conditions and the diabetes progression [18].
It is supposed that the imbalance between [eHSP72]/[iHSP70] ratio is associated with the risk of developing chronic diseases, such as diabetes. However, the effect of the association of HFD with PM2.5 in this specific parameter has not been addressed. Since alterations in antioxidant defense and [eHSP72]/[iHSP70] ratio can mark the process of tissue injury and metabolic changes, we used these variables to investigate the effects of exposure to air pollution associated with the consumption of unhealthy diet on nutrient metabolism-related tissues (NMRT). We hypothesized that sub-chronic exposure to PM2.5 in HFD-treated mice (susceptible to T2DM) would also be able to change NMRT cellular antioxidant defense, and the balance between iHSP70 and eHSP72 contents expressed as [eHSP72]/[iHSP70]ratio, predisposing for a major risk of cellular damage and development T2DM.
Materials and methods
Animals
Male (n = 59) just-weaned 21 days old B6.129SF2/J mice (approximately 14 g), from Animal Facility of Regional University of the Northwestern Rio Grande do Sul State (UNIJUÍ), were kept in semi-metabolic cages, under controlled conditions of temperature (22 ± 2 °C), relative humidity (50–60 %) and light-dark cycles (light from 7:00 a.m. to 7:00 p.m.). The animals received water and diet ad libitum. This protocol was approved by the Animal Ethics Committee of UNIJUÍ (CEUA 009/13).
Experimental design
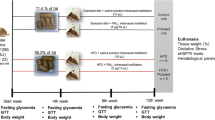
The mice were randomly divided into four groups: control (n = 15), PM2.5 (n = 15), HFD (n = 14), and HFD + PM2.5 (n = 15). Animals in control and PM2.5 groups received standard diet (11.4 % calories from fat) for 24 weeks, while animals from HFD and HFD + PM2.5 groups received a diet containing 58.3 % calories from fat for the same period (see below, please). At the 12th week of study, PM2.5 and HFD + PM2.5 groups received intranasal instillation of PM2.5 (5 μg/10 μL) daily, while control or HFD groups received saline solution, for the subsequent 12 weeks (up to 24 weeks). Experimental design that characterizes the sub-chronic protocol is summarized in Fig. 1. Sub-chronic exposure can be defined as the repeated exposure by inhalation route for more than 30 days up to 90 days, in typically used laboratory animal species [8].
Experimental design. After weaning, 59 mice were randomly divided into four groups: Animals in control and PM2.5 groups received standard diet (11.4 % calories from fat), and animals from HFD and HFD + PM2.5 groups received HFD (58.3 % energy from fat) for 24 weeks. At 12th week, PM2.5 and HFD + PM2.5 groups received intranasal instillation of PM2.5 (5 μg/10 μL) daily, while control or HFD groups received saline solution, for the subsequent 12 weeks (up to 24 weeks)
Diet
The standard pelleted diet, offered to control and PM2.5 groups, consisted of crude protein, mineral material, fibrous matter, and minerals (Nuvilab CR-1, commercially obtained from Nuvital Nutrientes SA.; total metabolizable energy 16.6 MJ/kg, being 11.4 % as fats, 62.8 % as carbohydrates, and 25.8 % as proteins). For the HFD groups, it was provided a lard-based diet (37.4 % w/w; total metabolizable energy 22.8 MJ/kg, being 58.3 % as fats, 24.5 % as carbohydrates, and 17.2 % as proteins) [2, 38]. All diet ingredients in the HFD (except for starch and lard) were adjusted to be present at the same amount as in the standard one. To meet this pattern, 13.7 % egg albumin, 7.4 % amino acid supplement (Aminomix™), and 1.1 % dried bone and oyster flour were used to freshly prepare the experimental diet on a weekly basis. The HFD was prepared weekly in the Laboratory of Biological Assays (UNIJUI) and was conserved under refrigeration (4–8 °C) during this period.
Characterization of particulate matter
The pollutant used in the experiment was PM2.5, collected in polycarbonate filter through a gravimetric collector, on the terrace of the Faculty of Medicine, University of São Paulo (USP), in São Paulo, Brazil, as previously described [22]. The exposure site was located close to a monitoring station of the State of São Paulo Sanitation Agency. It is estimated that at least 100,000 vehicles circulate daily on the main and lateral street (∼83 % cars, ∼10 % diesel vehicles, ∼6 % motorcycles). There are no industries or significant biomass sources in the surrounding area. Trace element determinations of PM content were carried out by neutron activation analysis, and their concentration was expressed by nanograms per cubic meter of air as follows: As, 12.91 ± 0.53; Br, 8.88 ± 0.39; Cl, 8.88 ± 0.39; Co, 1.14 ± 0.04; Fe, 1.15 ± 0.03; La, 2.33 ± 0.29; Mn, 27.5 ± 2.2; Sb, 8.73 ± 0.08; Sc, 0.141 ± 0009; and Th 0.351 ± 0.050. Likewise, PM sulfur concentration, determined by X-ray fluorescence analysis, was 1.424 ± 0.08 μm/m3. Briefly, after exposure (24 h), the filter was removed and retained particles were obtained by sonication, with ultrasound bath in seven sessions (50 min each) and resuspended in saline solution at a dose of 5 μg/10 μL. The process of nasotropic instillation was performed once a day (at 1:00 and 2:00 p.m.), for 12 weeks with an automatic pipette, with 10 μL of solution in the nostril of the animal. This procedure induces apnea reflex promoting the inhalation of the pollutant [24].
Food and water consumption
The water [volume offered minus remaining volume in the bottle (mL)] and food consumption [diet offered minus remaining diet in the box (g)] were monitored three times per week during the 24-week study.
Biometric profile
The biometric profile of animals was monitored once a week for 24 weeks. It was measured body weight (g) and length (cm), and carried out the calculation of the Lee Index [19]. Body weight was checked with semi-analytical scale and length was verified by naso-anal distance. The Lee Index consists to divide the cube root of body weight (g) by naso-anal distance (cm), the result of the weight/length division is multiplied by 1000 [19]. We also evaluate adiposity [% of epididymal white adipose tissue (EWAT)/body weight] and % of liver, gastrocnemius muscle, and pancreas weight in relation to body weight.
Glucose and lipid levels
Blood glucose, triacylglycerol, and cholesterol plasma levels were monitored every 2 weeks, after 12 h of fasting. Blood glucose was measured by Glucometer Optium Xceed (Abbott) (5 μL of blood), while triacylglycerol and total cholesterol levels were assessed with Accutrend® Plus System (Roche) (25 μL of blood) in a puncture of the distal part of the tail of mice. The results of glycemia were expressed in millimoles per liter, whereas triacylglycerol and cholesterol levels were expressed in milligrams per deciliter of blood. The response of glucose, triacylglycerol, and cholesterol levels over 24 weeks was assessed by calculating the incremental area under the curve (IAUC), calculated geometrically by applying the trapezoid rule [37]. To avoid stress, we monitored each variable in different mice subgroups once every 2 weeks. Thus, each experimental group (n = 15) was divided in five animals/group for each variable measurement (glycemia, triacylglycerols, and total cholesterol).
GTT
The glucose tolerance test was performed in the 4th, 8th, 12th, 16th, 20th, and 24th weeks of intervention in all animals. Food was withdrawn in the night before experiments (12 h before). Glycemia was measured as described above immediately before and at 30 and 120 min after glucose (1 g/kg in saline solution, i.p.) administration. The glycemic response during glucose tolerance test (GTT) was evaluated by the IAUC method [37].
Tissue preparation
At the end of the 24 weeks of intervention, the animals were euthanized. NMRT (liver, pancreas, gastrocnemius muscle, and EWAT) were dissected, weighed, freeze-clamped in liquid nitrogen, and stored for further homogenization. For the analysis of antioxidant activity enzymes superoxide dismutase (SOD) and catalase (CAT), a portion of the tissues was homogenized in potassium phosphate buffer pH 7.4. To determine iHSP70 expression by Western blotting, another portion of the tissues was homogenized in 0.1 % (w/v) sodium dodecyl sulfate (SDS) buffer.
All tissues were homogenized (still frozen) in respective buffers, containing protease and phosphatase inhibitor cocktail (Sigma) consisting of (final concentrations) leupeptin (4.2 μM), aprotinin (0.31 μM), TLCK (N-tosyl-l-lysine chloromethyl ketone, hydrochloride; 20 μM), PMSF (phenyl-methyl-sulfonyl fluoride, 100 μM), sodium orthovanadate (Na3VO4; 1 mM), sodium molybdate (Na2MoO4; mM), and β-glycerophosphate (1 mM). Afterward, the homogenates were centrifuged at 15000×g for 5 min at room temperature, and the supernatant fractions were saved for protein determination by the spectrophotometric method Bradford (1976) [3] at 595 nm, using bovine serum albumin as standard.
Evaluation of antioxidant activity and HSP70 content
Determination of SOD and CAT activity
SOD activity was performed by inhibition of auto-oxidation of pyrogallol [23]. Briefly, in a cuvette, 930 μL of 50 mM Tris/1 mM EDTA buffer (pH 8.2), 4 μL of catalase (CAT; 30 μM), and 50 μL of homogenate were added and mixed. After, pyrogallol (24 mM in HCl 10 mM) was added and SOD activity determined at 25 °C in spectrophotometer (420 nm) for 120 s. Results were expressed in units of SOD mg−1 of protein.
CAT activity was performed accordingly to Aebi [1]. In a quartz cuvette, 30 μL of homogenate and 2865 μL of phosphate buffer (50 mM, pH 7.4) were mixed, and after, 105 μL of hydrogen peroxide (0.01 M) was added and mixed. The decomposition of hydrogen peroxide by CAT activity was determined at 25 °C in spectrophotometer (240 nm) for 120 s. The results were expressed in pmol s−1 mg of protein.
HSP70 tissue expression (iHSP70)
iHSP70 expression was evaluated in NMRT by immunoblot analyses [15]. Equivalent amounts of protein from each sample (∼40 μg) were mixed with Laemmli’s gel loading buffer [50 mM Tris, 10 % (w/v) SDS, 10 % (v/v) glycerol, 10 % (v/v) 2-mercaptoethanol, and 2 mg/mL bromphenol blue] in a ratio of 1:1, boiled for 5 min, and electrophoresed in a 10 % polyacrylamide gel (5 h in 15 mA/gel). After, the proteins were transferred onto a nitrocellulose membrane (GE HealthCare) by electrotransfer (1 h in 100 V), and subsequently, transferred bands were visualized with 3 % (w/v) Red Ponceau S (Sigma-Aldrich).
The procedures were performed with SNAP i.d. (Merck Millipore) vacuum system for rapid immunoblot. Membranes were washed with water and then blocked in 0.5 % (w/v) nonfat dry milk in washing buffer [TEN-Tween 20 solution (0.1 %, w/v); TEN is 50 mM Tris, 5 mM EDTA, 150 mM NaCl, pH 7.4]. Membranes were washed three times with wash buffer and incubated for 15 min with monoclonal anti-HSP70 antibody (Sigma-Aldrich H5147, 1:1000). After three consecutive washings with wash buffer, peroxidase-labeled rabbit anti-mouse IgG (Sigma-Aldrich A9044) was utilized as secondary antibody, at 1:15000 dilution. For gel loading control, it was used anti β-actin containing peroxidase (Sigma-Aldrich A3854, dilution 1:15000) or Coomassie Blue (0.1 % Coomassie blue, 40 % methanol, 10 % acetic acid). Blot visualization was performed by using ECL-Prime Western blotting Reagent (GE Healthcare). Quantification of bands was performed using the ImageJ® software. The data are presented in arbitrary units of iHSP70, normalized in terms of β-actin.
Plasma HSP72 (eHSP72) quantification
A highly sensitive EIA method (EKS-715 Stressgen, Victoria, BC, Canada) was used to determine the amount of eHSP72 protein in plasma as previously described [36]. Absorbance was measured at 450 nm and a standard curve constructed from known dilutions of HSP72 recombinant protein to allow quantitative assessment of eHSP72 plasma concentration. Quantification was made using a microplate reader (Mindray MR-96A). Intra-assay coefficient of variation was identified as being <2 %.
[eHSP72]/[iHSP70] ratio
After eHSP72 (plasma) and iHSP70 (NMRT) measurements, extracellular to intracellular HSP70 ratio index (H-index) was established. This index reveals the immunoinflammatory status [10, 17, 18]. The rationale for this is that the higher eHSP70 amounts, the more inflammatory signals are coming into play, because eHSP70 is pro-inflammatory in nature. On the other hand, for each particular “j” situation, the more the cells are able to respond to stressful stimuli by enhancing iHSP70, the more such cells are facing a state of anti-inflammation.
Therefore, if one takes Rc = [eHSP70]c/[iHSP70]c as the HSP70 ratio in a control situation, whatever the techniques used to assess each eHSP70 and iHSP70, then H-index can be calculated as the quotient of any Rj = [eHSP70]j/[iHSP70]j by Rc, which will be therefore considered as the unity (Rc = 1), normalizing all the remaining results in this situation j. Hence, H-index = Rj/Rc may allow for the comparisons between any stressful situation j and the situation assumed as the control one. H-index can be applied to estimate immunoinflammatory status in many different situations, as dietary and/or environmental challenges responses related in each tissue.
Statistical analysis
Statistical analysis was developed using one-way analysis of variance (ANOVA). Post hoc multiple comparisons among groups were performed with the Tukey’s test. The Pearson’s correlation test was employed to test the associations between the variables in each experimental group (adiposity/body weight and IAUC response to GTT at the 24th week/body weight). All statistical analyses were performed using SPSS for Windows, version 19.0. The level of significance was set to P < 0.05. Results were expressed as mean ± standard deviation.
Results
HFD and PM2.5 association increased adiposity and promoted a decrease in both muscle and pancreatic mass
Figure 2a shows biometric profile of the animals studied herein. All groups presented similar body weight (body weight AUC; Fig. 2a) (P = 0.0626) and body length (P = 0.169; F 3,58 = 1.742) during the 24 weeks of the study, and consequently, all groups showed similar Lee index (P = 0.289; F 3,52 = 1.286). Likewise, no difference was observed in water consumption (mL/week/animal) (P = 0.747; F 1,10 = 1.154) or amount of food intake (g/week/animal) (P = 0.105; F 1,10 = 2.015). The animals treated with standard diet consumed the equivalent of 68.9 ± 6.6 kJ/day, and the animals that received high-fat diet consumed 62.8 ± 9.6 kJ/day with no interference by PM2.5 exposition (P = 0.2322; F 1,10 = 2.100).
Effect of PM2.5 exposure on body weight and percentage weight of tissues in mice treated with HFD during 24 weeks (mean ± SD). a Area under the curve of the body weight during the 24 weeks (P = 0.0641, F 3,52 = 2.591). b Percentage of adiposity (as described in methods) (*/†) vs control and PM2.5 (P < 0.001, F 3,51 = 40.82). c Percentage of pancreas weight/final body weight (*/†) vs control and PM2.5 (P = 0.008, F 3,54 = 5.604). d Percentage of gastrocnemius weight/final body weight (*) vs control (P = 0.028, F 3,55 = 3.275). n = 13–15 per group
Despite no difference that was observed in the variables listed above, animals that received HFD (HFD and HFD + PM2.5 groups) showed higher adiposity (2 % or more of EWAT weight/body weight) as compared to normal fed mice (control and PM2.5 groups) (Fig. 2b). In addition, HFD + PM2.5 group presented at least a 10 % decrease in both pancreas and muscle mass (Fig. 2c, d, respectively), while the liver mass was not influenced by any treatment (control 3.2 ± 0.3 g; PM2.5 3.1 ± 0.3 g; HFD 3.2 ± 0.2 g; HFD + PM2.5 3.1 ± 0.3 g; P = 0.270, F 3,57 = 1.343).
HFD changes the metabolic profile and PM2.5 potentiates these effects on glycemia
The effect of HFD + PM2.5 on metabolic profile was evaluated weekly during 24 weeks of the study. Fasting glycemia, triacylglycerol, and cholesterol plasma levels were not influenced by the experimental interventions at week 24 (Fig. 3a, c, e). However, we observed that HFD + PM2.5 promoted an increase in glycemic profile, as inferred from the AUC (glycemia x time) in the experimental period (Fig. 3b). Also, evaluating the AUC (triacylglycerols × time) in the experimental period, we found that HFD groups (HFD and HFD + PM2.5 groups) showed increased triacylglycerol levels during the 24 weeks (Fig. 3d). No modifications were observed in cholesterol levels during the study (Fig. 3f).
Effect of PM2.5 exposure in mice treated with HFD on the glycemia, triacylglycerol, and cholesterol during 24 weeks of study (mean ± SD). a Glycemia. b AUC of glycemia levels during 24 weeks (*) vs control (P = 0.0363, F 3,32 = 3.201). c Triacylglycerol. d AUC of Triacylglycerol levels during 24 weeks (*/†) vs control and PM2.5 (P = 0.0418, F 3,22 = 3.236). e Cholesterol. f AUC of cholesterol levels during 24 weeks (P = 0.252, F 3,22 = 1.461). n = 6–9 per group
Sub-chronic exposure to PM2.5 worsened the response to a glucose overload in animals treated with HFD
We evaluated the effect of HFD + PM2.5 on mouse metabolic response to glucose overload by IAUC-GTT before the PM2.5 intervention (at 4th, 8th, 12th weeks, Fig. 4a–c) and after the PM2.5 intervention (at 16th, 20th, and 24th week, Fig. 4d–f). From the 12th week of the study, animals that received HFD showed impaired metabolic response to a glucose overload (Fig. 4c–e, respectively). From the 20th week, the effect of the HFD + PM2.5 association becomes evident, by the worst metabolic response to the GTT, evaluated by IAUC-GTT in both 20th and 24th weeks (Fig. 4f).
Effect of PM2.5 exposure on glucose tolerance test in mice treated with HFD during 24 weeks. Glucose tolerance test (GTT) (mean ± SD). a Fourth week (P = 0.954, F 3,28 = 0.109). b Eighth week (P = 0.071, F 3,38 = 2.554). c Twelfth week (*) vs control (P = 0.004 F3,32 = 5.455). d Sixteenth week (*/†) vs control and PM2.5 (P < 0.001, F 3,29 = 20.665). e Twentieth week (*/†) vs control and PM2.5 (P = 0.002, F 3,28 = 6.748). f Twenty-fourth week (*) vs control (*/†) vs control and PM2.5 vs PM2.5 (P < 0.001, F 3,35 = 11.106). n = 7–8 per group
Altogether, the results indicated a positive correlation between adiposity and final body weight (Fig. 5a; r = 0.4461, P = 0.001). The HFD effect (more than 2 % of adiposity) is highlighted in Fig. 5a showed by right points from vertical line in the gray background area in the graph. Analyzing each group separately, only HFD + PM2.5 group presented positive correlation between adiposity and final body weight (HFD + PM2.5, r = 0.702, P = 0.005; control, r = 0.539, P = 0.070; PM2.5, r = 0.237, P = 0.459; HFD, r = 0.213, P = 0.484). In the same way, and considering metabolic status, we observed a positive correlation between adiposity and glucose intolerance (IAUC-GTT; r = 0.591, P = 0.003) (Fig. 5b). In this correlation, only HFD animals showed values higher than 300 min mmol−1 L−1 in the IAUC-GTT with adiposity equal or higher than 2 % (light and dark gray background in the Fig. 5b). Hence, this confirms the hypothesis that sub-chronic exposure to PM2.5 worsens metabolic dysfunction induced by HFD, since within the same range of adiposity (2 % or more), only the HFD + PM2.5 group showed values higher than 450 min mmol−1 L−1 in the IAUC-GTT (dark gray background on the Fig. 5b). The adiposity effect of HFD + PM2.5 combination can be observed directly in the mice carcass, as observed in Fig. 6.
Effect of PM2.5 exposure on adiposity (% of epididymal white adipose tissue weight/final body weight), final body weight, and IAUC response to GTT in mice treated with HFD during 24 weeks (mean ± SD). a Correlation between adiposity and final body weight. b Correlation between adiposity and IAUC response to GTT at the 24th week. n = 5–14 per group
Effect of PM2.5 exposure on adiposity (epididymal white adipose tissue (EWAT)) in mice treated with HFD during 24 weeks. Representative pictures of mice treated with a standard diet and intranasal instillation of saline (CONTROL), b standard diet and intranasal instillation of PM2.5 (PM2.5), c high-fat diet and intranasal instillation of saline (HFD), and d high-fat diet and intranasal instillation of PM2.5 (HFD + PM2.5)
HFD + PM2.5 modify the defense capacity of the NMRT and the [eHSP72]/[iHSP70] ratio
To evaluate the stress response induced by exposure to HFD + PM2.5 association, we evaluated the iHSP70 expression and CAT and SOD activities (Table 1) in NMRT. EWAT had higher iHSP70 expression in all experimental groups (PM2.5, HFD, and HFD+ PM2.5), on average 7.2-fold as compared to the control group (Table 1). Groups that received HFD (HFD and HFD + PM2.5) showed higher EWAT antioxidant enzyme activity (CAT and SOD). Specifically, HFD + PM2.5 promoted 4.0-fold increase in CAT activity and 2.7-fold increase in SOD activity compared to the others groups. In the liver, the iHSP70 expression was 8.0-fold higher in HFD + PM2.5 group and had lower CAT activity when compared to control group (Table 1). Groups that received HFD (HFD and HFD + PM2.5 groups) showed higher SOD activity (1.1-fold). On the contrary, pancreas showed lower iHSP70 expression in HFD groups (HFD and HFD + PM2.5 groups) (Table 1) and higher CAT activity in HFD + PM2.5 group (1.5-fold) as compared to control and PM2.5 groups, with no effects in SOD activity. In the gastrocnemius muscle, we did not find alterations in iHSP70 expression (Table 1) or in the activities of CAT and SOD.
We evaluated the eHSP72 levels in plasma and performed the [eHSP72]/[iHSP70] ratio considering the iHSP70 amount inside each tissue. Despite no alterations in plasma eHSP72 levels induced by PM2.5 or HFD or HFD + PM2.5 association, the [eHSP72]/[iHSP70] ratio (eHSP72-plasma/iHSP70-pancreas ratio) was increased 3.7-fold in HFD + PM2.5 as compared to control and PM2.5 groups (P = 0.006). No alterations in [eHSP72]/[iHSP70] ratio were found related to muscle, liver, or EWAT (Fig. 7).
Discussion
In this study, we confirm the hypothesis that sub-chronic exposure to PM2.5 can exacerbate the effects of HFD consumption, anticipating subclinical alterations for the development of metabolic disease in mice. In this context, we observed changes in cellular defense via HSP70 expression and antioxidant response. The strongly dangerous properties of air pollutants, especially PM2.5, may explain the multiple effects of these inhaled toxicants on different organs and disease overtness [29]. Therefore, we wonder how the metabolic tissues studied could be arming a cellular defense through the stress response, in the context of the risks posed by urbanization, as the consumption of HFD and exposure to air pollution.
Metabolic changes observed in our study are closely related to changes in NMRT. HFD + PM2.5 association resulted primarily in biometric changes, such as adiposity and loss of muscle mass. Muscle tissue plays a key role in the regulation of glucose metabolism [33], and the lean mass reduction can be involved with other biometric/metabolic alterations that configure susceptibility to the development of intolerance glucose. During the first 12 weeks of the study, the animals receiving HFD showed impaired response to glucose overload, and after this period, with the introduction of the PM2.5 by nasotropic instillation in this model of T2DM-susceptible mice, the impairment on the glucose metabolism became more evident when compared to other groups (HFD + PM2.5 group results in worse GTT response from the 20th to the last week of study). The HFD + PM2.5 association developed distinct biometric/metabolic characteristic in this group: mice with 2 % or more of adiposity, increased of fasting glycemia, triacylglycerol levels, and levels of blood glucose as 450 min mmol−1 L−1 in the IAUC-GTT.
It is worth mentioning that the effects of PM2.5 in our study were produced by low-dose (5 μg/10 μL) and sub-chronic exposure (more than 30 days and up to 90 days), while other studies have shown that long-term exposure to PM2.5 with previously HFD consumption may cause glucose intolerance in exposure to chambers or intratracheally PM inhalation experimental models [39, 40]. In fact, PM2.5 exposure harms whole-body insulin sensitivity and glucose homeostasis after a substantial latency period (>8 weeks) [20]. In our study, the PM2.5 was introduced at the 12th week of HFD treatment showing an effect on the GTT in the 20th weeks of the study (exactly 8 weeks of PM2.5), with most evident effect of HFD + PM2.5 at the 24th week, even at low doses. Taken together, these data suggest the potential of the PM2.5 to exacerbate previously established disturbances in metabolic status [2].
Xu et al. [39] discuss the term “meta-inflammation” suggesting that metabolic changes are related to the induction of reactive oxygen species (ROS) production and pro-inflammatory profile in response to the consumption of HFD, that is being enhanced by exposure to PM2.5, which led us to evaluate antioxidant enzyme activities and the stress response by iHSP70 expression and the H-index in such tissues.
Muscle, liver, and adipose tissues appear to be responsive to challenges due to antioxidant capacity. Submitted constantly to metabolic challenges, these tissues respond with preserved defense, even upon decreased muscle mass. In the present study, there was an increase in liver and adipose tissue iHSP70 expression induced by HFD + PM2.5 association, which is a remarkable evidence of a stress response that may contribute to the defense against dangerous extracellular injuring signals, such as ROS, adipokines, and even eHSP72. High levels of these signals are present in the circulation in obesity [32], exposure to PM2.5 [20, 35], and finally, in T2DM individually [31].
Despite that, obesity-induced iHSP70 suppression in the liver is positively correlated with poor maintenance of glucose homeostasis [6]; in the present study, liver and adipose tissue seemed to still have some degree of cellular defense capacity [2] and increased SOD activity. Bock et al. [2] discuss that higher expression of HSP70 in the liver of HFD animals may represent just a transitory response, while the insulin resistance is not severe, which was evidenced in our study by the association HFD + PM2.5. Unlike, pancreas had the content of iHSP70 reduced. Since the iHSP70 expression is required for protecting pancreatic islets [16, 17], a decrease in iHSP70 content (or its deficiency) may represent a major risk for the development of DM [14, 27]; indeed, this may be evidence that insulin resistance is only the beginning, as shown by other studies only with HFD [2].
Recent studies have indicated that HSP70 status is intimately correlated with glycemic control and vice versa [21]. In other words, alterations in blood glucose may also interfere in HSP70 expression and its export toward the extracellular space. HSP70 is able to induce antagonistic actions, depending on the location of the protein. For example, iHSP70 exerts anti-inflammatory effects, while eHSP72 activates pro-inflammatory pathways [10]. Increased eHSP72 is associated with inflammatory and oxidative stress conditions, whereas decreased iHSP70 levels are related to insulin resistance [6]. Thus, the imbalance in HSP70 status [18] is a strong indicative of the immunoinflammatory status and may be determinant to trigger a chronic pro-inflammatory state that leads to insulin resistance and T2DM development. Assuming the H-Index = 1 for the control group, we analyze each NRMT separately. Thus, HFD + PM2.5 association produces a shift in plasma/pancreas H-Index to up to 3.7, which is related with a poor pancreas cellular defense, indicating that the pancreas is under risk for dysfunction. No alterations in liver, muscle, and EWAT H-index suggests that other cellular defenses, perhaps SOD and CAT antioxidant enzyme activities, are still working to the maintenance cellular and metabolic homeostasis.
However, pancreatic β-cells are vulnerable to metabolic and oxidative stress, eventually becoming unresponsive to glucose and fatty acid stimulation and subsequently dying via apoptosis [5, 16], which can be evident to the pancreatic mass loss, producing significant effects on the tissue response. Hence, poor pancreas cellular defense in HFD + PM2.5 may suggest this association as a potential risk factor for T2DM, as evidenced in our study.
This scenario of defense response reveals that the nutrient metabolism-related tissue (NMRT) has different patterns of response to stress and different degrees of susceptibility to damage when subjected to stressor as the association HFD + PM2.5, and the HSP70 status may be an important marker of these conditions.
Overexpression of iHSP70 inhibits apoptosis and inflammatory cytokine production following cellular stresses [18]. Induced expression of iHSP70, by preconditioning animals with either a thermal or chemical stressor, protects against acute pancreatitis [13]. Knocking out heat shock transcription factor-1 (HSF-1) in mice inhibits HSP synthesis leading to more severe acute pancreatitis induced by cerulean, this occurring via upregulation of nuclear factor-kB (NF-kB) signaling, intrapancreatic trypsinogen activation, calcium overload, actin cytoskeleton, apoptosis, and autophagy. On the other hand, HSP70 is rapidly released following non-programmed cell death [13]. eHSP72 induces immune cell activation and pro-inflammatory response by binding to Toll-like receptor-2 (TLR2), Toll-like receptor-4 (TLR4), and α2-macroglobulin receptor (CD91). Administration of recombinant HSP70 to mice aggravates cerulein-induced acute pancreatitis in a TLR4-dependent manner [13]. Thus, the role of iHSP70 and eHSP72 in severe inflammatory pancreatic diseases highlights eHSP72/iHSP70 ratio, expressed as H-Index, as a potential biomarker in these damaging processes.
Conclusion
In conclusion, simulating urbanized conditions (HFD + PM2.5), our study showed that sub-chronic exposure to PM2.5, even at low doses, potentiates metabolic dysfunction in HFD-fed mice, which are T2DM-susceptible. Furthermore, our study showed that the combination of two relevant challenges to the organism, from different origins (environmental and dietary factors), promotes alterations in cell stress response (measurable by plasma/tissue H-index), reinforcing the chaperone balance ([eHSP72]/[iHSP70]) status as a biomarker of subtle changes of diabetes risk.
Abbreviations
- CAT:
-
Catalase
- CD91:
-
α2-Macroglobulin receptor
- DM:
-
Diabetes mellitus
- EWAT:
-
Epididymal white adipose tissue
- GTT:
-
Glucose tolerance test
- HFD:
-
High-fat diet
- HSP70:
-
The 70-kDa family of heat shock proteins
- eHSP70:
-
Extracellular 70-kDa heat shock proteins
- iHSP70:
-
Intracellular 70-kDa heat shock proteins
- IAUC:
-
Incremental area under the curve
- NF-kB:
-
Nuclear factor-kappa B
- NMRT:
-
Nutrient metabolism-related tissues
- PM:
-
Particulate matter
- PM2.5 :
-
Fine particulate matter
- PMSF:
-
Phenylmethanesulfonyl fluoride
- SDS:
-
Sodium dodecyl sulfate
- SOD:
-
Superoxide dismutase
- T2DM:
-
Type 2 diabetes
- TLCK:
-
Tosyl-l-lysine chloromethyl ketone hydrochloride
- TLR2:
-
Toll-like receptor 2
- TLR4:
-
Toll-like receptor 4
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Bock PM, Krause M, Schroeder HT, Hahn GF, Takahashi HK, Scholer CM, Nicoletti G, Neto LD, Rodrigues MI, Bruxel MA et al (2016) Oral supplementations with L-glutamine or L-alanyl-L-glutamine do not change metabolic alterations induced by long-term high-fat diet in the B6.129F2/J mouse model of insulin resistance. Mol Cell Biochem 411:351–362
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chen JC, Schwartz J (2008) Metabolic syndrome and inflammatory responses to long-term particulate air pollutants. Environ Health Perspect 116:612–617
Defronzo RA (2009) Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 58:773–795
Di Naso FC, Porto RR, Fillmann HS, Maggioni L, Padoin AV, Ramos RJ, Mottin CC, Bittencourt A, Marroni NA, de Bittencourt HPI Jr (2015) Obesity depresses the anti-inflammatory HSP70 pathway, contributing to NAFLD progression. Obesity 23:120–129
Emmerechts J, Hoylaerts MF (2012) The effect of air pollution on haemostasis. Hamostaseologie 32:5–13
Fajersztajn L, Veras M, Barrozo LV, Saldiva P (2013) Air pollution: a potentially modifiable risk factor for lung cancer. Nat Rev Cancer 13:674–678
Heck TG, Scholer CM, de Bittencourt HPI Jr (2011) HSP70 expression: does it a novel fatigue signalling factor from immune system to the brain? Cell Biochem Funct 29:215–226
Hoffmann B, Moebus S, Dragano N, Stang A, Mohlenkamp S, Schmermund A, Memmesheimer M, Brocker-Preuss M, Mann K, Erbel R et al (2009) Chronic residential exposure to particulate matter air pollution and systemic inflammatory markers. Environ Health Perspect 117:1302–1308
Hooper PL, Balogh G, Rivas E, Kavanagh K, Vigh L (2014) The importance of the cellular stress response in the pathogenesis and treatment of type 2 diabetes. Cell Stress Chaperones 19:447–464
Kang R, Lotze MT, Zeh HJ, Billiar TR, Tang D (2014) Cell death and DAMPs in acute pancreatitis. Mol Med 20:466–477
Keane KN, Cruzat VF, Carlessi R, de Bittencourt PI H Jr, Newsholme P (2015) Molecular Events Linking Oxidative Stress and Inflammation to Insulin Resistance and beta-Cell Dysfunction. Oxidative Med Cell Longev 2015:181643
Kolberg A, Rosa TG, Puhl MT, Scola G, da Rocha JD, Maslinkiewicz A, Lagranha DJ, Heck TG, Curi R, de Bittencourt HPI Jr (2006) Low expression of MRP1/GS-X pump ATPase in lymphocytes of Walker 256 tumour-bearing rats is associated with cyclopentenone prostaglandin accumulation and cancer immunodeficiency. Cell Biochem Funct 24:23–39
Krause M, Bock PM, Takahashi HK, de Bittencourt HPI Jr, Newsholme P (2015) The regulatory roles of NADPH oxidase, intra- and extra-cellular HSP70 in pancreatic islet function, dysfunction and diabetes. Clin Sci 128:789–803
Krause M, de Bittencourt HPI Jr (2008) Type 1 diabetes: can exercise impair the autoimmune event? The L-arginine/glutamine coupling hypothesis. Cell Biochem Funct 26:406–433
Krause M, Heck TG, Bittencourt A, Scomazzon SP, Newsholme P, Curi R, de Bittencourt HPI Jr (2015) The chaperone balance hypothesis: the importance of the extracellular to intracellular HSP70 ratio to inflammation-driven type 2 diabetes, the effect of exercise, and the implications for clinical management. Mediat Inflamm 2015:249205
Lee MO (1929) The Function of the Air Sacs in Holopneustic Insects. Science 69:334–335
Liu C, Ying Z, Harkema J, Sun Q, Rajagopalan S (2013) Epidemiological and experimental links between air pollution and type 2 diabetes. Toxicol Pathol 41:361–373
Ludwig MS, Minguetti-Camara VC, Heck TG, Scomazzon SP, Nunes PR, Bazotte RB, de Bittencourt HPI Jr (2014) Short-term but not long-term hypoglycaemia enhances plasma levels and hepatic expression of HSP72 in insulin-treated rats: an effect associated with increased IL-6 levels but not with IL-10 or TNF-alpha. Mol Cell Biochem 397:97–107
Maatz LF, Wood GJ, Rivero DH & Saldiva PH et al (2009) Tracheal instillation of urban PM(2.5) suspension promotes acute cardiac polarization changes in rats. Brazilian journal of medical and biological research = Revista brasileira de pesquisas medicas e biologicas/Sociedade Brasileira de Biofisica 42:207–213
Marklund S, Marklund G (1974) Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European journal of biochemistry/FEBS 47:469–474
Medeiros N Jr, Rivero DH, Kasahara DI, Saiki M, Godleski JJ, Koutrakis P, Capelozzi VL, Saldiva PH, Antonangelo L (2004) Acute pulmonary and hematological effects of two types of particle surrogates are influenced by their elemental composition. Environ Res 95:62–70
Mendez R, Zheng Z, Fan Z, Rajagopalan S, Sun Q, Zhang K (2013) Exposure to fine airborne particulate matter induces macrophage infiltration, unfolded protein response, and lipid deposition in white adipose tissue. Am J Transl Res 5:224–234
Miller MR, Shaw CA, Langrish JP (2012) From particles to patients: oxidative stress and the cardiovascular effects of air pollution. Futur Cardiol 8:577–602
Newsholme P, de Bittencourt HPI Jr (2014) The fat cell senescence hypothesis: a mechanism responsible for abrogating the resolution of inflammation in chronic disease. Current opinion in clinical nutrition and metabolic care 17:295–305
Pearson JF, Bachireddy C, Shyamprasad S, Goldfine AB & Brownstein JS (2010) Association between fine particulate matter and diabetes prevalence in the U.S..Diabetes care 33:2196–2201
Probst-Hensch NM (2010) Chronic age-related diseases share risk factors: do they share pathophysiological mechanisms and why does that matter? Swiss Med Wkly 140:w13072
Rajagopalan S, Brook RD (2012) Air pollution and type 2 diabetes: mechanistic insights. Diabetes 61:3037–3045
Rodrigues-Krause J, Krause M, O'Hagan C, De Vito G, Boreham C, Murphy C, Newsholme P, Colleran G (2012) Divergence of intracellular and extracellular HSP72 in type 2 diabetes: does fat matter? Cell Stress Chaperones 17:293–302
Schuster DP (2010) Obesity and the development of type 2 diabetes: the effects of fatty tissue inflammation. Diabetes, metabolic syndrome and obesity : targets and therapy 3:253–262
Sone H, Kagawa Y (2005) Pancreatic beta cell senescence contributes to the pathogenesis of type 2 diabetes in high-fat diet-induced diabetic mice. Diabetologia 48:58–67
Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S et al (2009) Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119:538–546
Van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC (2001) Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med 164:826–830
Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA (2001) Exercise increases serum Hsp72 in humans. Cell Stress Chaperones 6:386–393
WHO (1998) Carbohydrates in human nutrition. Report of a Joint FAO/WHO Expert Consultation FAO food and nutrition paper 66:1–140
Winzell MS, Ahren B (2004) The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53(Suppl 3):S215–S219
Xu X, Yavar Z, Verdin M, Ying Z, Mihai G, Kampfrath T, Wang A, Zhong M, Lippmann M, Chen LC et al (2010) Effect of early particulate air pollution exposure on obesity in mice: role of p47phox. Arterioscler Thromb Vasc Biol 30:2518–2527
Yan YH, Chou CC, Lee CT, Liu JY, Cheng TJ (2011) Enhanced insulin resistance in diet-induced obese rats exposed to fine particles by instillation. Inhal Toxicol 23:507–519
Acknowledgments
The authors would like to thank to E.G.P. Basso, E.T.C. Martins, M.M. Sulzbacher, R.D.B. Basso (all from UNIJUI), P.H.N. Saldiva (USP), and colleagues from the Laboratory of Oxidative Stress and Atmospheric Pollution (UFCSPA) for their technical support.
Author contribution
PBGF completed all the experiments described in this manuscript. PBGF, BS, and FGB performed biometric and metabolic profile. PBGF and ABS performed experiments on oxidative stress parameters. PBGF, BS, and FGB performed Western blot analyses. All authors were involved in analyzing the results. PBGF, TGH, and MSL co-wrote the paper. TGH and CRR designed the study. PIHBJ, TGH, MSL, and CRR provided experimental advice and helped with manuscript revision. All the authors had final approval of the submitted and published versions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare that they do not have competing financial interests.
Financial support
This work was supported by Federal University of Health Sciences of Porto Alegre (UFCSPA) and by grants from Research Support Foundation of the State of Rio Grande do Sul (PqG-2013 - FAPERGS, process: 002106-2551/13-5 to TGH). PBGF and FGB were recipients of scholarships from the Coordination for the Improvement of Higher Education Personnel (CAPES) and ABS from Research Support Foundation of the State of Rio Grande do Sul (FAPERGS).
Rights and permissions
About this article
Cite this article
Goettems-Fiorin, P.B., Grochanke, B.S., Baldissera, F.G. et al. Fine particulate matter potentiates type 2 diabetes development in high-fat diet-treated mice: stress response and extracellular to intracellular HSP70 ratio analysis. J Physiol Biochem 72, 643–656 (2016). https://doi.org/10.1007/s13105-016-0503-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0503-7