Abstract
Adiposopathy, or sick fat, refers to adipose tissue dysfunction that can lead to several complications such as dyslipidemia, insulin resistance, and hyperglycemia. The relative contribution of adiposopathy in predicting insulin resistance remains unclear. We investigated the relationship between adiposopathy, as assessed as a low plasma adiponectin/leptin ratio, with anthropometry, body composition (hydrostatic weighing), insulin sensitivity (hyperinsulinemic-euglycemic clamp), inflammation, and fitness level (ergocycle VO2max, mL/kgFFM/min) in 53 men (aged 34–53 years) from four groups: sedentary controls without obesity (body mass index [BMI] <25 kg/m2), sedentary with obesity (BMI > 30 kg/m2), sedentary with obesity and glucose intolerance, and endurance trained active without obesity. The adiponectin/leptin ratio was the highest in trained men (4.75 ± 0.82) and the lowest in glucose intolerant subjects with obesity (0.27 ± 0.06; ANOVA p < 0.0001) indicating increased adiposopathy in those with obesity. The ratio was negatively associated with adiposity (e.g., waist circumference, r = −0.59, p < 0.01) and positively associated with VO2max (r = 0.67, p < 0.01) and insulin sensitivity (M/I, r = 0.73, p < 0.01). Multiple regression analysis revealed fitness as the strongest independent predictor of insulin sensitivity (partial R 2 = 0.61). While adiposopathy was also an independent and significant contributor (partial R 2 = 0.10), waist circumference added little power to the model (partial R 2 = 0.024). All three variables remained significant independent predictors when trained subjects were excluded from the model. Plasma lipids were not retained in the model. We conclude that low fitness, adiposopathy, as well as adiposity (and in particular abdominal obesity) are independent contributors to insulin resistance in men without diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipose tissue (AT) is a powerful secretory organ releasing adipokines and inflammatory cytokines [7, 15, 23], many of which having significant effects on whole-body or tissue-specific insulin resistance (IR) [23]. However, state and functional differences exist between depots of different types (e.g., subcutaneous and omental visceral depots, e.g., [25]). Also, function not only varies greatly according to anatomical location but also by the type of adipocyte (white, brown, or beige) found within each depot [24], and can further be modified according to internal and external stresses (e.g., AT browning, inflammation). In this context, it is of interest to investigate the global secretory profile of whole-body AT and its relation to health.
Although conditions such as type 2 diabetes (T2D), hypertension, or dyslipidemia are generally associated with obesity, these conditions can occur in individuals without obesity or differ in individuals with similar adiposity [2]. This has led to the concept of adiposopathy, or sick fat, which states that AT function, independently from its quantity, can influence the metabolic state [5]. Thus, a modified adipocyte secretory profile can contribute to metabolic disorders such as hyperglycemia or dyslipidemia [6, 3]. In this context, the most often considered adipokines are adiponectin and leptin [11, 20]. Given their similar effects on insulin sensitivity (IS), but their inverse relationships with BMI and metabolic health, the adiponectin/leptin (A/L) ratio has been suggested and used as a marker of adiposopathy, i.e., a marker of the secretory “health” of adipocytes [18, 35].
Other factors such as inflammatory cytokines also impact the insulin response [15, 23, 4]. With obesity, there is an increased infiltration of white blood cells in AT, and these, along with adipocytes, can secrete anti- and pro-inflammatory molecules. Adiponectin is an example of the former, while interleukin-6 (IL-6), C-reactive protein (CRP), tumor necrosis factor alpha (TNF-α), chemokine (C-C motif) ligand 5 (RANTES), and monocyte chemotactic protein 1 (MCP-1) are examples of the latter [30]. TNF-α also has an impact on insulin sensitivity by diacylglycerol esterification [10, 19]. RANTES and MCP-1 are also increased with obesity, glucose intolerance, and T2D [22, 34].
Despite some work having studied the relationship between adiposopathy and the insulin response, many questions remain as to its relative contribution to IR when compared to other known contributors. The aim of this study was to investigate the relationship between inflammatory markers, adipokines, adiposopathy, and IR and to clarify how the fitness level (VO2max) and abdominal obesity could influence these relations in a cohort of middle-aged men without diabetes. We hypothesized that adiposopathy would independently contribute to IR.
Materials and methods
Subjects
Fifty-three men aged 34 to 55 years were recruited in four groups in a cross sectional study: sedentary (n = 11), obesity (n = 11), obesity and glucose intolerance (n = 16), and highly trained endurance active (n = 15). The first three groups and testing methods were previously described [33]. In brief, control sedentary subjects (defined by an absence of regular physical activities over the last 3 months as described in [9]) had a body mass index (BMI) ≤ 25 kg/m2. Subjects in the obesity group were sedentary and had a BMI > 30 kg/m2. The trained group consisted of subjects without obesity practicing high levels of regular aerobic physical activities. Subjects from these groups had normal glucose tolerance measured by oral glucose tolerance test (OGTT) (2 h glucose ≤ 7.8 mmol/L). Subjects from the final group, with obesity and glucose intolerance, differed from those in the group with obesity by presenting impaired glucose tolerance measured by OGTT (2 h glucose > 7.8 mmol/L). Exclusion and inclusion criteria are detailed in [33]. Briefly, persons with T2D, a ±2 kg body weight fluctuation in the previous 6 months, smokers, heavy alcohol consumers, asthmatics needing steroid therapy, or with any liver, renal, or uncontrolled thyroid disorders were excluded. Subjects using medication with steroid hormones, α- or β-blockers, diuretics, or other lipid metabolism modulators (e.g., thiazolidinediones, statins, and insulin) were also excluded. Individuals with a history or physical findings of coronary heart disease, peripheral vascular disease, hypertension, or an inability to perform the exercise test were also excluded. The research protocol was approved by the ethics committees of Université Laval (CERUL, approval 2004–2007) and the Centre de Recherche de l’Institut Universitaire de Cardiologie et Pneumologie de Québec (CRIUCPQ; approval 2013–2151), and all subjects provided written informed consent.
Body composition
Body weight was obtained to the nearest 0.1 kg with a calibrated scale including a tension gauge (Intertechnology Inc., Don Mills, ON, Canada) and a digital panel indicator (Beckman industrial series 600; Beckmann Coulter Canada Inc., Mississauga, ON, Canada). Height was measured to the nearest millimeter with a wall stadiometer. Waist circumference (WC) was taken in duplicate at the mid-distance between the iliac crest and last rib margin with a flexible steel metric tape to the nearest 0.1 cm. Body composition was obtained by hydrostatic weighing as described in [9] for precise assessment of fat and fat free masses.
Maximal aerobic power and cardiorespiratory fitness (VO2max)
The procedure was previously described [33]. In brief, the test was performed on a bicycle ergometer (Ergo-Metrics 800 s; SensorMedics, Yorba Linda, CA, USA), a 5-min warmup between 50 and 75 W followed by an incremental test starting between 100 and 150 W with an increase of 25 W every 2 min. Subjects were instructed to maintain a cadence of 70 rpm during the test. Direct measures of O2 (Ametek S-3A with a Zircon cell) and CO2 (Anarad AR-411 infrared gas analyzer) were obtained every 30 s. Perceived exertion, Borg analog RPE scale [8], and blood pressure were measured every 2 min. The end of the test occurred when the participants could not maintain the required cadence or upon the appearance of a usual indicator for the determination of exercise testing [13]. Since we were interested in a measure of the relative metabolic capacity of lean (muscle) tissue, we express VO2max adjusted for fat free mass.
Euglycemic-hyperinsulinemic clamp
Clamp conditions were previously described [29]. In brief, subjects fasted overnight (12 h) [27] and avoided exercise for 3 days preceding the clamp to minimize the acute effects of exercise on IS [26, 31]. All subjects also consumed a standardized diet adjusted to their isocaloric needs the day preceding the clamp [37]. An antecubital arm vein was cannulated with a catheter for infusion of insulin and 20 % glucose, and the contralateral arm vein was cannulated to allow sampling for determination of plasma insulin and glucose levels. Baseline fasting blood was drawn for measurements. A continuous infusion of insulin (Humulin; 40 mU/m2/min) was started, keeping arterial insulin in the upper physiologic range of approximately 500 pM. Blood samples were taken every 5 min for measurements of plasma glucose, and insulin levels were measured every 10 min. IS was measured as the insulin sensitivity index (M/I; mean glucose infusion rate/mean insulin concentration) calculated between times 90 and 120 min of the clamp.
Oral glucose tolerance test
After an overnight fast, an oral glucose tolerance test with a 75-g glucose load was performed. Blood samples were collected through a venous catheter from an antecubital vein at −15, 0, 30, 60, 90, and 120 min following glucose ingestion for the determination of plasma glucose and insulin concentrations [28]. Plasma glucose was measured enzymatically, whereas plasma insulin was measured by radioimmunoassay with polyethylene glycol separation [12, 32]. Subjects were classified as glucose tolerant if fasting plasma glucose level was <7.0 mmol/L and <7.8 2 h after bolus ingestion. Subjects were considered glucose intolerant if their plasma glucose level was ≥7.8 mmol/L 2 h after bolus ingestion. T2D was defined as a fasting plasma glucose concentration ≥7.0 mmol/L and/or a 2-h plasma glucose concentrations ≥11.1 mmol/L [1].
Blood analyses
An Elite Bayer glucometer (3903-E) was used to monitor glucose every 5 min during the clamp. Every 10 min, additional blood samples were collected, centrifuged to obtain plasma, and stored at −20 °C for later analyses. Glucose was measured using the hexokinase method and plasma insulin using a radioimmunoassay with polyethylene glycol separation as described previously [29]. The plasma lipid-lipoprotein profile was assessed as described previously [33]. Human high molecular weight (HMW) adiponectin was measured by ELISA (Millipore, Billerica, MA, USA). Leptin and inflammatory molecules were measured by Milliplex® Multiplex kits (Millipore, Billerica, MA, USA) using Luminex® technology. The adiponectin/leptin ratio was used as an indicator of AT function, a higher ratio indicating good, healthy function, and a lower ratio indicating increased adiposopathy. Except where specified, all measures were performed on plasma obtained before the beginning of the euglycemic-hyperinsulinemic clamp.
Statistical analysis
Statistical analyses were performed using the JMP® 9.0.2 software (2010 SAS Institute Inc., Cary, NC, USA). Values which were three standard deviations or more from the mean for normally distributed data were considered outliers and excluded from analyses. Group comparisons were performed with a one-way ANOVA followed by Tukey-Kramer HSD post hoc tests for group differences with normally distributed data. For data that lacked normality, non-parametric Wilcoxon/Kruskal-Wallis (Rank Sums) tests and non-parametric Wilcoxon comparisons for each pair were performed. Pearson correlations were used to assess relationships between variables. Stepwise (mixed) regression modeling was used to assess the independent contributions to IS (measured as M/I) of retained variables. Separate models with and without trained individuals were run. Data are presented as means ± SEM and are considered statistically different when p < 0.05.
Results
Subject characteristics
Subjects were generally well matched for age, although trained active men were slightly older than the glucose intolerant with obesity (Table 1). As expected, there were significant differences in body composition and anthropometric markers between subjects with and without obesity, the trained having lower body mass, body fat, and waist circumference. Also as expected, the glucose intolerant subjects with obesity had the highest fat free mass of any group. VO2max, expressed per kilogram of fat free mass to better reflect the metabolic function of lean (muscle) tissue, did not differ between the sedentary groups, although it tended to be lower in the glucose intolerant with obesity. As expected, values were much higher in trained subjects.
Plasma variables
Plasma glucose, insulin, lipid-lipoprotein profile, inflammatory markers, and adipokines levels are shown in Table 2. The glycemic profile shows no between group differences for fasting glucose levels, as expected, whereas fasting insulin levels were lower in sedentary subjects and lowest in the trained (2.5-fold less than the levels in glucose intolerant men with obesity). Glucose intolerant subjects with obesity were the most metabolically deteriorated, their glucose levels being almost two times higher and their insulin levels five times higher than the trained, 2 h following glucose bolus ingestion during the OGTT. IS (measured as M/I) was significantly different between groups, the lowest in glucose intolerant men with obesity, intermediary in controls without obesity, and the highest in trained individuals.
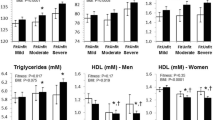
Subjects from the groups with obesity also displayed the most deteriorated lipid-lipoprotein profile, presenting higher NEFA and triacylglycerol levels, and lower HDL-cholesterol levels, than their counterparts without obesity. No differences in total or LDL-cholesterol were seen between groups.
As for inflammatory variables, no significant between group differences were seen for levels of TNF-α, MCP-1 and RANTES (Table 3). CRP levels were similar in the sedentary and trained groups, but were significantly greater in those with obesity (twofold higher) and glucose intolerance (fourfold higher). IL-6 levels were similar in subjects with obesity or obesity and glucose intolerance, sedentary subjects presenting intermediary values while trained men had the lowest levels (half those of the groups with obesity).
No significant differences were observed for adiponectin despite large differences in fat mass between groups. There was no difference between subjects with obesity or obesity and glucose-intolerance for leptin levels, whereas sedentary subjects had levels three to four times lower and trained ten times lower than individuals with obesity. The adiponectin/leptin ratio followed an inverse distribution pattern when compared to leptin, the trained having the highest (healthiest) value and subjects with obesity and glucose intolerance the lowest level (indicating increased adiposopathy).
Relationships between anthropometry, fitness, adipokines, inflammatory markers, and IS
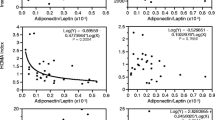
The results of Pearson correlation analyses between circulating inflammatory markers, anthropometry, fitness, adipokines, inflammatory markers, and clamp variables are presented in Table 4. Correlations were effected with and without the trained group to investigate how high fitness and regular physical activity could affect the relationships. CRP and IL-6 showed moderate (r range 0.3–0.6) but statistically significant correlations with increasing body weight and adiposity, all positive except for a negative correlation between IL-6 and lean body mass (data not shown). The negative relationships between fitness as well as IS with CRP and IL-6 were all moderate (r range −0.3 to −0.5) but statistically significant. No significant correlations were observed with RANTES, TNF-α, or MCP-1. Overall, this indicates that a deteriorated anthropometric, fitness, and IS profile is associated with increased inflammation measured as circulating IL-6 and CRP. Leptin levels were strongly positively correlated with markers of body weight and fat mass, and negatively correlated with lean body mass (data not shown). Leptin levels were also moderately to strongly negatively correlate with fitness and IS. No significant correlations with any of the variables of interest were seen with circulating adiponectin levels. Correlations with the adiponectin/leptin ratio were the mirror image of those seen with leptin. Of note, the single strongest correlation with IS was observed with the adiponectin/leptin ratio.
Multiple regression modeling of IS
In order to identify which variables independently predict IS, we entered the strongest single correlates from each family of variables into a stepwise multiple regression models (Table 5). We chose to include all subjects (sedentary and trained) in the first model to better reflect the general population. The second was run without trained subjects, in order to exclude any specific confounding effect of regular training on the studied relationships. In both models, fitness, adiposopathy, and waist circumference were found to be significant and independent predictors of IS. The relative importance of each variable in the model varied when trained subjects were excluded. While fitness level was the strongest predictor when all subjects were included (VO2max partial R 2 = 0.61) with waist circumference adding only very little power to the model (partial R 2 = 0.024), this was reversed when only sedentary subjects were analyzed with waist circumference becoming the strongest predictor (partial R 2 = 0.52) and VO2max losing importance (partial R 2 = 0.08). The contribution of adiposopathy was almost identical in both models (partial R 2 = 0.10 and 0.11, respectively). Leptin alone, or adiponectin alone, in lieu of the adiponectin/leptin ratio, were not retained in the regression models with or without the trained individuals (data not shown).
Discussion
In the present study, we investigated the relationship between adiposopathy and IS in men without diabetes, and attempted to quantify its relative contribution when compared to inflammatory state, fitness level, and AT distribution. Our findings reveal that, in a population of men without diabetes varying greatly for their adiposity and maximal aerobic capacity, fitness, adiposopathy, and abdominal obesity were independent predictors of IS.
The finding of a strong relationship between VO2max and IS when all subjects are included agrees with previous work that has highlighted the importance of cardiorespiratory fitness, even in the absence of regular training, in protecting against deteriorated metabolic health [36]. That the strength of this contribution was decreased in the model without trained subjects is not surprising, given the narrow range of VO2max values between subjects from the three sedentary groups.
Our inflammatory marker data are also in agreement with previous findings showing obesity as a chronic inflammatory state, which is further exacerbated in the presence of an increasing number of risk factors for the metabolic syndrome [15, 4]. The elevated levels of IL-6 and CRP we observe are concordant with the well known increased pro-inflammatory adipokine levels reported in numerous studies [15, 23, 4]. Inflammation in the context of obesity has also been shown to correlate with IR, and the negative relationships we observe between IS and markers of inflammation are in agreement. These relationships were weak, however, when compared to those seen with fitness and markers of adiposity or adiposopathy. Given their origin in AT, and the myriad of other factors modulating the insulin response that arise from this same tissue, it is perhaps not surprising that inflammation was not retained as an independent predictor of IS in various regression models we studied (data not shown). As the weakest single correlates of the different classes of variables to IS, inflammation was not used in the final regression model shown in Table 5 in order to limit the number of variables to within accepted guidelines for our number of subjects.
Anthropometric measures are highly valuable and reliable clinical measures, and strongly correlate with an individual’s metabolic profile. However, they cannot inform us on the actual functional state, for example the secretory profile, of the AT itself. Adiponectin, an adipokine exclusively secreted by adipocytes, has a positive action on IS by activating skeletal muscle β-oxidation of lipids, glucose uptake, and energy expenditure, and is often reported as being decreased with increasing adiposity [11]. While leptin has also a positive effect on IS by increasing muscle glucose uptake, storage as glycogen, and lipid β-oxidation [20], its levels are increased in subjects that are dysmetabolic or with obesity, the result of a compensatory mechanism aimed at reducing energy intake and improving the metabolic condition, but also as a result of the development of leptin resistance [21]. Although both are implicated in promoting insulin sensitization, these adipokines are generally reported to have opposite relationships to adiposity. The adiponectin/leptin ratio has been previously used as an indicator of overall AT function, and some studies show that it is a better predictor of IR than HOMA-IR [16, 17]. A high ratio is considered as a marker of good AT health, or low adiposopathy.
As expected, subjects presenting the most deteriorated metabolic profile were those who also had the highest level of adiposopathy (a low adiponectin/leptin ratio), and our data reveal a strong correlation between this ratio and IS. The most surprising and novel data from our study were the results of the multiple regression model we used to tease out the independent contributions of adiposity, adiposopathy, fitness and inflammation state on IS (using the strongest single correlates from each category in our models).
While finding that fitness (VO2max) was the strongest predictor of IS when all subjects are included was not surprising, though the magnitude of its predictive power perhaps was (R 2 = 0.60), we were surprised to find that the only other variable, besides waist circumference, with an important independent contribution in our model predicting IS, was adiposopathy. This occurred despite the well-described relationship between waist circumference (a very good surrogate for visceral adiposity) and metabolic dysfunction and cardiovascular disease (e.g., [14] [4]). Our data thus agree with the suggestion that adiposopathy might be a better predictor of metabolic dysfunction than AT distribution itself when the whole population is considered. The observation that the predictive power of the adiponectin/leptin ratio in the model with only the sedentary subjects remained essentially identical to that with all subjects, does suggest an important and constant contribution of adiposopathy to IS regardless of fitness and physical activity levels. The absence of an independent contribution of leptin or adiponectin in our regression models (data not shown) reinforces the notion that adiposopathy as measured as their ratio is a useful tool to inform us on the metabolic status of whole-body adipose tissue and its potential impact on metabolic health.
Our study does have certain limitations. A more detailed analysis of regional AT distribution, for example as assessed with DEXA or computed tomography, might provide a stronger predictor of IS and could have modulated our regression model results. Also, our study used only middle-aged men without diabetes, thus we cannot extrapolate our results to women, other age groups, or to those suffering from greater metabolic dysfunction. However, direct fitness (VO2max) assessment, measuring IS with the clamp (the gold-standard method to examine glucose-insulin homeostasis), obtained under well-controlled conditions, are clear strengths of this study.
Conclusion
In conclusion, our data show that fitness, adiposity (notably abdominal obesity), and adiposopathy are independent predictors of IS in middle-aged men without diabetes. From a clinical standpoint, albeit not an economical one for the time being, this suggests that measuring adiposopathy might be a useful diagnostic tool in subjects without diabetes, linking AT function to metabolic health. It would be of interest to examine this relationship in a longitudinal study, to assess whether increased adiposopathy predicts greater metabolic deterioration over time, or to assess the effects of exercise or caloric restriction on adiposopathy.
References
Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15:539–553
Bays H (2005) Adiposopathy: role of adipocyte factors in a new paradigm. Expert Rev Cardiovasc Ther 3:187–189
Bays HE (2011) Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol 57:2461–2473
Bays H (2014) Adiposopathy, “sick fat,” Ockham’s razor, and resolution of the obesity paradox. Curr Atheroscler Rep 16:409
Bays H, Stein EA (2003) Pharmacotherapy for dyslipidaemia—current therapies and future agents. Expert Opin Pharmacother 4:1901–1938
Bays H, Abate N, Chandalia M (2005) Adiposopathy: sick fat causes high blood sugar, high blood pressure and dyslipidemia. Future Cardiol 1:39–59
Bays HE, Gonzalez-Campoy JM, Bray GA, Kitabchi AE, Bergman DA, Schorr AB, Rodbard HW, Henry RR (2008) Pathogenic potential of adipose tissue and metabolic consequences of adipocyte hypertrophy and increased visceral adiposity. Expert Rev Cardiovasc Ther 6:343–368
Borg GA (1982) Psychophysical bases of perceived exertion. Med Sci Sports Exerc 14:377–381
Bouchard C, Leon AS, Rao DC, Skinner JS, Wilmore JH, Gagnon J (1995) The HERITAGE family study. Aims, design, and measurement protocol. Med Sci Sports Exerc 27:721–729
Bruce CR, Dyck DJ (2004) Cytokine regulation of skeletal muscle fatty acid metabolism: effect of interleukin-6 and tumor necrosis factor-alpha. Am J Physiol Endocrinol Metab 287:E616–621
Caselli C (2014) Role of adiponectin system in insulin resistance. Mol Genet Metab 113:155–160
Desbuquois B, Aurbach GD (1971) Use of polyethylene glycol to separate free and antibody-bound peptide hormones in radioimmunoassays. J Clin Endocrinol Metab 33:732–738
Fletcher GF, Balady GJ, Amsterdam EA, Chaitman B, Eckel R, Fleg J, Froelicher VF, Leon AS, Pina IL, Rodney R, Simons-Morton DA, Williams MA, Bazzarre T (2001) Exercise standards for testing and training: a statement for healthcare professionals from the American Heart Association. Circulation 104:1694–1740
Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D’Agostino RB Sr, O’Donnell CJ (2007) Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation 116:39–48
Galic S, Oakhill JS, Steinberg GR (2010) Adipose tissue as an endocrine organ. Mol Cell Endocrinol 316:129–139
Inoue M, Maehata E, Yano M, Taniyama M, Suzuki S (2005) Correlation between the adiponectin-leptin ratio and parameters of insulin resistance in patients with type 2 diabetes. Metabolism 54:281–286
Inoue M, Yano M, Yamakado M, Maehata E, Suzuki S (2006) Relationship between the adiponectin-leptin ratio and parameters of insulin resistance in subjects without hyperglycemia. Metabolism 55:1248–1254
Jung CH, Rhee EJ, Choi JH, Bae JC, Yoo SH, Kim WJ, Park CY, Mok JO, Kim CH, Lee WY, Oh KW, Park SW, Kim SW (2010) The relationship of adiponectin/leptin ratio with homeostasis model assessment insulin resistance index and metabolic syndrome in apparently healthy korean male adults. Korean Diabetes J 34:237–243
Katsuki A, Sumida Y, Murashima S, Murata K, Takarada Y, Ito K, Fujii M, Tsuchihashi K, Goto H, Nakatani K, Yano Y (1998) Serum levels of tumor necrosis factor-alpha are increased in obese patients with noninsulin-dependent diabetes mellitus. J Clin Endocrinol Metab 83:859–862
Khan M, Joseph F (2014) Adipose tissue and adipokines: the association with and application of adipokines in obesity. Scientifica (Cairo) 2014:328592
Kitahara CM, Trabert B, Katki HA, Chaturvedi AK, Kemp TJ, Pinto LA, Moore SC, Purdue MP, Wentzensen N, Hildesheim A, Shiels MS (2014) Body mass index, physical activity, and serum markers of inflammation, immunity, and insulin resistance. Cancer Epidemiol Biomarkers Prev 23:2840–2849
Kohno S, Ueji T, Abe T, Nakao R, Hirasaka K, Oarada M, Harada-Sukeno A, Ohno A, Higashibata A, Mukai R, Terao J, Okumura Y, Nikawa T (2011) Rantes secreted from macrophages disturbs skeletal muscle regeneration after cardiotoxin injection in Cbl-b-deficient mice. Muscle Nerve 43:223–229
Kwon H, Pessin JE (2013) Adipokines mediate inflammation and insulin resistance. Front Endocrinol (Lausanne) 4:13
Lee MJ, Wu Y, Fried SK (2013) Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med 34:1–11
Mauriege P, Joanisse DR, CasparBauguil S, Cartier A, Lemieux I, Bergeron J, Biron S, Marceau P, Richard D (2015) Gene expression of different adipose tissues of severely obese women with or without a dysmetabolic profile. J Physiol Biochem 71:719–732
Mikines KJ, Sonne B, Farrell PA, Tronier B, Galbo H (1988) Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol 254:E248–259
Oppert JM, Nadeau A, Tremblay A, Despres JP, Theriault G, Bouchard C (1997) Negative energy balance with exercise in identical twins: plasma glucose and insulin responses. Am J Physiol 272:E248–254
Piche ME, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J, Lemieux S (2005) Contribution of abdominal visceral obesity and insulin resistance to the cardiovascular risk profile of postmenopausal women. Diabetes 54:770–777
Pigeon E, Riou ME, St-Onge J, Couillard E, Tremblay A, Marette A, Weisnagel SJ, Joanisse DR (2009) Validation of a simple index (SIisOGTT) of insulin sensitivity in a population of sedentary men. Diabetes Metab 35:398–403
Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM (2001) C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286:327–334
Rice B, Janssen I, Hudson R, Ross R (1999) Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care 22:684–691
Richterich R, Dauwalder H (1971) Determination of plasma glucose by hexokinase-glucose-6-phosphate dehydrogenase method. Schweizerische medizinische Wochenschrift 101:615–618
Riou ME, Pigeon E, St-Onge J, Tremblay A, Marette A, Weisnagel J, Joanisse DR (2009) Cardiorespiratory fitness and components of the metabolic syndrome in sedentary men. Obes Facts 2:318–324
Sell H, Dietze-Schroeder D, Kaiser U, Eckel J (2006) Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology 147:2458–2467
Vega GL, Grundy SM (2013) Metabolic risk susceptibility in men is partially related to adiponectin/leptin ratio. J Obes 2013:409679
Wei M, Gibbons LW, Mitchell TL, Kampert JB, Lee CD, Blair SN (1999) The association between cardiorespiratory fitness and impaired fasting glucose and type 2 diabetes mellitus in men. Ann Intern Med 130:89–96
White MD, Bouchard G, Buemann B, Almeras N, Despres JP, Bouchard C (1985) Tremblay A (1996) reproducibility of 24-h energy expenditure and macronutrient oxidation rates in an indirect calorimeter. J Appl Physiol 80:133–139
Acknowledgments
We would like to express our heartfelt gratitude to the study participants. We would also like to thank Guy Fournier, Jean Doré, Marc Brunet, Linda Drolet, Nancy Parent, Marie Tremblay, Rollande Couture, Valérie-Ève Julien, Rachelle Duchesne, and Ginette Lapierre for their expert technical assistance in recruiting subjects and carrying out the clinical aspects of the study. This study was supported by grants from the Canadian Institutes of Health Research grant (MOP-68846) to DRJ and from the Fondation du Centre de Recherche de l’Institut Universitaire de Cardiologie et Pneumologie de Québec (CRIUCPQ) to DRJ and AM.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The research protocol was approved by the ethics committees of Université Laval (CERUL, approval 2004–2007) and the Centre de Recherche de l’Institut Universitaire de Cardiologie et Pneumologie de Québec (CRIUCPQ; approval 2013–2151), and all subjects provided written informed consent.
Rights and permissions
About this article
Cite this article
Huth, C., Pigeon, É., Riou, MÈ. et al. Fitness, adiposopathy, and adiposity are independent predictors of insulin sensitivity in middle-aged men without diabetes. J Physiol Biochem 72, 435–444 (2016). https://doi.org/10.1007/s13105-016-0488-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-016-0488-2