Abstract
Mitochondrial dysfunctions have been detected in non-alcoholic steatohepatitis, but less information exists regarding adaptation of mitochondrial function during the initiation of hepatic steatosis. This study aimed to determine in rat liver the sequence of mitochondrial and metabolic adaptations occurring during the first 8 weeks of a moderate high fat diet (HFD). Sprague–Dawley rats were fed a HFD during 2, 4, and 8 weeks. Mitochondrial oxygen consumption, respiratory chain complexes activity, and oxidative phosphorylation efficiency were assessed in isolated liver mitochondria. Gene expression related to fat metabolism and mitochondrial biogenesis were determined. Results were compared to data collected in a group of rats sacrificed before starting the HFD feeding. After 2 and 4 weeks of HFD, there was a development of fatty liver and a concomitant increase the expression of mitochondrial glycerol-3-phosphate acyltransferase (mtGPAT) and peroxisome proliferator-activated receptor γ. Higher serum β-hydroxybutyrate levels and enhanced hepatic pyruvate dehydrogenase kinase 4 expression suggested increased fatty acid oxidation. However, mitochondrial respiration and respiratory chain activity were normal. After 8 weeks of HFD, lower accumulation of liver triglycerides was associated with reduced expression of mtGPAT. At this time, oxygen consumption with palmitoyl-l-carnitine was decreased whereas oxidative phosphorylation efficiency (ATP/O) with succinate was enhanced. Hepatic levels of mtDNA were unchanged whatever the time points. This longitudinal study in rats fed a HFD showed that hepatic lipid homeostasis and mitochondrial function can adapt to face the increase in fatty acid availability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity and associated metabolic disorders such as type 2 diabetes, dyslipidemia, and non-alcoholic fatty liver diseases (NAFLD) have become a major health problem in industrialized and developing countries [11, 18]. Obesity results from a complex interaction between genetic, behavioral, and environmental factors that are involved in different proportions in each individual. The major environmental cause of obesity is a higher intake of diets enriched in fat and carbohydrate combined with lower energy expenditure due to a sedentary lifestyle [28]. High fat diet (HFD) represents a challenge for the different tissues involved in energy and lipid homeostasis as they must maintain whole body metabolic equilibrium and curb lipid accumulation despite higher fat intake. The liver is a key organ as regards energy homeostasis since it represents 20% of the whole body basal metabolic rate [20]. Within the hepatocytes, mitochondria are metabolic hubs by producing the energy needed for numerous cellular processes thanks to the degradation of different energetic metabolites (glucose, fatty acids, and amino acids). Currently, it is well recognized that mitochondrial dysfunction occurs in the context of obesity, insulin resistance [16], and non-alcoholic steatohepatitis (NASH) [7]. Hepatocyte mitochondria are the main site of β-oxidation of FFAs and adenosine triphosphate (ATP) production is one of the crucial issues in the understanding of NASH pathogenesis. It was previously reported that mitochondrial structural abnormalities, depletion of mitochondrial DNA and ATP, and mitochondrial dysfunction are characteristics of NASH patients as well as of diet-induced and animal models [7]. Over the last few years, there has been an increasing knowledge on the pathogenesis of fatty liver as well as disease progression to NASH and fibrosis by studies in different experimental models and in humans with this condition. Lipid overloading appear to play a central role not only as regulators of insulin sensitivity and development of fatty liver but also in the inflammatory process, mitochondrial apoptosis, cell death, and fibrosis. But few data are available on their impact on liver mitochondrial functions at the very early steps of steatosis. Numerous studies have shown that mitochondrial dysfunction is likely to constitute the “second hit” that greatly favors the progression of simple steatosis into full-blown steatohepatitis [7].
It is noteworthy that animal investigations dealing with the pathogenesis of NAFLD and NASH have been often conducted in genetic animal models [4], or with a diet deficient in choline and methionine [25] that can induce a loss of body fat mass. Thus, these models are not optimal in order to decipher the physiopathology of diet-induced NAFLD that are frequently observed in human. Of note, although studies using HFD rodent models are physiologically relevant they are usually performed at endpoint [2, 3], so that little information is available regarding the sequence of events leading to metabolic alterations and mitochondrial dysfunction during NAFLD progression. Indeed, although the liver normally presents a great metabolic flexibility in order to maintain energy homeostasis despite continuous feeding/fasting cycles, chronic intake of high amounts of fat can progressively deregulate this flexibility and favor lipid accumulation. Consequently, the aim of this study was to determine in rat liver the sequence of mitochondrial and metabolic adaptations during the first 8 weeks of a moderate HFD. To this end, investigations were performed after 2, 4, and 8 weeks of HFD in order to assess liver triglycerides, mitochondrial respiration with different substrates, respiratory chain complexes activity, mitochondrial DNA levels, and the expression of several genes involved in lipid homeostasis and mitochondrial biogenesis.
Methods
Animals
The present investigations were performed in accordance with the national guidelines for the care and use of animals in biomedical research. Male Sprague–Dawley rats were caged individually in a temperature-controlled room (20 ± 2°C) with a dark/light cycle of 12:12 h. Animals were provided with water ad libitum and a standard diet (U-A-R A04; SAFE, Epinay-sur-Orge, France) composed of (percent total weight) 16% protein, 3% fat, 60% carbohydrate, and 21% water, fiber, vitamins, and minerals. At the age of 2 months, the control group (without high fat diet) was sacrificed while the other groups were fed a HFD (UPAE, Jouy-en-Josas) containing (percent total weight) 22% protein, 35% fat (lard), 28% carbohydrate, and 15% water, fiber, vitamins, and minerals. Rats were fasted during the night before being killed after 2, 4, or 8 weeks of HFD feeding. We used six rats for each group throughout the study.
Mitochondrial respiration
Liver mitochondria were isolated as previously described [4]. Oxygen consumption was measured with a Clark oxygen electrode in a 2-ml glass cell thermostatically controlled at 37°C. Liver mitochondria (0.5 mg protein/ml) were incubated in a respiratory reaction medium, consisting of 120 mM KCl, 5 mM KH2PO4, 1 mM EGTA, 2 mM MgCl2, 3 mM HEPES, and 0.3% bovine serum albumin (w/v). The different oxidative substrates were 5 mM pyruvate + 2.5 mM malate, 40 μM palmitoyl-l-carnitine + 2.5 mM malate, or 5 mM succinate + 5 μM rotenone. The active state of respiration (state 3) was initiated by the addition of 300 μM ADP whereas the basal non-phosphorylating respiratory rate (state 4) was measured before addition of ADP. Respiratory control ratio (RCR) was calculated by dividing state 3 respiratory rate by state 4 respiratory rate.
Mitochondrial ATP synthesis
Mitochondria (0.5 mg/ml) were incubated in the respiratory reaction medium supplemented with 5 μM rotenone, 5 mM succinate, 20 mM glucose, and 125 μM ATP. State 3 of mitochondrial respiration was initiated by the addition of 175 mU/ml of hexokinase and ATP production was monitored by glucose-6-phosphate formation, as previously described [17]. After 2 min of oxygen consumption recording, aliquots were taken from the respiratory reaction medium and immediately placed in perchloric acid (6%) before centrifugation (8,000 × g, 3 min). The supernatant was subsequently removed and neutralized to pH 7. NADP+ (0.5 mM) and glucose 6-phosphate dehydrogenase (0.25 U) were then added and ATP production was monitored spectrophotometrically at 340 nm thanks to NADPH generation.
Mitochondrial enzyme activities
The activities of citrate synthase (CS), complex I, II, III, and IV were assessed spectrophotometrically at 37°C in isolated mitochondria thanks to an adaptation of the method described by Malgat et al. [13]. Briefly, CS activity was measured by monitoring the change in optical density of 5,5′-dithio-bis(2-nitrobenzoic acid) at 412 mm. The activity of NADH ubiquinone reductase (complex I) and succinate ubiquinone reductase (complex II) was determined by monitoring NADH oxidation at 340 mM and reduction of 2,6-dichlorophenolindophenol at 600 nm, respectively. Ubiquinone cytochrome c reductase (complex III) and cytochrome c oxidase (complex IV) activity was assessed by monitoring at 550 nm the reduction of cytochrome c and the oxidation of reduced cytochrome c, respectively. ATP synthase activity was measured thanks to a coupled assay using lactate dehydrogenase and pyruvate kinase by monitoring NADH oxidation at 340 mM [22].
Liver triglyceride content and serum parameters
Liver triglyceride (TG) content was determined using the method described by Xu et al. [29]. Briefly, 100 mg of liver tissue was homogenized in 1 ml of ethanol followed by a centrifugation at 15,000 × g for 10 min, and TG levels were measured with a Sigma kit. Blood glucose concentrations were measured with a glucometer. Serum insulin levels were measured using an enzyme-linked immunosorbent assay kit (Linco Research, St Charles, MO, USA). Serum free fatty acid and β-hydroxybutyrate concentrations were measured using enzymatic assay kits on a Cobas Mira automatic analyzer (Roche). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) were measured using the corresponding commercial enzyme kits (Bio Life Science Product, France). Left and right fat pads were isolated and weighted separately and then averaged for each animal.
Mitochondrial DNA quantification
Total DNA was extracted from liver using EZ1 DNA Tissue kit and a BioRobot EZ1 DSP workstation (Qiagen). The content of mitochondrial DNA (mtDNA) was calculated using real-time quantitative PCR by measuring the threshold cycle ratio (ΔCt) of a mitochondrial encoded gene (ND1) versus a nuclear encoded gene (β-globin).
Real-time quantitative RT–PCR analysis
Total RNA was extracted with the Trizol Reagent (Invitrogen). Levels of target mRNAs were measured after reverse transcription (RT) by real-time PCR using a LightCycler (Roche). A standard curve was systematically generated with six different amounts of purified target cDNA and each assay was performed in duplicate. Hypoxanthine guanine phosphoribosyl transferase (HPRT) was used as a reference gene in order to express the relative expression of each selected gene. Primer sequences and RT–qPCR conditions are available upon requested.
Statistical analysis
Results are expressed as mean ± SEM. The statistical significance of observed variations was assessed using one-way ANOVA, and differences between means were subsequently tested by Fisher post hoc test. A P value <0.05 was considered significant in all cases. All analyses were performed using Statview 5.0 (SAS Institute, Cary, NC, USA).
Results
Body weight and biochemical parameters
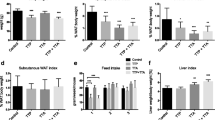
A progressive and significant increase in body weight was recorded during the whole duration of HFD feeding (Table 1). The mass of the epididymal fat pad was significantly higher during the first 4 weeks of HFD but remained stable afterwards. Serum insulin levels significantly enhanced after 2, 4, and 8 weeks of HFD compared to control. Glycemia was significantly increased after 4 and 8 weeks of HFD when compared to earlier time points. Serum non-esterified fatty acid (NEFA) levels remained unchanged during the 8 weeks of HFD. Serum β-hydroxybutyrate, a surrogate marker of hepatic mitochondrial β-oxidation, was significantly increased only after 4 weeks of HFD (Table 1). Liver triglyceride content was dramatically enhanced after 2 and 4 weeks of HFD, thus reflecting the presence of hepatic steatosis. However, although the accumulation of liver TG was still important after 8 weeks, it was significantly lower when compared with earlier time points (Fig. 1).
Mitochondrial oxygen consumption, ATP synthesis, respiratory chain complexes activities, and mtDNA levels
To determine the effect of HFD on liver mitochondrial respiratory activity, oxygen consumption was measured with various substrates in the presence (state 3) or the absence (state 4) of ADP (Table 2). Results showed that whatever the time point there was no difference in states 3 and 4 mitochondrial respiration using pyruvate + malate (complex I) or succinate + rotenone (complex II) as substrates. State 3 mitochondrial respiration using palmitoyl-l-carnitine + malate as substrates was significantly decreased after 8 weeks of HFD, thus indicating lower mitochondrial β-oxidation. As state 4 respiration with palmitoyl-l-carnitine + malate was unchanged, RCR was significantly reduced at this time point (Table 2). We also measured ATP synthesis during oxygen consumption (ATP/O) with succinate as substrate in order to assess oxidative phosphorylation efficiency (Fig. 2). ATP/O significantly increased after 8 weeks of HFD due to higher ATP synthesis. Interestingly, ATP synthase activity was significantly higher after 2, 4, and 8 weeks compared to control rats. Citrate synthase activity was significantly decreased after 4 and 8 weeks of HFD compared to control rats (Table 3). Activity of complex I, II, III, and IV remained unchanged during the whole duration of HFD feeding. Finally, hepatic mtDNA content was assessed b RT–PCR in the different groups of rats. We observed a highly stable mtDNA quantity during each step of the 8 weeks of regimen arguing against a hazardous or a proliferative process on mitochondria.
ATP synthesis/oxygen consumption ratio. ATP synthesis and oxygen consumption were measured at the same time with succinate + rotenone as respiratory substrate by using the hexokinase assay in liver mitochondria isolated from control rats and rats fed a HFD during 2, 4, and 8 weeks (n = 5–6). Results are expressed as mean ± SEM. P for ANOVA is 0.0541. *P <0.05 versus control and 2 weeks of HFD
Expression of genes involved in lipid metabolism and mitochondrial biogenesis
In order to evaluate the expression of key genes involved in lipid metabolism and mitochondrial biogenesis, real-time quantitative RT–PCR was performed in the different groups of rats (Fig. 3). Firstly, we measured gene expression of peroxisome proliferator-activated receptor γ coactivator 1 (PGC1) α and β, mitochondrial transcription factor A (TFAM), nuclear respiratory factor 1 (NRF1), and estrogen-related receptor α (ERRα) which are key regulatory factors involved in mitochondrial biogenesis and function (Fig. 3a). TFAM expression was significantly higher after 2 and 4 weeks of HFD when compared to the other time points. PGC1α expression tended to be lower after 2 and 4 weeks of HFD compared to control rats (P = 0.0955 and P = 0.0673, respectively), and was significantly decreased after 8 weeks of HFD. ERRα expression was significantly reduced after 4 and 8 weeks when compared with rats fed for 2 weeks with the HFD. In contrast, NRF1 and PGC1β expression remained constant during the whole period of HFD feeding.
Gene expression. Total RNA was extracted from liver of control rats and rats fed with a HFD for 2, 4, and 8 weeks of HFD (n = 5–6). Samples were analyzed by quantitative RT–PCR, and all PCR reactions were normalized for HPRT expression. The mean expression assessed in control liver was used as 100%. a Expression of genes involved in mitochondrial biogenesis and function. b Expression of genes involved in lipid synthesis. c Expression of genes involved in fatty acid and pyruvate oxidation. Results are expressed as mean ± SEM. *P <0.05 versus control, † P <0.05 versus control and HFD 8 weeks, ‡ P <0.05 versus HFD 2 weeks, a P = 0.0955, b P = 0.0673, c P = 0.0654, and d P = 0.0672 versus control
We also measured the hepatic expression of stearoyl-coenzyme A desaturase 1 (SCD1), mitochondrial glycerol-3-phosphate acyltransferase (mtGPAT), and peroxisome proliferator-activated receptor γ (PPARγ) which are involved in fatty acid and triglycerides synthesis (Fig. 3b). Whereas SCD1 expression remained unchanged between the different groups of rats, mtGPAT expression was significantly higher after 2 and 4 weeks of HFD when compared to the other groups. PPARγ expression tended to be higher after 4 weeks of HFD (P = 0.0672), and was significantly increased after 4 and 8 weeks.
Finally, we assessed the expression of carnitine palmitoyltransferase 1 (CPT1) and peroxisome proliferator-activated receptor α (PPARα) that are involved in fatty acid β-oxidation (Fig. 3c). However, CPT1 and PPARα expressions remained stable during the whole duration of HFD feeding. The expression of PDK4, which is involved in the mitochondrial oxidation of pyruvate, was increased only after 2 weeks of HFD (Fig. 3c).
Discussion
Although our understanding of NAFLD pathogenesis has significantly improved over the last few years, the whole spectrum of mitochondrial adaptations during the first steps of hepatic lipid accumulation remains to be characterized. In the present study, we aimed to investigate liver metabolism, in particular by studying the time course of mitochondrial function in a model of rats fed a HFD. Indeed, these investigations were performed in order to detect early metabolic adaptations setting up to face lipid overload and maintain energy homeostasis.
After 2 weeks of HFD, there were apparently no deleterious consequences of higher fatty acid supply on liver metabolism. The main and not surprising event at this stage was the increase in fatty acid storage which was illustrated by the accumulation of liver TG. Interestingly, we noticed higher expression of mtGPAT which is involved in the first and most likely rate-limiting step of TG synthesis [27]. There was also an increase in the expression of hepatic PPARγ. In addition to its major role in adipogenesis, increasing evidence has demonstrated that PPARγ plays a key role in the development of fatty liver [10]. Several murine models of NAFLD induced by obesity, diabetes [1, 14, 19], and HFD [12] are characterized by higher hepatic PPARγ expression. Interestingly, there was also an enhanced expression of PDK4, a kinase regulating an important metabolic switch between glycolysis and fatty acid oxidation [26]. Indeed, PDK4 activation inhibits the pyruvate dehydrogenase complex, and thus the principal source of acetyl-CoA becomes the fatty acid oxidation pathway. Therefore, there was an apparent metabolic orientation in order to remove the excess of fatty acids, and this may explain the trend toward higher serum β-hydroxybutyrate levels. Of note, there was at the same time a significant increase in ATP synthase activity. Unlike muscle, mitochondrial function in liver is regulated by ATP synthesis and not by the entry of the oxidative substrates into the respiratory chain [21]. Hence, enhanced ATP synthase activity could represent a mitochondrial adaptation in order to oxidize more substrates and/or to supply more ATP to face higher energy needs (e.g., acyl-CoA and TG synthesis).
After 4 weeks of HFD, liver TG accumulation was associated with a significant increase in serum β-hydroxybutyrate, thus strongly suggesting higher hepatic fatty acid oxidation. Conceivably, enhanced mitochondrial β-oxidation could be a direct consequence of higher fatty acid availability and entry within the β-oxidation pathway since there was no concomitant increase in both palmitoyl-l-carnitine oxidation and respiratory chain complexes activity. Of note, the increase in hepatic PDK4 expression was not maintained after 4 weeks of HFD. Since PDK4 expression is regulated by PGC1α and ERRα [30], decreased hepatic PDK4 mRNA levels could be related to the concomitant reduction of PGC1α and ERRα expression.
After 8 weeks of HFD, we observed an inversion of the early metabolic adaptation. Indeed, serum levels of β-hydroxybutyrate decreased to values identical to the control group and this was associated with lower state 3 respiration with palmitoyl-l-carnitine, thus indicating impaired mitochondrial β-oxidation pathway. The fact that respiration with succinate/rotenone and pyruvate/malate was not altered strongly suggested that the inhibition of mitochondrial β-oxidation was not related to respiratory chain dysfunction. Indeed, inhibition of complexes I and III respectively hampers the oxidation of NADH and FADH2 into NAD+ and FAD, two cofactors required for the mitochondrial dehydrogenases involved in the β-oxidation pathway [6]. Our data were confirmed by the constant activities of complex I, II, III, and IV along the whole duration of HFD feeding. Interestingly, ATP/O assessed with succinate was increased after 8 weeks of HFD as the result of higher ATP synthesis, but oxygen consumption was unaffected. Enhanced oxidative phosphorylation efficiency with succinate could be a metabolic adaptation to impaired mitochondrial fatty acid oxidation in order to maintain ATP synthesis with alternative substrates. Higher oxidative phosphorylation efficiency with some substrates entering in excess within mitochondria could subsequently increase ROS production and thus have deleterious consequences on the respiratory chain integrity [6, 8]. It is worth mentioning that reduced hepatic mtGPAT expression after 8 weeks compared to 2 and 4 weeks could induce a decrease in TG synthesis and thus explain the significant reduction of liver TG at the last time point. Thus, the concomitant association of impaired fatty acid oxidation and reduced TG synthesis could lead to the detrimental accumulation of intracellular free fatty acids and some of their highly toxic metabolites such as dicarboxylic acids [6].
In addition, mitochondrial biogenesis was assessed thanks to the measure of the hepatic expression of key regulatory genes, and mtDNA content. Of note, PGC1α expression tended to be lower after 2 and 4 weeks of HFD and was significantly decreased after 8 weeks, although we did not observe reduced expression in its downstream target, NRF1. Recent evidence shows that ERRα interacts with PGC1α to regulate mitochondrial gene expression, thus indicating that this nuclear receptor is an important modulator of mitochondrial function [15, 23]. Moreover, ERRα expression is regulated by PGC1α [24]. Interestingly, ERRα expression was decreased after 4 and 8 weeks of HFD compared to the earlier time points and this paralleled the reduction of PGC1α expression. Another downstream target of PGC1α is TFAM, which is key transcriptional activator that translocates to the mitochondria and activates mitochondrial DNA replication and transcription. Surprisingly, TFAM expression increased after 2 and 4 weeks of HFD but declined afterwards. However, although there was significant variation in the expression of PGC1α, ERRα, and TFAM, mtDNA levels remained constant over the whole period of HFD feeding. Of note, our study did not include a group of rats fed a standard chow. However, except for some biological parameters such as body weight and fat mass which can vary with age, other parameters such as blood glucose, insulinemia, and liver triglycerides can be fairly stable for several months in the context of normal feeding [5, 9]. During such a time frame, the hepatic expression of several genes involved in lipid and mitochondrial homeostasis seems also constant in control rats [5]. The results support a model in which during the development of NAFLD, overflow of FFA to the liver is associated with an increased mitochondrial β-oxidation and ATPase activity resulting in a tilt of the balance towards energy formation and energy demand (Fig. 4). This could lead during the following weeks of overfeeding to the development of the second hit of steatosis—characterized by mitochondrial oxidative stress—morphological abnormalities, and finally hepatocyte apoptosis.
Early effect of increasing lipid load on liver mitochondria. Oversupply of free fatty acid to hepatocyte led to increase β-oxidation machinery and ATP production to supply energy demand for triglyceride storage and droplet growth. Until a limit step, where mitochondria are uncoupled from β-oxidation, triglycerides storage started to decrease. Later, it is hypothesized that intermediate lipids could accumulate and lead to mitochondrial stress and cell apoptosis characterizing the second hit of steatosis
Conclusions
In summary, in the first time of HFD (2–4 weeks), there was a dramatic accumulation of liver triglycerides and an apparent increase of fatty acid oxidation which could be set up in order to get rid of the excess of fatty acids. However, this first regulation is transient and there is a subsequent metabolic shift which leads to a decrease in fatty acid oxidation and triglyceride synthesis. Although reduced triglyceride synthesis could be at first glance favorable for the liver, concomitant impairment of both fatty acid esterification and mitochondrial β-oxidation could favor on the long term the accumulation of free fatty acids that can have major deleterious effects on cell homeostasis [6]. It should be pointed out that there was no alteration of mitochondrial respiratory chain itself illustrating that until 8 weeks of HFD liver mitochondria can preserve their functionality despite fatty acid overload. Thus, this longitudinal study in rats fed a HFD for 8 weeks allowed us to show that liver mitochondria (and more broadly lipid homeostasis) are able to set up different types of metabolic adaptation in the context of lipid overload.
Abbreviations
- HFD:
-
High fat diet
- RCR:
-
Respiratory control ratio
- CS:
-
Citrate synthase
- TG:
-
Triglyceride
- mtDNA:
-
Mitochondrial DNA
- HPRT:
-
Hypoxanthine guanine phosphoribosyl transferase
- PGC1α and β:
-
Peroxisome proliferator-activated receptor γ coactivator 1 α and β
- TFAM:
-
Mitochondrial transcription factor A
- NRF1:
-
Nuclear respiratory factor 1
- ERRα:
-
Estrogen-related receptor α
- SCD1:
-
Stearoyl-coenzyme A desaturase 1
- mtGPAT:
-
Mitochondrial glycerol-3-phosphate acyltransferase
- PPARα and γ:
-
Peroxisome proliferator-activated receptor α and γ
- CPT1:
-
Carnitine palmitoyltransferase 1
- PDK4:
-
Pyruvate dehydrogenase kinase 4
References
Bedoucha M, Atzpodien E, Boelsterli UA (2001) Diabetic KKAy mice exhibit increased hepatic PPARgamma1 gene expression and develop hepatic steatosis upon chronic treatment with antidiabetic thiazolidinediones. J Hepatol 35:17–3
Ciapaite J, Bakker SJ, Van Eikenhorst G et al (2007) Functioning of oxidative phosphorylation in liver mitochondria of high-fat diet fed rats. Biochim Biophys Acta 1772:307–316
Crescenzo R, Bianco F, Falcone I et al (2008) Alterations in hepatic mitochondrial compartment in a model of obesity and insulin resistance. Obesity (Silver Spring) 16:958–64
Flamment M, Arvier M, Gallois Y et al (2008) Fatty liver and insulin resistance in obese Zucker rats: no role for mitochondrial dysfunction. Biochimie 90:1407–13
Forcheron F, Abdallah P, Basset A et al (2009) Nonalcoholic hepatic steatosis in Zucker diabetic rats: spontaneous evolution and effects of metformin and fenofibrate. Obesity 17:1381–9
Fromenty B, Pessayre D (1995) Inhibition of mitochondrial beta-oxidation as a mechanism of hepatotoxicity. Pharmacol Ther 67:101–54
Fromenty B, Robin MA, Igoudjil A et al (2004) The ins and outs of mitochondrial dysfunction in NASH. Diabetes Metab 30:121–38
García-Ruiz C, Colell A, Morales A et al (1995) Role of oxidative stress generated from the mitochondrial electron transport chain and mitochondrial glutathione status in loss of mitochondrial function and activation of transcription factor nuclear factor-kappa B: studies with isolated mitochondria and rat hepatocytes. Mol Pharmacol 48:825–34
Gauthier MS, Favier R, Lavoie JM (2006) Time course of the development of non-alcoholic hepatic steatosis in response to high-fat diet-induced obesity in rats. Br J Nutr 95:273–81
Gavrilova O, Haluzik M, Matsusue K et al (2003) Liver peroxisome proliferator-activated receptor gamma contributes to hepatic steatosis, triglyceride clearance, and regulation of body fat mass. J Biol Chem 278:34268–76
Hedley AA, Ogden CL, Johnson CL et al (2004) Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA 291:2847–50
Inoue M, Ohtake T, Motomura W et al (2005) Increased expression of PPARgamma in high fat diet-induced liver steatosis in mice. Biochem Biophys Res Commun 336:215–22
Malgat M, Durrieu G, Mazat JP (1999) Enzymatic and polarographic measurements of the respiratory chain complexes. In: Lestienne P (ed) Mitochondrial diseases. Springer, Paris, pp 357–77
Memon RA, Tecott LH, Nonogaki K et al (2000) Up-regulation of peroxisome proliferator-activated receptors (PPAR-alpha) and PPAR-gamma messenger ribonucleic acid expression in the liver in murine obesity: troglitazone induces expression of PPAR-gamma-responsive adipose tissue-specific genes in the liver of obese diabetic mice. Endocrinology 141:4021–31
Mootha VK, Handschin C, Arlow D et al (2004) Erralpha and Gabpa/b specify PGC-1alpha-dependent oxidative phosphorylation gene expression that is altered in diabetic muscle. Proc Natl Acad Sci USA 101:6570–5
Morino K, Petersen KF, Shulman GI (2006) Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes 55:S9–S15
Nogueira V, Piquet MA, Devin A et al (2001) Mitochondrial adaptation to in vivo polyunsaturated fatty acid deficiency: increase in phosphorylation efficiency. J Bioenerg Biomembr 33:53–61
Prentice AM (2006) The emerging epidemic of obesity in developing countries. Int J Epidemiol 35:93–9
Rahimian R, Masih-Khan E, Lo M et al (2001) Hepatic over-expression of peroxisome proliferator activated receptor gamma2 in the ob/ob mouse model of non-insulin dependent diabetes mellitus. Mol Cell Biochem 224:29–37
Rolfe DF, Brown GC (1997) Cellular energy utilization and molecular origin of standard metabolic rate in mammals. Physiol Rev 77:731–58
Rossignol R, Letellier T, Malgat M et al (2000) Tissue variation in the control of oxidative phosphorylation: implication for mitochondrial diseases. Biochem J 347:45–53
Rustin P, Chretien D, Bourgeron T et al (1994) Biochemical and molecular investigations in respiratory chain efficiencies. Clin Chim Acta 228:35–51
Schreiber SN, Emter R, Hock MB et al (2004) The estrogen-related receptor alpha (ERRalpha) functions in PPARgamma coactivator 1alpha (PGC-1alpha)-induced mitochondrial biogenesis. Proc Natl Acad Sci USA 101:6472–7
Schreiber SN, Knutti D, Brogli K et al (2003) The transcriptional coactivator PGC-1 regulates the expression and activity of the orphan nuclear receptor estrogen-related receptor alpha (ERRalpha). J Biol Chem 278:9013–8
Serviddio G, Bellanti F, Tamborra R et al (2008) Alterations of hepatic ATP homeostasis and respiratory chain during development of non-alcoholic steatohepatitis in a rodent model. Eur J Clin Invest 38:245–52
Sugden MC (2003) PDK4: a factor in fatness? Obes Res 11:167–9
Sul HS, Wang D (1998) Nutritional and hormonal regulation of enzymes in fat synthesis: studies of fatty acid synthase and mitochondrial glycerol-3-phosphate acyltransferase gene transcription. Annu Rev Nutr 18:331–51
World Health Organization (2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ. Tech Rep Ser 894: i-xii, 1–253
Xu H, Wilcox D, Nguyen P et al (2006) Hepatic knockdown of mitochondrial GPAT1 in ob/ob mice improves metabolic profile. Biochem Biophys Res Commun 349:439–48
Zhang Y, Ma K, Sadana P et al (2006) Estrogen-related receptors stimulate pyruvate dehydrogenase kinase isoform 4 gene expression. J Biol Chem 281:39897–906
Acknowledgments
Melissa Flamment was supported by a grant from "Conseil Régional Pays de la Loire/Inserm". The authors thank Jerome Roux, Pierre Legras, and Laurent Buffet for animal care.
Authors’ contributions
Mélissa Flamment conducted all the study, with the help of Naig Gueguen and Gilles Simard for mitochondrial respiration. Jennifer Rieusset and Hubert Vidal did the gene expression quantifications. Yves Malthiery, Bernard Fromenty, and Pierre-Henri Ducluzeau were involved in the design and the manuscript writing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Flamment, M., Rieusset, J., Vidal, H. et al. Regulation of hepatic mitochondrial metabolism in response to a high fat diet: a longitudinal study in rats. J Physiol Biochem 68, 335–344 (2012). https://doi.org/10.1007/s13105-012-0145-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-012-0145-3