Abstract
Ketogenesis is the branch of mammalian metabolism concerned with the synthesis of ketone bodies. In this process, the small, water-soluble compounds acetoacetate, D-3-β-hydroxybutyrate and propanone are produced by the liver in response to reduced glucose availability. Although ketone bodies are always present at a low level in healthy individuals, dietary manipulation and certain pathological conditions can increase the levels of these compounds in vivo. In some instances, such as in refractory epilepsy, high levels of ketone bodies can be beneficial—in this instance, by exerting an anticonvulsant effect. Conversely, if the levels of ketones rise to supraphysiological levels, as can occur in diabetes mellitus, a state of ketoacidosis can occur, which has serious consequences for cellular function. More recently, research has identified a possible link between ketogenesis and free radical-mediated pathologies, highlighting the potential application of ketogenic diets to the treatment of conditions such as Alzheimer's disease. Overall, an understanding of ketone body metabolism and its links to human disease may prove to be vital in developing new regimens for the treatment of human disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mammals have evolved to utilize carbohydrate as their primary source of metabolic fuel, extracting energy through a series of intricate biochemical pathways. Yet, since the early days of medicine, it has been known that by restricting our dietary intake of carbohydrate, we can significantly alter the course of certain diseases, such as epilepsy. In his work Epidemics, a volume within the Hippocratic Corpus, Hippocrates stated that “epilepsy is cured as quickly as it had appeared, through complete abstinence of food and drink” [40]. While Hippocrates had certainly no concept of the metabolic pathways he was manipulating, his early theories have stood the test of time, and research into low carbohydrate, high fats diets has enjoyed a renaissance in recent years. To fully appreciate the clinical utility of such diets, we must first understand the changes in mammalian biochemistry brought about by such a regime. This involves a study of the basic pathways of mammalian metabolism.
Mammalian metabolism
Normal metabolism
In the well-fed state, mammals derive much of their metabolic energy from carbohydrates, with glucose serving as the principle substrate for energy (ATP) production, which is achieved via. the three well-known pathways: glycolysis, tricarboxylic acid (TCA) cycle and the electron transport chain (ETC). A crucial reaction linking the glycolytic pathway with the TCA cycle involves the intermediate compound acetyl-CoA, a thioester that can be considered as the gatekeeper of mammalian metabolism, since under normal conditions, the majority of ATP production requires the passage of two-carbon units into the TCA cycle. In particular, acetyl-CoA is the major product of fatty acid catabolism, and thus plays a major role in ketogenesis, i.e., the formation of ketone bodies [10].
Ketone body metabolism
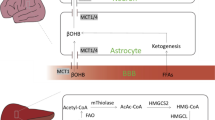
Ketone bodies are a group of small organic molecules produced in the mitochondria of perivenous hepatocytes under conditions of reduced glucose availability, with healthy adults producing up to 185 g of ketones per day. The two principal ketone bodies are acetoacetate and D-3-β-hydroxybutyrate; the former compound undergoes slow nonenzymicFootnote 1 decarboxylation to produce the third and least abundant ketone body, propanone. The primary physiological role of ketone bodies is the transfer of lipid-derived energy from the liver to the peripheral tissues [39], which is particularly important for the brain during periods of hypoglycemia, since this organ cannot utilize fatty acids for energy production.
Overall, ketogenesis is driven by the availability of acetyl-CoA, which in terms of ketone body production, is derived primarily from the β-oxidation of fatty acids [17]. Under hypoglycemic conditions, oxaloacetate is used preferentially for gluconeogenesis, and therefore acetyl-CoA produced from fatty acid oxidation cannot be funneled into the TCA cycle (since this requires oxaloacetate), but is instead converted into ketone bodies (Fig. 1). During ketogenesis, acetyl-CoA is converted to 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) by mitochondrial HMG-CoA synthase, which is also used for cytosolic cholesterol synthesis. However, since hepatic mitochondria express higher levels of HMG-CoA synthase, ketogenesis is favored over the isoprenoid pathway. The ketones so-formed are then released by the liver and delivered to the tissues for use in energy production through ketolysis.
Metabolic pathways in ketogenesis. Under reduced glucose availability, acetyl-CoA is channeled towards ketone body formation in the liver. β-Ketothiolase (1) mediates the condensation of two acetyl-CoAs, forming acetoacetyl-CoA, which then undergoes a further condensation with acetyl-CoA mediated by HMG-CoA synthase (2), forming HMG-CoA. This compound is cleaved (3) to acetoacetate, the first ketone body, which is then reduced by β-hydroxybutyrate dehydrogenase (4), yielding D-3-β-hydroxybutyrate. In extra hepatic tissues, ketone bodies are activated by acetoacetate:succinyl CoA CoA transferase and converted to acetyl-CoA by β-ketothiolase (5), which permits their passage into the TCA cycle. α-Ketoglutarate can be funneled towards glutamate synthesis either directly via glutamate dehydrogenase (6) or by transamination (7). The glutamate so-formed may be used for GABA synthesis, or be converted to the tripeptide glutathione; however, this latter process is heavily dependent on cysteine availability
Ketolysis can be considered as essentially the reverse of ketogenesis, although separate enzyme systems have evolved to mediate the process. The conversion of acetoacetate back into acetoacetyl-CoA by succinyl-CoA-oxoacid transferase (SCOT) is the rate-limiting step in this process, with the expression of the SCOT gene being downregulated by high intracellular levels of acetoacetyl-CoA [37]. The acetoacetyl-CoA so-produced is then cleaved by methylacetoacetyl-CoA thiolase (MAT), producing free acetyl-CoA, which can then enter the TCA cycle. Interestingly, high levels of MAT activity have been demonstrated in hepatic tissues, unlike SCOT that is virtually undetectable in hepatocytes [15]. It seems likely that hepatic MAT activity supplements production of acetoacetyl-CoA.
The role of ketone bodies during fasting
General metabolism during fasting
The metabolic changes that occur during fasting have been well characterized [28]. Initially, hepatic reserves of glycogen are broken down by glycogenolysis, liberating glucose, which is exported into the circulation. After 1–2 days, the carbohydrate supply becomes largely dependent upon gluconeogenesis (in reality, the glycogen stores won't have become depleted until several days of fasting, but gluconeogenesis works in unison with glycogenolysis to meet the energy requirements of the organism). The main substrate for gluconeogenesis at this stage is 3-monoacylglycerol (MAG) [45], which is released following the hydrolysis triglycerides in adipose tissue. The fatty acids released during lipolysis cannot generally be converted to glucose in mammals, due to an inability to produce oxaloacetate independently of glycolysis. The only exception to this is the catabolism of odd-chain fatty acids that yield propinoyl-CoA, a precursor of succinly-CoA. In plants and microbes, the glyoxylate cycle provides a means of producing oxaloacetate independently of glycolysis [11, 40].
Lipogenesis vs. ketogenesis
Ordinarily, in the well-fed state when metabolic fuel is abundant, the liver converts glucose to fatty acids and incorporates these (as triglycerides) within very low density lipoproteins (VLDL), which are then secreted by the liver for transport to other tissues [16]. In the fasting state, however, the liver preferentially converts fatty acids to ketone bodies, not VLDL. This metabolic switch is made at the level of the hepatic mitochondria [20], since this is the location of ketone body synthesis and β-oxidation. In order for fatty acids to enter the mitochondrial matrix, they must be esterified to carnitine by carnitine acyl transferase I, which is only present on the outer face of the mitochondrial membrane and is inhibited by malonyl-CoA—the key intermediate of fatty acid synthesis. Thus, when long-chain fatty acids are being synthesized, the levels of malonyl-CoA will be high and carnitine acyl transferase I will be inhibited [12]. Since fatty acids are blocked from entering the mitochondrial matrix, they remain in the cytosol and are converted to triglycerides and phospholipids. Conversely, in the fasting state, when glucagon and β-adrenergic receptor agonists stimulate lipolysis, the level of malonyl-CoA will be low, and fatty acids are converted to fatty acyl carnitine and traverse the mitochondrial matrix membrane for synthesis of ketone bodies. Of course, since the liver lacks SCOT, it cannot utilize ketone bodies for energy production. Instead, the liver derives much of its ATP from keto acids, derived from the degradation of amino acids [44].
The starvation timeline
The average, well-nourished 70-kg man would be expected to have the fuel reserves described in Table 1 [5]. Assuming that the basal energy demand for a 24-hour period is approximately 1,600 kcal, these fuel reserves would be sufficient to ensure survival for about 3 months. Carbohydrate reserves are exhausted within 24 h, yet inspection of Fig. 2 shows that blood glucose is maintained at a relatively constant level after this time has elapsed. This observation arises from the brain's and red blood cells' absolute requirement for glucose, and thus the first priority of metabolism during starvation is to provide fuel for these systems [38]. The potential energy liberated form glucose synthesis is about 25,600 kcal (1,600 kcal from glycogenolysis and 24,000 kcal from protein-derived gluconeogenesis). Since, from an evolutionary point-of-view, survival is largely dependent upon being able to move quickly, which is in turn dependent on muscle mass, gluconeogenesis from amino acids released by protein breakdown is a last resort. Luckily, however, the vast majority of a mammal's energy reserves are contained within the fatty acyl moieties of triglycerides stored in adipose tissues. Therefore, if survival is to continue, the energy contained within triglycerides must be converted into a form usable by the brain and red blood cells [31].
In the first day of starvation, low blood sugar levels lead to a reduction in insulin secretion and an increase in circulating glucagon. This hormone activates two processes: (i) increased lipolysis in adipose tissue and (ii) hepatic gluconeogenesis [46]. The increases in acetyl-CoA and citrate brought about by these two processes switch off glycolysis, sparing glucose. Cellular glucose uptake is also reduced by glucagon, and muscle shifts from using glucose to fatty acids for fuel. This inhibits conversion of pyruvate to acetyl-CoA, and results in the export of pyruvate and lactate to the liver for gluconeogenesis. A small amount of proteolysis releases amino acids, such as alanine, which are also exported to the liver for gluconeogenesis.
After 3 days of starvation, the TCA cycle is unable to oxidize all of the acetyl-CoA produced by fatty acid catabolism, since the levels of oxaloacetate have been depleted by gluconeogenesis. Consequently, excess acetyl-CoA is utilized for ketogenesis, and the subsequently produced ketone bodies are released by the liver for use by the brain and other tissues. After several weeks' starvation, ketone bodies are the principal fuel source for the brain, and thus the length of survival is roughly dependent upon the size of the triglyceride reservoir [14].
Ketone bodies, diabetes and ketoacidosis
Pathologies leading to abnormalities of glucose metabolism, principally poorly-controlled diabetes mellitus, can lead to increased ketone body production. Cellular glucose uptake is mediated by the pancreatic hormone insulin, which activates the GLUT transporter proteins. In type 1 diabetes, which is characterized by complete insulin deficiency, increased blood glucose levels can prove lethal, and this condition must be treated with exogenous insulin. In type 2 diabetes, insulin is produced, but various biological factors have induced a state of insulin resistance. Under conditions of low insulin, hormone sensitive lipase activity is increased, leading to increased lipolysis and thus ketone body production [47] (Fig. 3). If the levels of ketone bodies are allowed to increase, their acidic nature overwhelms the buffering capacity of blood, decreasing its pH, and leading to a condition known as diabetic ketoacidosis (DKA). The production of ketone bodies in this fashion accounts for the fruity smell on the breath of patients with diabetes who have poorly controlled blood sugar (this is due to propanone, which is a volatile ketone; its boiling point is only 56.5°C) [54].
Control of ketogenesis. The activation of hormone-sensitive lipase (HSL) by adrenaline via protein-kinase A (PKA) stimulates lipolysis, liberating molecules of fatty acyl CoA that are exported from adipocytes to the hepatic tissues. Following metabolism by β-oxidation, acetyl-CoA enters the ketogenic pathway. Conversely, if insulin levels are high, HSL is maintained in an unphosphorylated form, and is inactive. In this case, fatty acids are stored as triglycerides
Although DKA is a more common complication of type 1 diabetes, it can also occur in type 2 diabetes, particularly when the patient is experiencing additional metabolic stressors—e.g., infection, surgery or even psychological stress [36]. A similar state of ketoacidosis can occur in cases of salicylate (aspirin) poisoning. This arises from the ability of salicylates to inhibit the enzymes of the TCA cycle (specifically succinate dehydrogenase and α-ketoglutarate dehydrogenase). This leads to increased formation of lactate, which is then converted into ketone bodies [32].
Ketogenic diets
General features of a ketogenic diet
A diet high in fat and low in carbohydrate and protein is generally described as a ‘ketogenic diet’. The classic ketogenic diet, developed in 1921 by Russell Wilder at the Mayo Clinic in Minnesota, USA, was based on the consumption of fats and carbohydrates in the ratio of 4:1, with the fat complement consisting mainly of long-chain saturated triglycerides. In his early work, Wilder noticed that ketogenic diets had an anticonvulsant effect in children with refractory epilepsy (i.e., epilepsy that does not fully respond to anticonvulsant drugs) [2, 35]. Since production of ketone bodies is a key feature of ketogenic diets, Wilder hypothesized that ketones were directly responsible for the observed anticonvulsant effect. While it is likely that ketone bodies have some anticonvulsant role in refractory epilepsy, ketogenic diets also promote alterations in the production of free fatty acids [50], amino acids [53], purines [30] and in the utilization of glucose. In particular, ketogenic diets appear to promote the production of γ-aminobutyric acid (GABA), a key inhibitory neurotransmitter in the mammalian brain [52]. The mechanism for increased GABA production would seem to be linked to the increased turnover of the TCA cycle, with a portion of α-ketoglutarate being used for production of glutamate, which is then available for conversion to GABA. The so-called ‘GABAergic hypothesis’ states that ketosis favors increased glutamic acid decarboxylase activity, promoting GABA synthesis and thus dampening hyperexcitability throughout the brain.
Medium-chain triglycerides ketogenic diet
Medium-chain triglycerides are formed from the esterification of glycerol with medium-chain fatty acids (between six and 12 carbon atoms in length), and are found in high abundance in coconut and palm kernel oils. They are rapidly absorbed into the portal circulation, and are thus metabolized much faster than long-chain triglycerides (such as those found in animal fat). In the 1960s, it was discovered that medium-chain triglycerides were much more ketogenic than long-chain triglycerides, and later in 1971, Huttenlocher introduced a variant of the classic 4:1 ketogenic diet using MCT [22]. Although this diet offered excellent seizure control in refractory epilepsy, gastrointestinal side-effects lead to a decline in use. Despite this, well-administered MCTs diets are associated with fewer clinical side-effects (kidney stones, hypoglycemia and acidosis) than classic ketogenic diets, and the highly ketogenic nature of MCT permits more carbohydrate intake than the classic ketogenic diets, and thus increased palatability. However, the MCT diet has a number of limitations. Firstly, it is not suitable for patients taking valproate (a common antiepileptic drug) since this may increase the risk of liver failure. Secondly, it takes longer to achieve seizure control with the MCT diet, since the levels of MCT oil must be increased slowly to minimize GI side-effects, and finally, MCT oil is much more expensive than alternative diet regimens [27], reducing the availability of such treatments.
The Atkins diet
Of all the high fat low carbohydrate diets, the Atkins diet is by far the most infamous. Popularized in the early 1990s, the Atkins diet is essentially a very low carbohydrate diet with the daily energy requirements being met almost exclusively by fat metabolism. Although a diet high in fat obviously leads to ketogenesis, the efficacy of the Atkins diet is now believed to be due to the restrictive food choice, and not ketogenesis [3]. Early discussions of the Atkins diet proposed that strict adherence could lead to ketoacidosis [48]. This was typified by a case report published in the Lancet, where Chen et al. described a 40-year-old female, who after following an Atkins style diet for 1 month, displayed a serum β-hydroxybutyrate concentration of 390 μg/mL (normal range: 0–44 μg/mL) and an apparently associated state of ketoacidosis [6]. Although the levels of β-hydroxybutyrate in this patient are clearly in excess of the normal range, it could not promote ketoacidosis, since this can only occur through insulin deficiency/insufficiency. Multiple authors [29] highlighted the misleading nature of this article, pointing out that insufficient detail was provided to enable correct evaluation of the case report.
Further criticism of Atkins style diets focuses on the consequences for cardiovascular health, since many of these diets contain high levels of saturated fat [8]. In vivo, dietary fat is transported to the liver by chylomicrons, where triglycerides are repackaged and secreted as nascent VLDL, which, via. an extensive delipidation cascade, are metabolized to low density lipoprotein (LDL). Thus, the higher the dietary fat intake, the more VLDL will be produced by the liver, and the more LDL will be generated by the delipidation cascade. However, the evidence seems to suggest that Atkins style diets may decrease total cholesterol and triglycerides, and increase the levels of high density lipoprotein (HDL), particularly in hyperlipidaemic patients. The reasons for this seem unclear; however, replacing saturated fatty acids with mono- or polyunsaturated fatty acids is known to decrease LDL, possibly by increasing its removal from the circulation [16]. Furthermore, the altered utilization of acetyl-CoA during ketogenesis may affect the isoprenoid pathway, resulting in reduced de novo cholesterol synthesis (c.f. Fig. 1). It would, therefore, seem that when comparing high fat low carbohydrate diets, care must be taken to compare ‘like with like’, since even the composition of the fats consumed in such a diet may have considerable bearing on whether it increases or decreases cardiovascular risk.
Weight loss mechanisms in ketogenic diets
The cellular and biochemical mechanisms responsible for weight loss in ketogenic diets, such as the Atkins diet, are still uncertain, despite extensive investigation. The incongruent results are most likely due to the prevalence of underpowered studies, since large, multicenter investigations have reported more consistent findings [25]. Overall, it certainly seems that initial weight loss can be attributed to dieresis; ketone body excretion (ketonuria) increases renal sodium and hence urinary water loss. In addition, glycogenolysis, which is a prominent feature of the early stage of a ketogenic diet, is associated with concomitant water release (for every 1 g of glycogen stored, approximately 2 g of water are stored also) [9]. Later, weight loss is more difficult to explain. Some authors have suggested that ketones act on the satiety center of the brain and suppress hunger [13], while others believe that it is simply the physical form of the carbohydrate consumed that affects satiety [33]. Increased lipolysis (due to reduced insulin secretion) does promote weight loss [43], but probably not to a significant extent. It would seem that the weight loss observed in such diets follows a biphasic pattern—initial weight loss is due to metabolic alterations, while later weight loss is more than likely attributable to restrictive food choice. The current consensus is that an effective ketogenic diet should contain slightly more protein than previously used, and maintain a total fat content around 30%, and to include approximately 50% low glycaemic carbohydrate [1]. This dietary profile is certainly less ‘severe’ than previously reported ketogenic diets and would undoubtedly improve long-term compliance due to its increased palatability.
Ketone bodies and oxidative stress
Free radical chemistry and antioxidants
Oxidative stress is a key feature of a variety of pathophysiological processes, including development of neurodegenerative conditions such as Alzheimer's disease, as well as cardiovascular disease. In general, it can be regarded as an imbalance between oxidant species (mostly free radicals) and antioxidant defenses [51]. In biological systems, most oxidants fall into the category of ‘reactive oxygen species’, which includes the superoxide (O •−2 ) and hydroxyl (HO•) radicals, and singlet oxygen (1O2). The superoxide anion is produced by a variety of biochemical reactions in vivo, including the electron transport chain, and it undergoes spontaneous disumtation to hydrogen peroxide. In 1894, Fenton first proposed the iron-catalyzed formation of hydroxyl radicals from superoxide (ii). However, since any system that produces superoxide will also produce hydrogen peroxide, it seems likely that the reducing power for this reaction is first supplied from the reaction of iron(III) with superoxide (i), and the iron(II) so-formed reacts with hydrogen peroxide to produce hydroxyl radicals in a sequence known as the Haber–Weiss reaction (iii):
-
(i)
Fe3+ + O •−2 → Fe2+ + O2
-
(ii)
Fe2+ + H2O2 → Fe3+ + HO• + HO−
-
(iii)
O •−2 + H2O2 → HO• + HO− + O2.
Hydroxyl radicals produced in this way are powerful oxidizing agents (E°′ = +1.06 V) and lipids, proteins and nucleic acids are all indiscriminately oxidized by this species. Taking this into account, it seems likely that much of the oxidative damaged inflicted by reactive oxygen species is mediated by hydroxyl radicals.
The various effects of free radicals can be controlled to some extent through the presence of antioxidants, such as ascorbate, α-tocopherol and glutathione. In general, antioxidants can function through one of two mechanisms—they can reduce free radical species through proton donation (proton-donating antioxidants) or they can interrupt the free radical chain reaction which occurs with many biological substrates (chain-breaking antioxidants). Proton-donating antioxidants are best represented by water-soluble compounds, e.g., ascorbate and certain plant polyphenols, while chain-breaking antioxidants tend to be lipophilic or amphipathic in nature, and are represented by fat-soluble compounds such as α-tocopherol and its analogues (e.g., Trolox). While much of the oxidative damage observed in vivo occurs through a free radical chain reaction, both types of antioxidant appear to operate synergistically to ameliorate the actions of reactive oxygen species. This is certainly the case in lipid peroxidation (Fig. 4), where α-tocopherol reduces lipid peroxyl radicals to lipid hydroperoxides (iv). The α-tocopheryl radical formed is then reduced by ascorbate (v), forming dehydroascorbate, which is in turn recycled by biradical quenching (vi) [19]:
-
(iv)
TOH + LOO• → TO• + LOOH
-
(v)
TO• + AscH− → TOH + Asc•−
-
(vi)
2Asc•− + H+ → Asc + AscH−.
Lipid peroxidation. An unsaturated fatty acid undergoes free radical attack by hydroxyl radicals, in which an allylic hydrogen atom is abstracted from a cis-cis pentadiene center, producing a lipid pentadienly radical, which quickly isomerises to a cis-trans conjugated diene. Addition of molecular oxygen forms a lipid peroxyl radical, which may react with a further unsaturated fatty acid, propagating the chain reaction, or undergo termination, in which lipid hydroperoxides and cyclic peroxides are formed as the terminal products of lipid peroxidation
By considering these reactions, it is clear that a defining characteristic of an antioxidant is its ability to participate in redox reactions, typically by donating a proton to the oxidative milieu. Ketone bodies certainly have the ability to act as Brønsted–Lowry acids (pK a's ~4.7), and taking into account that reduction of acetoacetate to D-3-β-hydroxybutyrate has E°′ = −0.291 V [26], it is tempting to speculate that this species could reduce the α-tocopheryl radical, for example. Indeed, a quick calculation for this system gives ΔE°′ = + 0.209 V, which would be thermodynamically favorable under physiological conditions. Thus, it seems that ketone bodies have the chemical potential to be effective antioxidants. However, the experimental evidence is far from persuasive.
Ketone bodies—antioxidant or prooxidant?
In 1904, Holleman [21] demonstrated that pyruvate (an α-keto acid) could react with hydrogen peroxide to produce water, and thus function as an antioxidant:
Taking this into consideration, it seems likely that ketone bodies could also act as antioxidants, since they share a certain amount of structural homology with pyruvate (ketone bodies are β-keto acids). Accordingly, it is possible that ketone bodies may confer an ‘antioxidant effect’ and thus prove beneficial in pathologies associated with oxidative stress, such as Alzheimer's disease, cardiovascular disease and cancer.
Unfortunately, the experimental evidence for an antioxidant role of ketone bodies is conflicting, and it would seem that a division lies between experiments conducted in vivo and those conducted in vitro. For example, Haces et al. [18] investigated the free radical scavenging properties of acetoacetate and 3-β-hydroxybutyrate against six reactive oxygen species. Their findings demonstrated that acetoacetate was able to quench the activity of singlet oxygen, hypochlorous acid and peroxynitrite, while 3-β-hydroxybutyrate was only able to quench the activity of hydroxyl radicals. When this was extended to a rat model, it was found that 3-β-hydroxybutyrate ameliorated lipid peroxidation in the rat hippocampus, which was attributed to either restoration of the ATP supply, or a direct antioxidant effect. However, since acetoacetate can also supplement ATP production, and this latter compound showed no significant effect on lipid peroxidation, it would seem likely that some other ‘antioxidant’ property of 3-β-hydroxybutyrate was responsible.
It has been shown elsewhere [23] that acetoacetate increases lipid peroxidation in human endothelial cells. In this investigation, Jain et al. demonstrated a dose-responsive relationship between acetoacetate concentration and the formation of superoxide, which was significantly enhanced by iron(II) ions. The authors suggest that iron(II) catalyzes the addition of two protons across both carbonyl groups of acetoacetate, followed by autooxidation, releasing hydrogen peroxide. Although the involvement of a reactive enol intermediate is an appealing idea, such a mechanism would be more likely to lead to the decarboxylation of acetoacetate, producing propanone and carbon dioxide. Taking into account that the carbon dioxide so-produced could then react with perxoynitrite to form (inter alia) nitrocarbonate anions [41], we could speculate that it is the metal-catalyzed formation of carbon dioxide which may actually be a source of oxidative stress (Fig. 5). This may also go some way to explain why 3-β-hydroxybutyrate appears more ‘antioxidant’, while acetoacetate seems more ‘prooxidant’.
Decarboxylation of acetoacetate. Acetoacetate may be stabilized by weak electrostatic interactions with a metal ion, M(II), which promotes decarboxylation independently of Schiff-base formation (as in an enzyme-mediated mechanism). The product would predominately exist as the keto tautomer, propanone. The carbon dioxide formed in the process may react with peroxynitrite, forming a variety of radical species, including the nitrocarbonate anion
A role for ketone bodies in improving mitochondrial function and reducing systemic oxidative stress has been identified [7], and a state of ketosis has also been shown to be favorable in Alzheimer's disease, ostensibly through a similar mechanism [49]. The pathophysiology of Alzheimer's disease involves the formation and deposition of β-amyloid, which may be initiated by an inappropriate inflammatory response. In murine models of the disease, a ketogenic diet rich in saturated fats and low in carbohydrates has been shown to reduce the formation of β-amyloid [42], which may be linked to the increased expression of monocarboxylic acid transporter-1 (MCT-1) [34], which will help maintain a positive energy balance in the brain. Of further interest are the high levels of iron and copper present in the Alzheimer's brain, both capable of undergoing Haber–Weiss chemistry to produce hydroxyl radicals, which may be subsequently involved in oxidative modification of biomolecules [24]. In light of these and similar findings, it would seem prudent to move towards an ‘oxidative hypothesis’ for Alzheimer's disease, in which ketogenic diets may have a role to play.
The antioxidant/prooxidant dichotomy of ketone bodies is most likely due to the vastly complex nature of mammalian metabolism. While chemically a ketone body may have the potential to act as an antioxidant, e.g., by reducing hydroxyl radicals, the metabolism of ketone bodies in vivo may actually result in more free radicals being formed, thus tipping the balance towards a state of oxidative stress. This latter conjecture is consistent with the increased synthesis of glutathioneFootnote 2 observed during ketone body metabolism—a metabolic attempt at maintaining the balance between oxidants and antioxidant defenses. Therefore, although explorations of ketone bodies' chemistry may help us understand the mechanisms involved, it is their physiological role which is ultimately important.
Conclusion
Mammalian metabolism is a complex series of interrelated biochemical pathways, each exerting considerable influence on one another. During reduced glucose availability (or uptake), ketone bodies serve as the primary currency of fat-derived metabolic fuel. Yet despite almost a century of investigation and clinical use, the molecular mechanisms involved in ketogenic diets are still poorly understood. The ancient physicians of the classical world were strong advocates of the ketogenic diet, even though they had no awareness of ketone body metabolism. Among them, the royal physician Erasistratus said that “one inclining to epilepsy should be made to fast without mercy and be put on short rations” [40]. While it would no longer be considered good dietetic practice to make someone “fast without mercy”, the manipulation of the diet to increase ketone body production still has clinical applications in epilepsy, but if we are to fully understand the anticonvulsant effects afforded by these diets, considerable effort must be made to elucidate the underlying mechanisms.
Notes
Decarboxylation is also possible via. the cytosolic enzyme acetoacetate decarboxylase.
Glutathione (γ-glutamylcysteinylglycine) has the largest negative reduction potential found in vivo (E°′ = −1.5 V) making it the most potent naturally occuring antioxidant in biological systems. See Ref. [4] for an excellent treatment of the thermodynamics of reduction by glutathione.
References
Abete I, Astrup A, Martínez JA, Thorsdottir I, Zulet MA (2010) Obesity and the metabolic syndrome: role of different dietary macronutrient distribution patterns and specific nutritional components on weight loss and maintenance. Nutr Rev 68:214–231
Bough KJ, Rho JM (2007) Anticonvulsant mechanisms of the ketogenic diet. Epilepsia 48:43–58
Bravata DM, Sanders L, Huang J, Krumholz HM, Olkin I, Gardner CD (2003) Efficacy and safety of low-carbohydrate diets: a systematic review. JAMA 289:1837–1850
Buettner GR (1993) The pecking order of free radicals and antioxidants: lipid peroxidation, α-tocopherol, and ascorbate. Arch Biochem Biophys 300:535–543
Cahill CF (1976) Starvation in man. Clin Endocrinol Metab 5:398–417
Chen TY, Smith W, Rosenstock JL, Lessnau KD (2006) A life-threatening complication of Atkins diet. Lancet 367:958
Crujeiras AB, Parra D, Goyenechea E, Abete I, González-Muniesa P, Martínez JA (2008) Energy restriction in obese subjects impact differently two mitochondrial function markers. J Physiol Biochem 64:211–219
Dashti HM, Al-Zaid NS, Mathew TC, Al-Mousawi M, Talib H, Asfar SK, Behbahani AI (2006) Long term effects of ketogenic diet in obese subjects with high cholesterol level. Mol Cell Biochem 286:1–9
Denke MA (2001) Metabolic effects of high-protein, low-carbohydrate diets. Am J Cardiol 88:59–61
Devlin TM (1997) Textbook of biochemistry with clinical correlations. Wiley, New York
Eastmond PJ, Graham IA (2001) Re-examining the role of the glyoxylate cycle in oilseeds. Trends Plant Sci 6:72–77
Eaton S, Bartlett K, Pourfarzam M (1996) Mammalian mitochondrial β-oxidation. Biochem J 320:345–357
Erlanson-Albertsson C, Mei J (2005) The effect of low carbohydrate on energy metabolism. Int J Obes (Lond) 29(Suppl 2):S26–S30
Fehm HL, Kern W, Peters A (2006) The selfish brain: competition for energy resources. Prog Brain Res 153:129–140
Fenselau WK (1974) Acetoacetate substrate inhibition of CoA transferase from various rat tissues. Life Sci 15:811–818
Fernandez ML, West KL (2005) Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr 135:2075–2078
Garber J, Menzel PH, Boden G, Owen OE (1974) Hepatic ketogenesis and gluconeogenesis in humans. J Clin Invest 54:981–989
Haces ML, Hernandez-Fonseca K, Medina-Campos ON, Montiel T, Pedraza-Chaverri J, Massieu L (2008) Antioxidant capacity contributes to protection of ketone bodies against oxidative damage induced during hypoglycemic conditions. Exp Neurol 211:85–96
Halliwell B, Gutteridge JMC (1984) Lipid peroxidation, oxygen radicals, cell damage and antioxidant therapy. Lancet I:1396
Hardie DG (1992) Regulation of fatty acid and cholesterol metabolism by the AMP-activated protein kinase. Biochem et Biophys Acta 1123:231–238
Holleman MAF (1904) Notice sur l'action de l'eau oxygénée sur les acides α-cétoniques et sur les dicétones. Rec Trav Chim Pays Bas Belg 23:169–171
Huttenlocher PR, Wilbourn AJ, Signore JM (1971) Medium-chain triglycerides as a therapy for intractable childhood epilepsy. Neurology 21:1097–1103
Jain SK, Kannan K, Lim G (1998) Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic Biol Med 25:1083–1088
Jomova K, Vondrakova D, Lawson M, Valko M (2010) Metals, oxidative stress and neurodegenerative disorders. Mol Cell Biochem 345:91–104
Larsen TM, Dalskov SM, van Baak M, Jebb SA, Papadaki A, Pfeiffer AF, Martinez JA, Handjieva-Darlenska T, Kunešová M, Pihlsgård M, Stender S, Holst C, Saris WH, Astrup A (2010) Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 363:2102–2113
Lemastersg JJ, Grunwald R, Emaus RK (1984) Thermodynamic limits to the ATP/site stoichiometries of oxidative phosphorylation by rat liver mitochondria. JBC 259:3058–3063
Liu YM (2008) Medium-chain triglyceride (MCT) ketogenic therapy. Epilepsia 49:33–36
MacDonald A, Webber J (1995) Feeding, fasting and starvation: factors affecting fuel utilization. Proc Nutr Soc 54:267–274
Martínez JA, Parra MD, Manninen AH, Hilton PJ, McKinnon W (2006) Life-threatening complications of the Atkins diet? Lancet 368:23–24
Masino SA, Geiger JD (2008) Are purines mediators of the anticonvulsant/neuroprotective effects of ketogenic diets? Trends Neurosci 31:273–278
McCall AL (2004) Cerebral glucose metabolism in diabetes mellitus. Eur J Pharmacol 490:147–158
Mogilevskaya E, Demin O, Goryanin I (2006) Kinetic model of mitochondrial Krebs cycle: unraveling the mechanism of salicylate hepatotoxic effects. J Biol Phys 32:245–271
Pan A, Hu FB (2011) Effects of carbohydrates on satiety: differences between liquid and solid food. Curr Opin Clin Nutr Metab Care 14:385–390
Pifferi F, Tremblay S, Croteau E, Fortier M, Tremblay-Mercier J, Lecomte R, Cunnane SC (2011) Mild experimental ketosis increases brain uptake of 11C-acetoacetate and 18F-fluorodeoxyglucose: a dual-tracer PET imaging study in rats. Nutr Neurosci 14:51–58
Porta N, Vallee L, Boutry E, Fontaine M, Dessein AF, Joriot S, Cuisset JM, Cuvellier JC, Auvin S (2009) Comparison of seizure reduction and serum fatty acid levels after receiving the ketogenic and modified Atkins diet. Seizure 18:359–364
Rutter GA (2000) Diabetes: the importance of the liver. Curr Biol 10:R736–R738
Serra D, Casals N, Asins G, Royo T, Ciudad CJ, Hegardt FG (1993) Regulation of mitochondrial 3-hydroxy-3-methylglutarylcoenzyme A synthase protein by starvation, fat feeding and diabetes. Arch Biochem Biophys 307:40–45
Simpson IA, Carruthers A, Vannucci SJ (2007) Supply and demand in cerebral energy metabolism: the role of nutrient transporters. J Cereb Blood Flow Metab 27:1766–1791
Sumithran P, Proietto J (2008) Ketogenic diets for weight loss: a review of their principles, safety and efficacy. Obes Res Clin Pract 2:1–13
Temkin O (1971) Falling sickness: history of epilepsy from the Greeks to the beginnings of modern neurology, 2nd edn. Johns Hopkins, USA
Uppu RM, Pryor WA (1996) Carbon dioxide catalysis of the reaction of peroxynitrite with ethyl acetoacetate: an example of aliphatic nitration by peroxynitrite. Biochem Biophys Res Comm 229:764–769
Van der Auwera I, Wera S, Van Leuven F, Henderson ST (2005) A ketogenic diet reduces amyloid beta 40 and 42 in a mouse model of Alzheimer's disease. Nutr Metab (Lond) 17:2–28
Volek JS, Sharman MJ (2004) Cardiovascular and hormonal aspects of very-low-carbohydrate ketogenic diets. Obes Res 12(Suppl 2):115S–123S
Wagenmakers AJ (1998) Protein and amino acid metabolism in human muscle. Adv Exp Med Biol 441:307–319
Wang S, Soni KG, Semache M, Casavant S, Fortier M, Pan L, Mitchell AG (2008) Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab 95:117–126
Wasserman DH (2009) Four grams of glucose. Am J Physiol Endocrinol Metab 296:E11–E21
Watt MJ, Steinberg GR (2008) Regulation and function of triacylglycerol lipases in cellular metabolism. Biochem J 414:313–325
White AM, Johnston CS, Swan PD, Tjonn SL, Sears B (2007) Blood ketones are directly related to fatigue and perceived effort during exercise in overweight adults adhering to low-carbohydrate diets for weight loss: a pilot study. J Am Diet Assoc 107:1792–1796
Yao J, Chen S, Mao Z, Cadenas E, Brinton RD (2011) 2-Deoxy-d-glucose treatment induces ketogenesis, sustains mitochondrial function, and reduces pathology in female mouse model of Alzheimer's disease. PLoS One 6:e21788
Young C, Gean PW, Chiou LC, Shen YZ (2000) Docosahexaenoic acid inhibits synaptic transmission and epileptiform activity in the rat hippocampus. Synapse 37:90–94
Young IS, Woodside JV (2001) Antioxidants in health and disease. J Clin Pathol 54:176–186
Yudkoff M, Daikhin Y, Horyn O, Lazarow A, Luhovyy B, Wehrli S, Nissim I (2005) Response of brain amino acid metabolism to ketosis. Neurochem Int 47:119–128
Yudkoff M, Daikhin Y, Nissim I, Grunstein R (1997) Effects of ketone bodies on astrocyte amino acid metabolism. J Neurochem 69:685–692
Zammi V (1994) Regulation of ketone body metabolism. Diabetes Reviews 132–155
Acknowledgements
This work was partially supported by the Northern Ireland R&D Office (Extension to RRG 5.42). The authors would like to thank Dr DJ Timson, School of Biological Sciences, Queen's University Belfast, for useful discussions of mammalian metabolism.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
McPherson, P.A.C., McEneny, J. The biochemistry of ketogenesis and its role in weight management, neurological disease and oxidative stress. J Physiol Biochem 68, 141–151 (2012). https://doi.org/10.1007/s13105-011-0112-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-011-0112-4