Abstract
During prolonged maximal exercise, oxygen deficits occur in working muscles. Progressive hypoxia results in the impairment of the oxidative resynthesis of ATP and increased degradation of purine nucleotides. Moreover, ATP consumption decreases the conversion of UDP to UTP, to use ATP as a phosphate donor, resulting in an increased concentration of UDP, which enhances pyrimidine degradation. Because the metabolism of pyrimidine nucleotides is related to the metabolism of purines, in particular with the cellular concentration of ATP, we decided to investigate the impact of a standardized exercise with increasing intensity on the concentration of uridine, inosine, hypoxanthine, and uric acid. Twenty-two healthy male subjects volunteered to participate in this study. Blood concentrations of metabolites were determined at rest, immediately after exercise, and after 30 min of recovery using high-performance liquid chromatography. We also studied the relationship between the levels of uridine and indicators of myogenic purine degradation. The results showed that exercise with increasing intensity leads to increased concentrations of inosine, hypoxanthine, uric acid, and uridine. We found positive correlations between blood uridine levels and indicators of myogenic purine degradation (hypoxanthine), suggesting that the blood uridine level is related to purine metabolism in skeletal muscles.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Uridine is an important nucleoside precursor in the pyrimidine nucleotide salvage pathway (Urd → UMP → UDP → UTP). Except for erythrocytes, the liver, and kidney, which maintain de novo pyrimidine biosynthesis and supply other tissues with uridine for salvage, most normal tissues in adults rely on the salvage of uridine from plasma [22].
Yamamoto et al. [26, 27] observed increased concentrations of uridine in plasma after ingestion of ethanol, fructose, or xylitol infusion. Metabolism of these compounds in the liver contributes to increased degradation of ATP, the donor of phosphate in the reaction catalyzed by nucleoside diphosphate kinases. Therefore, a decrease in ATP concentrations leads to a decrease in the concentration of UTP with a simultaneous increase in UDP and UMP. The decrease in ATP and increases in UDP and UMP enhanced pyrimidine degradation, which resulted in increased concentrations of uridine in plasma.
During maximal exercise, oxygen deficits occur in working muscles. Progressive hypoxia results in the impairment of oxidative resynthesis of ATP and the increased degradation of purine nucleotides. It has been demonstrated that the concentration of ATP in the muscle after high-intensity physical effort is reduced by about 17% compared to resting values, and a 9% decrease in ATP concentrations has been observed even 90 min after exercise [10].
Pyrimidine nucleotide metabolism is linked to the metabolism of purines, in particular the cellular concentration of ATP. Uridine 5′-monophosphate kinase catalyzes the reversible transfer of the γ phosphoryl group from ATP to UMP. The resulting UDP is further phosphorylated by nucleoside diphosphate kinase to UTP. If during intense physical effort degradation of purines coexists with increased degradation of pyrimidines, one could expect an increased concentration of uridine in blood, a product of catabolism of pyrimidine nucleotides. We also know that, during vigorous exercise, blood flow in visceral circulation is reduced. Experimental studies performed in recent years have established that ischemia induces the release of purine and pyrimidine catabolites [17, 18].
So far, there have been no reports on the influence of physical exercise at maximum intensity on the concentration of uridine in the blood after exercise and during recovery. At this point, it is worth quoting Yamamoto et al. [24] who in a study on a group of five persons showed increased concentrations of uridine in arterial and venous blood (collected from the femoral vein and radial artery) immediately after intense exercise. The results presented by Yamamoto et al. [24] apply only to posteffort changes in concentrations of tested metabolites (uridine, hypoxanthine, xanthine, uric acid). In this study, we also determined the changes in blood concentrations of uridine 30 min after recovery.
Hypoxanthine is regarded as an indicator of tissue hypoxia [2, 20, 21]. It is also considered a marker of adenine nucleotide breakdown in muscles and a marker of energy stress which accompanies high-intensity physical efforts [15]. If hypoxanthine is treated as an indicator of ATP degradation in skeletal muscle contraction, then uridine could be considered an indicator of UTP degradation.
Therefore, the purpose of this study was to investigate the effect of muscular exercise on the concentration of uridine, inosine, hypoxanthine, and uric acid. Concentrations of the metabolites were determined in blood immediately after exercise and during recovery. We studied the relationship between the levels of uridine and indicators of myogenic purine degradation.
Methods
Subjects
Twenty-two healthy male subjects volunteered to participate in his study. Their age, height, weight, and peak oxygen consumption were 21.9 ± 2.33 years, 179.8 ± 8.33 cm, 75.6 ± 7.89 kg, and 45.8 ± 4.11 ml kg−1 min−1, respectively.
The subjects were fully informed of any risk and discomfort associated with the experimental procedures before giving their consent to participate. The study was approved by the local ethics committee.
Exercise protocol
The participants were subjected to a single effort test with a progressively increasing intensity (until refusal) on a bicycle cycloergometer (Kettler X-7, Germany). The test was preceded by a 5-min warm-up on the cycloergometer (25 W). The tested individuals began the test at 70-W maintaining 70 rpm. The effort was continued with an increasing load (+20 W every 3 min) until refusal, or until the participant was not able to maintain the required frequency of rotation. In the final 15 s of each 3-min period at a given load, arterialized blood was collected to assess the lactic acid concentration. Other determinations during the increasing load were used to assess individual lactic thresholds. Lactate concentration was determined using a Dr Lange Lp-20 analytical kit (Lange, Germany). A Polar S610 heart rate monitor (Polar, Finland) was used to record heart rate throughout the exercise. An Oxycon gas analyzer (Jaeger, Germany) was used to assess oxygen uptake during the exercise. Table 1 shows the characteristics of the studied anthropometric and physiological parameters.
Venous blood analysis
Tests were performed on venous blood taken from the ulnar vein at the following intervals: immediately before the exercise test (rest), immediately after (postexercise), and 30 min after exercise (recovery). Using high-performance liquid chromatography, nucleoside (inosine (Ino), uridine (Urd)) and oxypurine (hypoxanthine (Hyp), uric acid (UA)) concentrations in whole blood were determined.
The samples (500 μL) of heparinized blood were deproteinized with equal volumes of 1.3 M HClO4, mixed, and then centrifuged at 20,000×g for 5 min at 4°C. The supernatant (400 μL) was neutralized with 130–160 μL of 1 M K3PO4 (to pH 5–7). The neutralized extract was again centrifuged and filtered through a 0.22-μm nylon filter. The clear filtrate was then used for HPLC assay or stored at −80°C until analysis.
Chromatographic analysis was performed using a Hewlett-Packard series 1050 chromatograph according to Smolenski et al. [16]. The concentrations of nucleosides and oxypurines are expressed as micromoles per liter of whole blood.
Comments to methods
Hellsten-Westing et al. [10] showed that the distribution of Hyp in plasma versus erythrocytes was similar ranging from 2 to 60 μmol/L. The curve equation was y = 1.003x − 1.203, and the correlation between the concentration of Hyp in plasma versus erythrocytes was r 2 = 0.898 (P < 0.001). These results suggest that whole blood values of Hyp can be used to represent plasma values.
Statistical analysis
All values are reported as mean ± SE. ANOVA with repeated measurements was employed to compare data over time. When ANOVA was significant, (RIR) Tukey’s post hoc tests were used to localize the difference. The level of probability to reject a null hypothesis was set at P < 0.05. In order to demonstrate whether the observed correlations were statistically significant, a Spearman's rank correlation coefficient was used.
Results
In the examined individuals, the average duration of the exercise test was 24.0 ± 5.97 min (minimum 15.0 min, maximum 31.0 min.), the pulse rate recorded before the exercise was 72.1 ± 8.03 bpm, and its maximum value was 195.6 ± 6.60 bpm. The maximum power value was 218 ± 40.77 W while the anaerobic threshold was determined at 134.5 ± 26.39 W. The oxygen consumption at maximum load was 45.8 ± 4.11 ml/min/kg (minimum 38.70 ml/min/kg, maximum 60.20 ml/min/kg), which indicates that the subjects had a good aerobic endurance for this age group compared to reference data (Astrand and Rodhal [1]; Table 1).
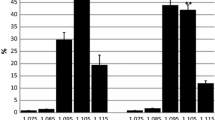
Figure 1a shows that concentrations of inosine measured in the blood immediately after the exercise reached a value of 0.5 ± 0.26 μmol/L and did not differ significantly from the value measured before the exercise (0.1 ± 0.04 μmol/L); however, inosine concentration was significantly higher 30 min after exercise, both in comparison with the resting (P < 0.001) and postexercise values (P < 0.001).
a Blood Ino concentration at rest, postexercise, and after 30 min of recovery. Values are means ± SE, n = 22. #P < 0.01, significantly different from postexercise. ***P < 0.0001, significantly different from rest. b Blood Hyp concentration at rest, postexercise, and 30 min of recovery. Values are means ± SE, n = 22. ***P < 0.0001, significantly different from rest. c Blood UA concentration at rest, postexercise, and after 30 min of recovery. Values are means ± SE, n = 22. ##P < 0.001, significantly different from postexercise. **P < 0.001, significantly different from rest. d Blood Urd concentration at rest, postexercise, and after 30 min of recovery. Values are means ± SE, n = 22. ***P < 0.0001, significantly different from rest
Physical exercise contributed to a nearly 8-fold increase in hypoxanthine concentration in the blood in relation to resting levels (from 2.2 ± 1.61 to 16.3 ± 7.86 μmol/L; P < 0.0001). High concentrations of hypoxanthine were observed even 30 min after exercise (18.5 ± 8.78 μmol/L). This increase was still significant in relation to resting values (P < 0.0001) but not to postexercise ones (Fig. 1b).
Figure 1c shows the concentration of uric acid. A comparison of the data reveals that the concentration of uric acid measured 30 min after the physical effort reached 245.5 ± 34.24 μmol/L and was significantly higher both in relation to resting values (202.8 ± 36.17 μmol/L; P < 0,001) and to those observed immediately after exercise (188.0 ± 39.60 μmol/L; P < 0.0001). There were no significant differences in uric acid concentration immediately after exercise compared to resting values.
Uridine concentration immediately after the exercise reached 3.9 ± 0.60 μmol/L and was significantly higher (P < 0.002) compared to resting values (3.0 ± 0.82 μmol/L). This concentration was maintained for 30 min after the exercise without any change from its postexercise value (3.9 ± 0.76 μmol/L; Fig. 1d). Although the concentration of uridine immediately after the exercise increased only 1.5 times compared with the 8-fold increase in hypoxanthine concentration, both uridine and hypoxanthine (coming from the conversions of pyrimidines and purines) significantly increased and sustained this level after the effort.
Blood uridine levels positively correlated with blood hypoxanthine levels (Fig. 2) but not with uric acid levels.
Discussion
Uridine is a pyrimidine nucleoside that forms part of RNA, which is not only necessary for the endogenous synthesis of nucleic acids but also plays an important role in the synthesis of glycogen via UDP-glucose. Uridine also takes part in the regulation of several physiological and pathological processes. In the absence of sugars, it can serve as a crucial precursor for both carbohydrate metabolism and nucleic acid synthesis [22, 23]. In the nervous system, uridine is a physiological regulator of the sleep function and exhibits the ability to maintain brain metabolism during ischemia and severe hypoglycemia [3]. Uridine is also used in the clinical treatment of autism with seizures [13] and pyrimidine-deficient genetic diseases such as orotic aciduria [11]. Moreover, uridine levels in plasma and other tissues are also crucial for the success of anticancer therapies based on pyrimidine antimetabolites. A hypotensive effect of uridine is also known [28].
Available literature lacks data on the effect of exercise on the metabolism of pyrimidine nucleotides, so we decided to examine the influence of standardized exercise on the concentration of uridine and purine degradation products.
Intensive efforts contribute to the reduction of oxygen concentration in relation to demand, leading to bioenergy hypoxia, increased anaerobic glycolysis, and ATP degradation accompanied by the accumulation of IMP [8, 19]. Most of the IMP is extremely quickly resynthesized to ATP during recovery, although part of the IMP is dephosphorylated and results in the production of purine bases, inosine, and hypoxanthine [19]. Purine bases, not recovered via intramuscular purine salvage, efflux the muscle and are deposited in plasma, mainly as hypoxanthine [2, 7, 29].
A postexercise increase in the concentration of inosine and hypoxanthine in the plasma has already been observed by many researchers [2, 8, 9, 21, 29]. The degree of loss of muscle nucleotides not only depends on exercise intensity, duration, and recovery interval but also on the number of exercise repetitions.
Many studies have demonstrated that inosine and hypoxanthine are produced in muscles during moderate to intense exercise, and a large individual variation of changes exist in plasma concentrations of hypoxanthine from intense exercise [2, 5, 14]. According to Bangsbo et al. [2] and Hellsten-Westing et al. [9], the serum levels of hypoxanthine increased to five times the resting levels after arm cranking and more than ten times after maximum short distance running. Hellsten-Westing et al. [6] and Sahlin et al. [14] showed that maximum hypoxanthine increase occurs 20 min after the end of the physical effort.
Hellsten-Westing et al. [8] showed that after a 100-s intense exercise, femoral venous inosine, and hypoxanthine concentrations increased, and significant net release of inosine and hypoxanthine was observed after 70 s. In recovery, venous inosine and hypoxanthine further increased, and the release of inosine and hypoxanthine peaked after 12–20 min and was still significant after 90 min of recovery.
In this study, a significant increase in hypoxanthine concentration in the blood immediately after exercise, with no significant changes in the concentration of inosine, shows the existence of differences in the rate of entry of these metabolites into the plasma. Zhao et al. [29] in studies on muscle adenine nucleotide metabolism during and in recovery from maximal exercise in humans showed a significant increase in the concentration of inosine and hypoxanthine in plasma, respectively, at 5 and 10 min of recovery, while Hellsten-Westing et al. [10] showed multiple significant increases in plasma concentrations of hypoxanthine immediately after high-intensity intermittent exercise. These differences may arise, as we have mentioned earlier, for example, from the different plot of an exercise or its duration.
The significant increase in the concentration of extracellular markers of muscle adenine nucleotide catabolism (inosine and hypoxanthine) indicates the occurrence of the severe energy stress that took place during the exercise. The observed multiple increases in the concentration of hypoxanthine immediately after exercise and 30 min of recovery point to a significant increase in ATP degradation. The results show that hypoxanthine was already being released from working muscles during exercise and also 30 min after its completion.
The test with increasing intensity until refusal also contributed to a significant increase in the concentration of uridine in the blood immediately after exercise. The results of our study confirm the results obtained by Ohno et al. [12] and Yamamoto et al. [24]. Ohno et al. [12] observed an increase in the concentration of purine bases and uridine in plasma after physical exercise. Yamamoto et al. [24] showed that muscular exercise increased plasma concentrations of uridine in blood samples from the femoral vein and the radial artery from 3.96 ± 0.78 to 5.04 ± 0.72 μmol/L (P < 0.01) and from 3.51 ± 0.59 to 5.12 ± 0.52 μmol/L (P < 0.05), respectively. We have shown that the concentration of uridine in the blood is still significantly higher as late as 30 min after the exercise. Thus, uridine, similarly to hypoxanthine, is released from working muscles during exercise and up to 30 min after its completion. Since uridine is a product of the degradation of pyrimidine nucleotides, our results suggest that physical activity not only contributes to the degradation of purines but also pyrimidines. It seems that the intensity of exercise and its duration has an impact on the degree of degradation of purines and also of pyrimidines. It has been demonstrated that a 2-min-long intensive exercise leads to increased concentrations of hypoxanthine but not uridine in plasma, while the approximately 20-min-long exercise in this study was sufficient to increase both the concentrations of hypoxanthine and uridine.
Yamamoto et al. [26], in their study on the effect of ethanol and fructose on purine and pyrimidine metabolism, showed an increase in the concentration of purine bases and uridine in plasma. Both ethanol and fructose increased the plasma concentration of uridine together with an increase in the plasma concentration of oxypurines, whereas fructose also increased the plasma concentration of uric acid, while ethanol did not. In ethanol ingestion and fructose infusion, an increase in the plasma concentration of purine bases correlated with that of uridine. These results strongly suggest that an increase in the plasma concentration of uridine is ascribable to increased pyrimidine degradation following purine degradation increased by ethanol and fructose.
A positive correlation between the concentration of uridine and hypoxanthine in blood was also shown by Hamada et al. [4], who suggested that plasma uridine levels in hypertensive patients reflects purine degradation and insulin resistance in skeletal muscles.
In this study, we found positive correlations between blood uridine levels and indicators of myogenic purine degradation (hypoxanthine), suggesting that the blood uridine level is related to purine metabolism in skeletal muscle.
The elevations in plasma hypoxanthine after intense exercise have a direct implication on plasma uric acid levels, as the liver oxidizes hypoxanthine to uric acid and releases it into the bloodstream at a rate proportional to the increase in plasma hypoxanthine [10]. Based on the results of Yamamoto et al. [25] which showed that the plasma concentration of uridine could be a marker of uric acid production in the body and that it could easily be used to divide patients with gout into underexcretion and overexcretion types, we decided to examine the relationship between the concentrations of uridine and uric acid. We found no significant relationship between these parameters, which indicates that the level of uridine is not an indicator of hyperuricemia which accompanies peak exercises.
In summary, it can be concluded that the standardized physical exercise with increasing intensity leads to increased levels of both indicators of muscle degradation of purines and uridine. Therefore, this study suggests that the abrupt loss of ATP causes the degradation of pyrimidines. The processes of adenine nucleotide catabolism coexist with the catabolism of pyrimidine nucleotides and are interdependent.
References
Astrand PO, Rodhal K (1986) Textbook of work physiology: physiological bases of exercise. McGraw-Hill, New York
Bangsbo J, Sjödin B, Hellsten-Westing Y (1992) Exchange of hypoxanthine in muscle during intense exercise in man. Acta Physiol Scand 146:528–533
Cansev M (2006) Uridine and cytidine in the brain: their transport and utilization. Brain Res Rev 52:389–397
Hamada T, Mizuta E, Yanagihara K, Kaestu Y, Sugihara S, Sonoyama K, Yamamoto Y, Kato M, Igawa O, Shigemasa Ch, Inokuchi T, Yamamoto T, Shimada T, Ohtahara A, Ninomiya H, Hisatome I (2007) Plasma levels of uridine correlate with blood pressure and indicators of myogenic purine degradation and insulin resistance in hypertensive patients. Circ J 71:354–356
Hellsten Y, Sjödin B, Richter A, Bangsbo J (1998) Urate uptake and lowered ATP levels in human muscle after high-intensity intermittent exercise. Am J Physiol Endocrinol Metab 274:600–606
Hellsten Y, Richter EA, Kiens B, Bangsbo J (1999) AMP deamination and purine exchange in human skeletal muscle during and after intense exercise. J Physiol 520:909–920
Hellsten-Westing Y, Ekblom B, Sjödin B (1989) The metabolic relation between hypoxanthine and uric acid in man following maximal short-distance running. Acta Physiol Scand 137:341–345
Hellsten-Westing Y, Balsom PD, Norman B, Sjödin B (1993) Changes in plasma hypoxanthine and free radical markers during exercise in man. Acta Physiol Scand 149:405–412
Hellsten-Westing Y, Norman B, Balsom PD, Sjödin B (1993) Decreased resting levels of adenine nucleotides in human skeletal muscle after high intensity training. J Appl Physiol 74:2523–2528
Hellsten-Westing Y, Ekblom B, Kajser I, Sjödin B (1994) Exchange of purines in human liver and skeletal muscle with short-term exhaustive exercise. Am J Physiol 266:81–86
Nyhan WL (2005) Disorders of purine and pyrimidine metabolism. Mol Genet Metab 86:25–33
Ohno M, Ka T, Inokuchi T, Moriwaki Y, Yamamoto A, Takahashi S, Tsutsumi Z, Tsuzita J, Yamamoto T, Nishiguchi S (2008) Effects of exercise and grape juice ingestion in combination on plasma concentrations of purine bases and uridine. Clin Chim Acta 388:167–172
Page T, Yu A, Fontanesi J, Nyhan WL (1997) Developmental disorder associated with increased cellular nucleotidase activity. Proc Natl Acad Sci USA 94(21):11601–11606
Sahlin K, Ekberg K, Cizinsky S (1991) Changes in plasma hypoxanthine and free radical markers during exercise in man. Acta Physiol Scand 142:275–281
Sahlin K, Tonkonogi M, Söderlund K (1999) Plasma hypoxanthine and ammonia in humans during prolonged exercise. Eur J Appl Physiol 80:417–422
Smolenski RT, Lachno DR, Ledingham SJM, Yacoub MH (1990) Determination of sixteen nucleotides, nucleosides and bases using high-performance liquid chromatography and its application to the study of purine metabolism in heart for transplantation. J Chromatogr 527:414–420
Smolenski RT, Lachno DR, Yacoub MH (1992) Adenine nucleotide catabolism in human myocardium during heart and heart–lung transplantation. Eur J Cardiothorac Surg 6:25–30
Smoleński RT, de Jong JW, Janssen M, Lachno DR, Zydowo MM, Tavenier M, Huizer T, Yacoub MH (1993) Formation and breakdown of uridine in ischemic hearts of rats and humans. J Mol Cell Cardiol 25:67–74
Stathis CG, Febbraio MA, Carey MF, Snow RJ (1994) Influence of sprint training on human skeletal muscle purine nucleotide metabolism. J Appl Physiol 76:1802–1809
Stathis CG, Zhao S, Carey MF, Snow RJ (1999) Purine loss after repeated sprint bouts in humans. J Appl Physiol 87:2037–2042
Sutton JR, Toews CJ, Ward R (1980) Purine metabolism during strenuous exercise in man. Metabolism 29:254–260
Traut TW, Jones ME (1996) Uracil metabolism—UMP synthesis from orotic acid or uridine and conversion of uracil to β-alanine: enzymes and cDNAs. Prog Nucleic Acid Res Mol Biol 53:1–78
Wice BM, Kennell D (1982) Ribose-1-P is the essential precursor for nucleic acid synthesisin animal cells growing on uridine in the absence of sugar. J Biol Chem 257(5):2578–2583
Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Yamakita J, Higashino K (1997) Effect of muscular exercise on the concentration of uridine and purine bases in plasma—adenosine triphosphate consumption-induced pyrimidine degradation. Metabolism 46:1339–1342
Yamamoto T, Moriwaki Y, Takahashi S, Tsutsumi Z, Yamakita J, Higashino K (1997) Is the plasma uridine level a marker of the overproduction of uric acid. Metabolism 46:801–804
Yamamoto T, Moriwaki Y, Takahashi S, Yamakita J, Tsutsumi Z, Ohata H, Hiroishi K, Nakano T, Higashino K (1997) Effect of ethanol and fructose on plasma uridine and purine bases. Metabolism 46:544–547
Yamamoto T, Moriwaki Y, Takahashi S, Yamakita J, Nakano T, Hiroishi K, Higashino K (1998) Xylitol-induced increase in the plasma concentration and urinary excretion of uridine and purine bases. Metabolism 47:739–743
Yilmaz MS, Coskun C, Suzer O, Yalcin M, Mutlu D, Savci V (2008) Hypotensive effects of intravenously administered uridine and cytidine in conscious rats: involvement of adenosine receptors. Eur J Pharmacol 584:125–136
Zhao S, Snow RJ, Stathis G, Febbraio MA, Carey MF (2000) Muscle adenine nucleotide metabolism during and recovery from maximal exercise in humans. J Appl Physiol 88:1513–1519
Acknowledgments
The study was supported by grant no. N404 281337 from the State Committee for Scientific Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dudzinska, W., Lubkowska, A., Dolegowska, B. et al. Blood uridine concentration may be an indicator of the degradation of pyrimidine nucleotides during physical exercise with increasing intensity. J Physiol Biochem 66, 189–196 (2010). https://doi.org/10.1007/s13105-010-0023-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13105-010-0023-9