Abstract
The antifibrinolytic enzyme carboxypeptidase U (CPU, TAFIa, CPB2) is an appealing target for the treatment of acute ischemic stroke (AIS). Increased insights in CPU activation and inactivation during thrombolysis (rtPA) with or without endovascular thrombectomy (EVT) are required to develop CPU inhibitors as profibrinolytic agents with optimal benefits/risks. Therefore, CPU kinetics during ischemic stroke treatment were evaluated. AIS patients with documented cerebral artery occlusion receiving rtPA (N = 20) or rtPA + EVT (N = 16) were included. CPU activation during thrombolysis was measured by an ultrasensitive HPLC-based CPU activity method and by an ELISA measuring both CPU and inactivated CPU (CPU + CPUi). Intravenous blood samples were collected at admission and throughout the first 24 h. Additional in situ blood samples were collected in the rtPA + EVT cohort proximal from the thrombus. The NIHSS score was determined at baseline and 24 h. CPU activity and CPU + CPUi levels increased upon rtPA administration and reached peak values at the end of thrombolysis (1 h). High inter-individual variability was observed in both groups. CPU activity decreased rapidly within 3 h, while CPU + CPUi levels were still elevated at 7 h. CPU activity or CPU + CPUi levels were similar in in situ and peripheral samples. No correlation between CPU or CPU + CPUi and NIHSS or thrombus localization was found. The CPU system was rapidly activated and deactivated following thrombolysis and thrombectomy in stroke patients, suggesting that a CPU inhibitor would have to be administered during rtPA infusion and over the next few hours. The high CPU generation variability suggests that some patients may not respond to the treatment. EudraCT number 2017-002760-41.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ischemic stroke is the first cause of disability in the Western world and the second cause of death [1]. Rapid blood flow restoration is crucial to limit adverse outcomes [2]. To date, the only approved pharmacological recanalization treatment is the recombinant tissue-type plasminogen activator (rtPA) [3]. However, recanalization by rtPA is achieved in less than 50% of patients with large artery occlusions [4,5,6]. Although the breakthrough of endovascular thrombectomy (EVT) drastically improved recanalization rates in patients with large vessel occlusion [7], still 2 out of 3 patients treated with EVT display limited microvascular reperfusion even though recanalization is obtained [8]. Besides, only specialized stroke centers are capable of performing the EVT procedure. Thus, more efficacious pharmacological agents are needed.
Procarboxypeptidase U (proCPU), also denoted thrombin-activatable fibrinolysis inhibitor (TAFI), is a proenzyme synthesized by the liver that, after activation into CPU (TAFIa) by the key enzymes of coagulation (thrombin) and fibrinolysis (plasmin) is a potent attenuator of fibrinolysis. CPU is unstable at 37 °C (half-life 7–15 min) and is rapidly inactivated into CPUi (TAFIai) [9,10,11].
Several studies confirmed that CPU is activated in ischemic stroke patients treated with intravenous thrombolysis and suggest that it can decrease the efficacy of thrombolytic therapy [12,13,14,15,16]. The development of CPU (TAFIa) inhibitors as profibrinolytic agents in combination with rtPA is, therefore, an attractive concept [10]. However, profibrinolytic therapy may lead to an increased bleeding risk. So far, CPU inhibition has not been associated with major bleeding complications but has not yet been tested in the clinical setting in combination with rtPA [17,18,19,20,21]. When determining the dosage and timeframe of the administration of an inhibitor, it is crucial to take into account the kinetics and the amplitude of CPU activation and inactivation (CPUi) during fibrinolysis.
While many studies described the mechanism of action of CPU in vivo, little is known about the exact kinetics of activation and inactivation of the enzyme in stroke patients. As its instability makes direct measurement challenging [22, 23], in most studies on stroke patients, proCPU or the released activation peptide were measured rather than CPU [14, 15, 24,25,26]. Only a few clinical studies evaluated the kinetics of CPU activation-inactivation in stroke patients receiving rtPA thrombolysis. One measured enzymatic CPU activity in only 4 patients [26]. The other measured CPU + CPUi antigen levels by ELISA [15]. Contrary to the CPU activity assay, the ELISA does not discriminate CPU from its inactivated form CPUi. It is thus difficult to interpret the amount and kinetics of CPU generation solely based on CPU + CPUi measurement as the in vivo half-life of CPUi is unknown. Therefore, the level and duration of CPU activation assessed in some studies using CPU + CPUi ELISA may have been overestimated.
Furthermore, the potential impact of thrombectomy on CPU generation is largely unknown as it has not been evaluated in previous studies. CPU generation may decrease after clot removal or be sustained when the clot retriever is passed several times through the clot.
The primary objective of our observational study was to evaluate both plasma CPU and CPU + CPUi kinetics in patients treated with thrombolysis (rtPA cohort) without or with additional EVT (rtPA + EVT cohort) and to assess the inter-individual distribution of CPU and CPU + CPUi levels. We also compared differences in CPU kinetics according to the occlusion site, the stroke severity, and several hemostasis parameters. In the rtPA + EVT cohort, CPU and CPU + CPUi levels in the peripheral circulation were compared with in situ peri-thrombus levels.
Methods
Compliance with Ethical Standards
Ethical approval was obtained from the respective ethics committees of the investigational sites and the study was approved by the competent authorities. It was registered at https://eudract.ema.europa.eu (2017–002760-41). All patients received standard-of-care treatment according to current clinical guidelines [27]. Clinical management was not influenced by the study design. All patients or their legal representatives gave their informed consent before participating in the trial. An abbreviated informed consent was obtained before inclusion to not delay patient care. The complete informed consent was obtained from the patient and/or an authorized representative within 12 h (see supplementary material).
Study Design
Adult patients hospitalized in the study centers (supplementary material) within 4.5 h after acute ischemic stroke (AIS) symptom onset and with a confirmed cerebral artery occlusion in the anterior circulation (CT or MRI) were screened. Their eligibility for either a thrombolytic treatment (IV rtPA, 0.9 mg/kg) alone (rtPA cohort) or followed by EVT (rtPA + EVT cohort) was assessed according to the locally applicable clinical guidelines and standard-of-care [27]. Pregnant patients or patients with any known serious disease (including active malignancy and active infection) likely to interfere with the conduct of the study were not included (full inclusion/exclusion criteria are enlisted in the supplementary material).
The study design is summarized in Fig. 1. Samples to evaluate CPU and hemostasis parameters were collected at baseline and 6 (rtPA cohort) or 7 (rtPA + EVT cohort) additional time points over 24 h. An additional blood sample was collected in the rtPA + EVT cohort through the stent retriever catheter, close to the thrombus, just before the first pass. Clinical evaluations including NIHSS scores (detailed in supplementary material) were performed throughout the time-course of the study.
Study design—investigation schedule. Patients were included in the IV-rtPA group or in the group with an additional endovascular thrombectomy (rtPA + EVT). At baseline, blood samples for CPU (+ CPUi) and hemostasis parameters were collected together with the determination of NIHSS. Additional sampling for CPU (+ CPUi) is indicated with green checkmarks. In the rtPA + EVT arm, a peripheral venous sample and an in situ arterial sample near the clot site were collected at the start of EVT. Additional sampling for hemostasis parameters is indicated with a blue H; NIHSS determination with a black N. CPU, carboxypeptidase U; ER, emergency room; NIHSS, National Institute of Health Stroke Scale; IV, intravenous; rtPA, recombinant tissue plasminogen activator; one patient from IV-rtPA cohort was excluded from the analysis (see consort diagram Fig. 2)
Laboratory Analyses
All CPU and CPUi measurements were performed in the Laboratory of Medical Biochemistry (University of Antwerp, Belgium). CPU activity was determined with high-performance liquid chromatography (HPLC)-based method using the substrate Bz-o-cyano-Phe-Arg as described in the supplementary material [22, 23]. CPU + CPUi antigen levels were determined by the Asserachrom® TAFIa/ai ELISA.
Hemostasis parameters (plasmin-α2-antiplasmin); Glu-plasminogen, fibrinogen, and D-dimer) were assessed by FIRALIS (Huningue, France) as detailed in the supplementary material.
Influence of Hemolysis
Forty percent of the study samples were visually hemolyzed. High oxyhemoglobin (oxyHb) concentrations interfere with CPU generation leading to an underestimation of the measurements [28]. However, when oxyHb is ≤ 0.6 g/L, the underestimation of CPU activity does not exceed 20%. Besides, CPU + CPUi ELISA is not significantly influenced for oxyHb ≤ 10.3 g/L. OxyHb levels were dosed in all samples and these thresholds were used to select samples for reliable CPU or CPU + CPUi measurements.
Statistics
Statistical analyses and data plotting were performed by Graphpad Prism® version 8.4.1 (GraphPad Software, CA, USA). For the assessment of CPU-related differences between patients with mild to moderate and severe neurological severity scores, data were dichotomized based on the NIHSS score as follows: NIHSS ≤ 13 (mild to moderate neurological deficit) or NIHSS > 13 (severe neurological deficit). Similarly, for the assessment of differences based on the occlusion site, data were dichotomized based on the occurrence of proximal (internal carotid artery (ICA), 1st segment of the middle cerebral artery (M1), or ICA + M1) or distal (pericallosal artery, 2nd or 3rd segment of the middle cerebral artery (M2, M3)) occlusions. CPU activities and CPU + CPUi antigen levels were compared using non-parametric tests between the two treatment cohorts (rtPA vs. rtPA + EVT). In the rtPA + EVT cohort, CPU activity and CPU + CPUi antigen levels in peripherally collected intravenous samples and in situ collected intra-arterial samples were compared by a Wilcoxon signed-rank test. Results with P < 0.05 were considered statistically significant. When similar time points were compared between groups, a Bonferroni correction was applied (P < 0.007).
Sample Size Calculations
No formal calculation of sample size was performed as this study was exploratory. Based on previous data observed in patients with acute ischemic myocardial infarction [29] and acute ischemic stroke [15], 20 patients per cohort were deemed sufficient to study CPU kinetics, the primary objective of the study.
RESULTS
Baseline Characteristics
Twenty patients were included in the rtPA cohort and 17 in the rtPA + EVT cohort (Fig. 2). One patient (rtPA + EVT) did not receive the planned dose of rtPA and was excluded from further analysis. The baseline characteristics of the patients are shown in Table 1. All patients were Caucasian. The mean age (± SD) was 76.3 ± 15.1 years (58.3% of patients were over 80 years) and 52.8% were males. The majority of strokes were cardioembolic (52.8%). The thrombolysis was initiated within 40 min (range: 15–78 min) after hospital admission within an average time of 2.5 ± 1.7 h after stroke onset.
Both patient cohorts were well-balanced regarding most of the baseline characteristics, except for the site of occlusion and NIHSS score. In the rtPA cohort, 70% of patients (N = 14) had a distal M2 or M3 occlusion of the middle cerebral artery (MCA). On the contrary, 15/16 (93.8%) patients eligible for thrombectomy presented with a large vessel occlusion involving the internal carotid artery (ICA) or M1-MCA. The NIHSS at admission was lower in the rtPA cohort (median 9 (range 3–23)) than in the rtPA + EVT cohort (median 17.5 (range 8–23)).
CPU and CPU + CPUi Kinetics After Thrombolysis with and Without Thrombectomy
At arrival in the emergency room, CPU was detected in 17 (47.2%) patients. The CPU activity increased then markedly upon rtPA administration and reached peak levels at the end of thrombolysis (Fig. 3, Table S1). A rapid decline was observed within 3 h. CPU remained then low or undetectable until the end of the measurements. Similar to CPU activity, CPU + CPUi antigen levels increased markedly during rtPA administration in both treatment cohorts. A progressive decline was observed thereafter with CPU + CPUi antigen levels still elevated at 7 h (Fig. 3).
Kinetics of CPU activity (red) and CPU + CPUi antigen (blue) in plasma of both treatment cohorts. Panel a rtPA cohort (thrombolysis only), panel b rtPA + EVT cohort (thrombolysis with additional EVT). Arrows indicate median start and end time of EVT and the sample that was collected 30 min after the end of EVT (rtPA + EVT cohort only). The time interval of the rtPA administration is shown in gray. Data are presented as mean ± SEM. EVT, endovascular thrombectomy
Inter-individual Variability in CPU and CPU + CPUi
Marked variability in CPU activity and CPU + CPUi antigen levels was observed between patients in both cohorts (Fig. 4, Suppl. Figure S1). Peak levels in CPU activity ranged from 0.2 to 15.6 U/L in the rtPA cohort and from 0.2 to 14.6 U/L in the rtPA + EVT cohort. CPU + CPUi peak levels ranged from 22 to 1552 ng/mL in the rtPA cohort and from 77 to 1241 ng/mL in the rtPA + EVT cohort.
Comparison of the peak of CPU activity and CPU + CPUi antigen levels between the rtPA and the rtPA + EVT cohorts. Panel a Peak levels of CPU activity and panel b Peak levels of CPU + CPUi antigen. Data are presented as scatter plots, whiskers indicate median and interquartile range. Wilcoxon test, ns, not significant
In the overall population, a modest CPU activation upon rtPA administration (CPU around 0.5 U/L and CPU + CPUi peak levels < 50 ng/mL) was observed in 3 patients (8%), whereas 11 patients (31%) displayed marked CPU activation (CPU activity > 2U/L and CPU + CPUi peak levels > 200 ng/mL), the remaining patients being in between. In the rtPA + EVT cohort, the proportion of patients with a marked CPU generation was higher than in the rtPA cohort (N = 8, 50% versus N = 3, 15%).
The kinetic pattern of CPU + CPUi differs between patients with some showing a plateau or a slow decrease following the rise observed at the end of rtPA infusion, while others displayed a more rapid decrease (Suppl. Figure S1).
Effect of Thrombectomy on CPU and CPU + CPUi Levels
No difference was found between both cohorts regarding the CPU or CPU + CPUi peak activity or level respectively (Fig. 4).
CPU/CPU + CPUi Levels in Peripheral Venous and In Situ Arterial Samples
Median CPU activity and CPU + CPUi antigen levels were similar in peripheral venous blood samples and arterial peri-thrombus samples (1.23 U/L vs. 1.35 U/L P = 0.1 for CPU; and 94.22 ng/mL vs. 110.4 ng/mL P = 0.5 for CPU + CPUi; Fig. 5a–b). Besides, the individual pairing of peripheral venous samples and those collected intra-arterially via the thrombectomy device did not show any general tendency (Fig. 5c–d).
CPU activity and CPU + CPUi antigen levels in relationship to sampling collection site. Panel a–b Overview of CPU activity (a) and CPU + CPUi levels (b) in intravenous (IV) peripheral or intra-arterial (IA) samples collected near the occlusion site through the thrombectomy device. All samples were collected at the start of the EVT procedure (before the first pass). Data are presented as scatter plots, whiskers indicate median and interquartile range. Wilcoxon test, ns, not significant. Panel c–d paired individual CPU activities (c) and CPU + CPUi levels (d) collected peripherally (IV) or near the occlusion site (IA)
CPU/CPU + CPUi Levels According to Cerebral Artery Occlusion Site (Distal vs. Proximal)
In the pooled cohorts (rtPA and rtPA + EVT combined), no significant differences in CPU activity or CPU + CPUi antigen levels at baseline or peak were noted on the basis of thrombus location (distal or proximal) (Suppl. Figure S2a-b and d-e). At 24 h, the levels of CPU + CPUi antigen, but not CPU, were higher in patients with a proximal occlusion than in patients with a distal occlusion (median 68 ng/mL vs. 35 ng/mL; P < 0.05; Suppl. Figure S2c,f).
CPU/CPU + CPUi Levels According to Stroke Severity
Patients with a severe neurological deficit at admission (baseline NIHSS > 13; N = 19) had significantly higher (P < 0.05) CPU + CPUi antigen peak levels than patients with mild or moderate deficit (baseline NIHSS ≤ 13; N = 17) (median 101.4 vs 333.4 ng/mL) (Figure S3b), but this was not reflected in the CPU peak activity (Suppl. Figure S3a).
The CPU peak activity or CPU + CPUi antigen peak levels (Figure S3c-d) did not differ between patients according to their NIHSS at 24 h.
Kinetics and Individual Variation of Hemostasis Parameters
The kinetics of fibrinogen, Glu-plasminogen, plasmin-antiplasmin, and D-dimer levels were similar in both treatment groups. Fibrinogen and plasminogen levels decreased consecutively to rtPA administration and increased thereafter to return close to baseline at 24 h. On the opposite, the plasmin-antiplasmin levels increased during the rtPA administration and decreased gradually during the following 24 h. A low variability was observed between patients. D-dimer levels increased in both cohorts reaching maximal levels at 3 h and then decreased slowly during the rest of the observation period (Suppl. Figure S4a-d). No correlation was found between individual CPU or CPU + CPUi peak level and hemostasis parameters (fibrinogen, Glu-plasminogen, plasmin-antiplasmin, and D-dimer peak levels).
CPU/CPU + CPUi Levels According to Clinical Outcomes
Three patients developed severe hemorrhagic events (two-stroke hemorrhagic transformations and one rectal hemorrhage). The CPU levels in these patients were either close to or superior to the study median level (CPU: 1.33 U/L) (Suppl. Table S2).
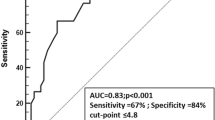
Recanalization was evaluated at 24 h in 9 patients from the rtPA + EVT cohort. Eight displayed sustainable full recanalization at 24 h. Five patients had CPU levels below median levels and three higher levels. The patient with TICI Grade 2A at 24 h (no full recanalization) had a CPU peak level higher than the median (5.17 U/L) (Suppl. Table S3).
Discussion
This observational study in ischemic stroke patients showed that CPU is rapidly generated during thrombolysis and decreases also rapidly after the end of rtPA infusion, while CPU + CPUi levels remain elevated for several hours. There was marked inter-patient variability in CPU generation, with some patients experiencing limited activation and others extensive activation. In general, no significant difference between in situ (intra-arterial) and peripheral (intravenous) CPU activity, or CPU + CPUi levels, was observed. Finally, no correlation between CPU or CPU + CPUi and NIHSS, thrombus localization, or hemostasis parameters was found.
The short CPU half-life makes it difficult to measure reliably, especially in acute settings such as AIS. We therefore, used an in-house, sensitive, specific, and validated HPLC-assisted assay to measure enzymatic CPU activity. Particular attention was paid to preanalytical and analytical issues associated with measurements of ultra-low levels of CPU. Ex vivo proCPU activation by plasmin and thrombin was counteracted by the addition of d-phenylalanyl-l-prolyl-l-arginine chloromethyl ketone (PPACK) and aprotinin to the collection tubes which was not always the case in previously published studies. Additionally, samples were placed in iced water immediately after sample collection and centrifugation was performed at 4 °C to avoid ex vivo degradation of the thermolabile CPU [22, 23, 26]. By applying these meticulous pre-analytical procedures and using the sensitive in-house assay, we were able to measure CPU in all samples after thrombolysis, demonstrating the feasibility of this complex method in real-life studies.
The net benefit of an anticoagulant or a profibrinolytic therapy depends on the balance between its efficacy (recanalization) and safety (bleeding risk). It is, therefore, necessary to ensure that it is administered at the right dose and, at the right time, to maximize the benefits and limit the risks. By measuring CPU + CPUi with the same ELISA as in our study, Alessi and co-workers indirectly studied CPU activation in 41 AIS patients [15]. Similar to our study, their results showed that CPU activation peaked at the end of the rtPA infusion and that CPU + CPUi remained elevated up to 7 h. They suggested therefore that this could represent the favorable time period for a CPU inhibitor administration. Our direct CPU activity measurement confirmed that CPU + CPUi measurements overestimate the duration of CPU activity post rtPA in AIS as CPU decrease rapidly to reach baseline within 3 h and that the favorable time window for CPU inactivation may be shorter. This discrepancy can be explained as the CPUi half-life is unknown and could be longer than those of CPU. The direct measurement of CPU activity appears thus to better reflect the CPU activity during fibrinolysis. Additionally, it could also identify some patients that displayed reactivation or continuous activation of the CPU system.
Importantly, very high inter-individual differences (up to 7.5-fold) in the CPU activation have been observed in this study (Figure S2). Some patients hardly showed any CPU activation, whereas several others presented with extensive activation. CPU and CPU + CPUi results were consistent excluding a measurement artifact. This high variability has also been noted in a subset of patients presenting with acute myocardial infarction but not receiving thrombolysis [29]. Similarly, CPU generation during in vitro clot lysis measured in the plasma of healthy volunteers was also highly variable [30].
Several factors may influence CPU generation. ProCPU is activated by thrombin, the thrombin-thrombomodulin complex, or plasmin. Plasmin is eightfold more potent than thrombin, but still, approximately 155-fold less than the thrombin-thrombomodulin complex [31, 32]. As expected upon rtPA administration, plasmin was rapidly activated by tPA as shown by the parallel decrease in Glu-plasminogen levels and the rapid increase of plasmin-antiplasmin levels (Figure S5). Low variability in plasmin-antiplasmin peak levels (range: 37–46 μg/ml), that were not correlated with CPU peak activity, was observed. Therefore, the variability in CPU generation cannot exclusively be explained by differences in plasmin generation.
Other factors influencing CPU generation and activity include baseline proCPU values and CPU half-life. A positive dependency was seen between the proCPU concentration and the CPU activity peak in an in vitro study by Leenaerts et al. [30]. ProCPU levels vary significantly in healthy subjects [33] and numerous single-nucleotide polymorphisms (SNPs) located within the proCPU gene (CPB2) have been described, but common variants in the 5’ and the 3’ region explain only a modest amount of proCPU variation [34]. Such variability may explain some of the variations in CPU generation, but proCPU levels have not been measured in the current study.
The Thr325Ile polymorphism extends the CPU half-life from 8 to 15 min at 37 °C and the 325Ile variant can lead to a 60% increase in CPU activity [35]. CPU peaks were observed at the end of thrombolysis in all patients. Even though the CPU half-life could impact the observed CPU levels, it is unlikely to fully explain the marked differences observed in this population.
The size of the thrombus can also play a role in the variability in CPU generation upon rtPA administration. No difference was observed between patients with a thrombus localized in proximal arteries (larger thrombi expected) as compared with distal occlusions. However, the exact size and composition of the thrombi were not assessed and need to be explored in relation to CPU system activation.
Contrary to previous observations of Leenaerts et al. who measured up to eightfold higher CPU activity levels near the clot site in patients with acute myocardial infarction compared to peripherally collected samples [29], we did not observe such difference in stroke patients. This discrepancy can potentially be attributed to the difficulty of sample collection through the thrombectomy device in the cerebral artery.
Relationships between CPU levels and artery recanalization at 24 h, as well as hemorrhagic risk, were explored to assess the potential benefits and risks of a pharmacological CPU inhibitor. The high level of recanalization in the rtPA + EVT cohort and the high variability in CPU generation prevented however any definitive conclusion to be drawn with regard to the benefit. Noteworthy, the high CPU levels observed in hemorrhagic patients do not support the hemorrhagic risk hypothesis, but larger sample sizes are required to confirm this observation.
A link between CPU activity, especially the peak CPU levels, and stroke severity has been suggested by several studies [13]. However, we only observed a higher CPU + CPUi peak in patients with a higher NIHSS at baseline and did not observe any other relation between NIHSS score and CPU levels. While Alessi et al. were also able to detect a significant difference in CPU + CPUi level according to NIHSS in a study with a similar sample size, the high variability of CPU generation we observed in our study—that included patients with more severe stroke—may have defined the need for a higher sample size to detect such difference [15].
Contrary to CPU activity that decreased within 3 h in all patients, CPU + CPUi antigen kinetic profiles differ between patients. In particular, two patterns were observed: one showing a rapid decrease after the peak at the end of thrombolysis, while the second displayed a slow decrease or a plateau. These differences may reflect delayed CPUi elimination and/or ongoing CPU activation. Indeed, in some patients, CPU activity was detected at T = 3 h, T = 7 h, or T = 24 h (for most at a single time point) evoking ongoing CPU generation possibly due to delayed fibrinolysis or rethrombosis. Coagulation activity was however not assessed making it impossible to correlate CPU reactivation to late rethrombosis. Of note, in one patient, the increase in CPU activity at later time points (T = 7 h and T = 24 h) coincided with a sudden decrease in fibrinogen levels (approximately 90% decrease vs. baseline) which could reflect thrombin activation and rethrombosis. However, no overall tendency regarding fibrinogen and CPU levels was observed in the study. Additionally, the relatively low level of late CPU activation in most patients does not fully support it as responsible for the differences in CPU + CPUi kinetics.
Our study demonstrates that a CPU inhibitor employed as a synergistic adjuvant of fibrinolysis may accelerate thrombolysis during and within a few hours after rtPA injection, as the CPU peak occurred at the end of rtPA infusion. However, not all patients displayed an important CPU activation. Noteworthy, CPU exerts its activity in a threshold-dependent manner, and limited activation of proCPU (1–2%) may be sufficient to counteract fibrinolysis [36, 37]. However, as this threshold is variable and dependent on the plasmin—and thus tPA—concentration, it may be hypothesized that not all patients will benefit from such treatment. As a consequence, the clinical development of a CPU inhibitor requires the ability to select the subset of responder patients. So far, two CPU inhibitors were evaluated in pulmonary embolism (AZD9684 and DS1040) and failed to show any statistically significant effect on lung scintigraphy score or thrombus size reduction [17, 38]. Our results and those already published suggest that the endogenous CPU generation potential is the composite effect of multiple factors. Therefore, it may be difficult to characterize the responder patients population without delaying treatment administration, especially in an emergency situation like AIS.
Limitations
This exploratory study is limited in its sample size. However, it was deemed sufficient to explore the kinetic of CPU activation. Nonetheless, the high inter-individual variability as well as the hemolysis of some samples decreased its power and made some secondary data analyses impossible.
Conclusion
In conclusion, this study demonstrates the feasibility to detect very low levels of CPU activity in a clinical setting especially in the challenging context of AIS with an appropriate CPU activity assay. As hypothesized, an ELISA assay measuring both CPU and CPUi overestimated the duration of CPU activation that peaks at the end of thrombolysis and declined shortly thereafter, without any impact of thrombectomy on the kinetics of it. The high heterogeneity observed between patients in CPU system activation defines the need to identify possible responders before developing a CPU inhibitor.
Data Availability
Anonymized patient-level, study-level clinical trial data (including clinical study report) and study protocol, underlying the results reported in this article will be shared in agreement with the Servier Data Sharing Policy available at https://clinicaltrials.servier.com/data-request-portal/.
Code Availability
Not applicable.
References
Donkor ES. Stroke in the 21st century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. Hindawi; 2018;2018.
Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38:967–73.
Coutts SB, Berge E, Campbell BCV, Muir KW, Parsons MW. Tenecteplase for the treatment of acute ischemic stroke: a review of completed and ongoing randomized controlled trials. Int J Stroke. 2018;13:885–92.
Bhatia R, Hill MD, Shobha N, Menon B, Bal S, Kochar P, et al. Low rates of acute recanalization with intravenous recombinant tissue plasminogen activator in ischemic stroke: real-world experience and a call for action. Stroke. 2010;41:2254–8.
Christou I, Felberg RA, Demchuk AM, Scott Burgin W, Malkoff M, Grotta JC, et al. Intravenous tissue plasminogen activator and flow improvement in acute ischemic stroke patients with internal carotid artery occlusion. J Neuroimaging. 2002;12:119–23.
Zangerle A, Kiechl S, Spiegel M, Furtner M, Knoflach M, Werner P, et al. Recanalization after thrombolysis in stroke patients: predictors and prognostic implications. Neurology. 2007;68:39–44.
Hasan TF, Todnem N, Gopal N, Miller DA, Sandhu SS, Huang JF, et al. Endovascular thrombectomy for acute ischemic stroke. Curr Cardiol Rep. Current Cardiology Reports; 2019;21.
Wollenweber FA, Tiedt S, Alegiani A, Alber B, Bangard C, Berrouschot J, et al. Functional outcome following stroke thrombectomy in clinical practice. Stroke. 2019;50:2500–6.
Bajzar L, Morser J, Nesheim M. TAFI, or plasma procarboxypeptidase B, couples the coagulation and fibrinolytic cascades through the thrombin-thrombomodulin complex. J Biol Chem. 1996;271:16603–8.
Claesen K, Mertens JC, Leenaerts D, Hendriks D. Carboxypeptidase U (CPU, TAFIa, CPB2) in thromboembolic disease: what do we know three decades after its discovery? Int J Mol Sci. 2021;22:883.
Foley JH, Kim PY, Mutch NJ, Gils A. Insights into thrombin activatable fibrinolysis inhibitor function and regulation. J Thromb Haemost. 2013;11:306–15.
Brouns R, Heylen E, Sheorajpanday R, Willemse JL, Kunnen J, De Surgeloose D, et al. Carboxypeptidase U (TAFIa) decreases the efficacy of thrombolytic therapy in ischemic stroke patients. Clin Neurol Neurosurg. 2009;111:165–70.
Brouns R, Heylen E, Willemse JL, Sheorajpanday R, De Surgeloose D, Verkerk R, et al. The decrease in procarboxypeptidase U (TAFI) concentration in acute ischemic stroke correlates with stroke severity, evolution and outcome. J Thromb Haemost. 2010;8:75–80.
Fernandez-Cadenas I, Alvarez-Sabin J, Ribo M, Rubiera M, Mendioroz M, Molina C-A, et al. Influence of thrombin-activatable fibrinolysis inhibitor and plasminogen activator inhibitor-1 gene polymorphisms on tissue-type plasminogen activator-induced recanalization in ischemic stroke patients. J Thromb Haemost. 2007;5:1862–8.
Alessi M-C, Gaudin C, Grosjean P, Martin V, Timsit S, Mahagne M-H, et al. Changes in activated thrombin-activatable fibrinolysis inhibitor levels following thrombolytic therapy in ischemic stroke patients correlate with clinical outcome. Cerebrovasc Dis. 2016;42:404–14.
Martí-Fàbregas J, Borrell M, Cocho D, Martínez-Ramírez S, Martínez-Corral M, Fontcuberta J, et al. Change in hemostatic markers after recombinant tissue-type plasminogen activator is not associated with the chance of recanalization. Stroke. 2008;39:234–6.
Eriksson H, Sandset PM, Jensen E, Wall U, Andersson M, Nerme V, et al. CPU inhibition with AZD9684: profibrinolytic effects in acute pulmonary embolism patients [Abstract]. J Thromb Haemost. 2007;5:P-S-367.
Zhou J, Limsakun T, Yin O, Warren V, Zamora C, Atiee G, et al. First-in-human study to assess the safety, pharmacokinetics, and pharmacodynamics of an oral formulation of DS-1040, an inhibitor of the activated form of thrombin-activatable fibrinolysis inhibitor, in healthy subjects. J Clin Pharmacol. 2019;59:1669–77.
Zhou J, Kochan J, Yin O, Warren V, Zamora C, Atiee G, et al. A first-in-human study of DS-1040, an inhibitor of the activated form of thrombin-activatable fibrinolysis inhibitor, in healthy subjects. J Thromb Haemost. 2017;15:961–71.
Limsakun T, Dishy V, Mendell J, Pizzagalli F, Pav J, Kochan J, et al. Safety and pharmacokinetics of DS-1040 drug-drug interactions with aspirin, clopidogrel, and enoxaparin. J Clin Pharmacol. 2020;60:691–701.
Petit Dop F, Latreille M, Guicherd L, Mertens J., Claesen K, Hendriks D, et al. Favourable safety profile of S62798, a potent TAFIa (activated thrombin-activatable fibrinolysis inhibitor) inhibitor, in first-in-man study in healthy subjects [Abstract]. Eur Heart J. 2020;41.
Mertens JC, Leenaerts D, Yperzeele L, Sim Y, Van Der Veken P, Hendriks D. Comparison and characterization of two assays (TAFIa/ai ELISA vs. activity based selective CPU assay) for the monitoring of carboxypeptidase U (CPU, TAFIa, CPB2) levels in patients. Res Pract Thromb Haemost. 2017;1:607.
Heylen E, Van Goethem S, Augustyns K, Hendriks D. Measurement of carboxypeptidase U (active thrombin-activatable fibrinolysis inhibitor) in plasma: challenges overcome by a novel selective assay. Anal Biochem. 2010;403:114–6.
Ladenvall C, Gils A, Jood K, Blomstrand C, Declerck PJ, Jern C. Thrombin activatable fibrinolysis inhibitor activation peptide shows association with all major subtypes of ischemic stroke and with TAFI gene variation. Arterioscler Thromb Vasc Biol. 2007;27:955–62.
Pedersen A, Redfors P, Lundberg L, Gils A, Declerck PJ, Nilsson S, et al. Haemostatic biomarkers are associated with long-term recurrent vascular events after ischaemic stroke. Thromb Haemost. 2016;116:537–43.
Willemse JL, Brouns R, Heylen E, De Deyn PP, Hendriks DF. Carboxypeptidase U activity is induced in vivo in ischemic stroke patients receiving thrombolytic therapy. J Thromb Haemost. 2008;6:200–2.
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018;49:e46-110.
Mertens JC, Claesen K, Leenaerts D, Sim Y, Lambeir A-M, Hendriks D. Inhibition of the procarboxypeptidase U (proCPU, TAFI, proCPB2) system due to hemolysis. J Thromb Haemost. 2019;17:878–84.
Leenaerts D, Bosmans JM, van der Veken P, Sim Y, Lambeir AM, Hendriks D. Plasma levels of carboxypeptidase U (CPU, CPB2 or TAFIa) are elevated in patients with acute myocardial infarction. J Thromb Haemost. 2015;13:2227–32.
Leenaerts D, Aernouts J, Van Der Veken P, Sim Y, Lambeir A, Hendriks D. Plasma carboxypeptidase U (CPU, CPB2, TAFIa) generation during in vitro clot lysis and its interplay between coagulation and fibrinolysis. Thromb Haemost. 2017;117:1498–508.
Wu C, Kim PY, Manuel R, Seto M, Whitlow M, Nagashima M, et al. The roles of selected arginine and lysine residues of TAFI (Pro-CPU) in its activation to TAFIa by the thrombin-thrombomodulin complex. J Biol Chem. 2009;284:7059–67.
Kim PYG, Kim PY, Hoogendorn H, Giles AR, Nesheim ME. Activated thrombin-activatable fibrinolysis inhibitor is generated in vivo at levels that can substantially affect fibrinolysis in chimpanzees in response to thrombin generation. J Thromb Haemost. 2008;6:1600–2.
Chetaille P, Alessi MC, Kouassi D, Morange PE, Juhan-Vague I. Plasma TAFI antigen variations in healthy subjects. Thromb Haemost. 2000;83:902–5.
Stanne TM, Olsson M, Lorentzen E, Pedersen A, Gummesson A, Gils A, et al. A genome-wide study of common and rare genetic variants associated with circulating thrombin activatable fibrinolysis inhibitor. Thromb Haemost. 2018;118:298–308.
Schneider M, Boffa M, Stewart R, Rahman M, Koschinsky M, Nesheim M. Two naturally occurring variants of TAFI (Thr-325 and Ile-325) differ substantially with respect to thermal stability and antifibrinolytic activity of the enzyme. J Biol Chem. 2002;277:1021–30.
Leurs J, Nerme V, Sim Y, Hendriks D. Carboxypeptidase U prevents lysis from proceeding into the propagation phase through a threshold-dependent mechanism. J Thromb Haemost. 2004;2:416–23.
Walker JB, Bajzar L. The intrinsic threshold of the fibrinolytic system is modulated by basic carboxypeptidases, but the magnitude of the antifibrinolytic effect of activated thrombin-activable fibrinolysis inhibitor is masked by its instability. J Biol Chem. 2004;279:27896–904.
Daiichi Sankyo. Study to assess the safety, pharmacokinetics, and pharmacodynamics of DS-1040b in subjects with acute ischemic stroke. [Internet]. ClinicalTrials.gov. [cited 2021 May 7]. Available from: https://clinicaltrials.gov/ct2/show/NCT02586233. Accessed 9 September 2020
Acknowledgements
The authors thank Y. Sim and L. Saudemont for their excellent technical assistance.
Funding
The study was funded by the Institut de Recherches Internationales Servier (IRIS, Paris, France). J.M. was a research fellow of the Research Foundation Flanders during the conduct of the study (grant: 1137719 N).
Author information
Authors and Affiliations
Contributions
Methodology: V.B.G., D.H., B.T., C.M., J.M.; formal analysis: J.M., V.B.G.; writing—original draft preparation: J.M., V.B.G., B.T.; writing—review and editing: all authors.
Corresponding author
Ethics declarations
Ethics Approval
The study was conducted according to the Declaration of Helsinki revised in Fortaleza and followed the Good Clinical Practices. Ethical approval was obtained from the respective ethics committees of the different centers and the study was approved by the competent authorities.
Consent to Participate
All patients or their legal representatives gave their informed consent before participating in the trial.
Consent for Publication
Not applicable.
Conflicts of Interest
VBG and BT are employees of IRIS. DH received consultant honoraria from IRIS.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Mertens, J.C., Blanc-Guillemaud, V., Claesen, K. et al. Carboxypeptidase U (TAFIa) Is Rapidly Activated and Deactivated Following Thrombolysis and Thrombectomy in Stroke Patients. Transl. Stroke Res. 13, 959–969 (2022). https://doi.org/10.1007/s12975-021-00962-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-021-00962-w