Abstract
As an integral part of the innate immune system of the brain, resident microglia must react rapidly to the onset of brain injury and neurological disease. These dynamic cells then continue to shift their phenotype along a multidimensional continuum with overlapping pro- and anti-inflammatory states, allowing them to adapt to microenvironmental changes during the progression of brain disorders. However, the ability of microglia to shift phenotype through nimble molecular, structural, and functional changes comes at a cost, as the extreme pro-inflammatory states may prevent these professional phagocytes from clearing toxic debris and secreting tissue-repairing neurotrophic factors. Evolution has strongly favored heterogeneity in microglia in both the spatial and temporal dimensions—they can assume diverse roles in different brain regions, throughout the course of brain development and aging, and during the spatiotemporal progression of brain injuries and neurological diseases. Age and sex differences add further diversity to microglia functional status under physiological and pathological conditions. This article reviews recent advances in our knowledge of microglia with emphases on molecular mediators of phenotype shifts and functional diversity. We describe microglia-targeted therapeutic opportunities, including pharmacologic modulation of phenotype and repopulation of the brain with fresh microglia. With the advent of powerful new tools, research on microglia has recently accelerated in pace and may translate into potential therapeutics against brain injury and neurological disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microglia were initially characterized over a century ago by Pío Del Río-Hortega, a graduate of the Cajal school, as the main immune cells in the brain [1]. Microglia reside throughout the neuraxis, accounting for approximately 0.5–16.6% of the total cell population in the human brain and 5–12% in the mouse brain [2, 3]. Microglia shape neural circuits by modulating synaptic transmission and sculpting neuronal synapses, especially during development, but also across the lifespan. By continually surveying and interacting with essential central nervous system (CNS) components, microglia regulate normal brain function and attempt to maintain tissue integrity under both physiological and pathological conditions [4, 5]. Recent studies have uncovered the spatiotemporal heterogeneity of microglia, raising new questions about their regional and time-dependent roles, particularly when homeostasis is interrupted after the onset of brain injury or disease.
As part of the innate immune system, microglia are activated promptly after injury but their activation can persist for a long time as a double-edged sword [6]. Microglia deploy an array of pro-inflammatory factors, including reactive oxygen species, with the potential to exacerbate neuronal damage and brain dysfunction. On the other hand, microglia are professional phagocytes and facilitate brain repair by clearing toxic debris, releasing neurotropic factors, and resolving brain inflammation [6]. Emerging studies have sharpened our focus on the complex phenotypic changes of these adaptable cells, and many new subpopulations of microglia with distinct molecular signatures have been recently identified [7]. By leveraging their unique phenotype dynamics, microglia regulate the progression of brain injury and restoration, including but not limited to white matter repair, neurogenesis, angiogenesis, and synaptic plasticity [6,7,8]. These diverse and critical roles of microglia after brain injury justify further exploration of microglia phenotype shifts and their underlying molecular mediators.
This review reassesses our current understanding of the diversity of microglia, with a focus on functional diversity. Microglial phenotype switches and the known molecular mechanisms underlying and modulating these switches are defined. We also discuss the influence of sex and age on microglia heterogeneity under physiological and pathological conditions. A better understanding of microglia biology may uncover new targets and opportunities for the development of therapeutic strategies against brain disorders.
Physiological Functions of Microglia in the Brain
Microglia in Brain Development and Homeostasis
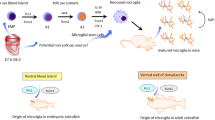
Microglia are myeloid cells that regulate early development of the CNS and maintain its homeostasis throughout life [9]. Microglial cells are derived from erythromyeloid progenitors in the yolk sac and begin to arise by embryonic day 8.5 [10]. They migrate to the brain from the yolk sac prior to the formation of blood-brain barrier (BBB) at embryonic days 13.4 to 14.5. Following BBB closure, microglia begin local self-renewal and spatial distribution across the brain and spinal cord [11, 12].
Unique transcriptional signatures of microglia in the normal adult brain have been identified with RNA sequencing and other techniques. Microglia signature genes include but are not limited to purinergic P2 receptors Y12 (P2RY12), transmembrane protein 119 (Tmem119), sialic acid–binding Ig-like lectin H (Siglech), and probable G protein–coupled receptor 34 (Gpr34) [13,14,15]. These molecules are helpful to distinguish brain resident microglia from peripheral myeloid cells. Depending on activation status, microglia assume ramified, primed, reactive, or amoeboid morphologies. Functionally, microglia play vital and complex roles in maintaining homeostasis in the adult brain. In the developing brain, microglia engulf non-functional synapses and axons, which is essential for shaping neural circuits [4, 5]. Microglia also secrete neurotrophic factors, such as brain-derived neurotrophic factor (BDNF), to promote learning-dependent synapse formation [16]. In the early postnatal stages, activated microglia promote neurogenesis and oligodendrogenesis by releasing cytokines or via phagocytic removal of excess newborn cells [17, 18]. In the adult brain, microglia exist along a continuum of resting or activated states, depending on the brain microenvironment, thereby serving as the first line of defense in the CNS.
Phenotypical and Functional Heterogeneity of Microglia in the CNS
The heterogeneity of microglia has been recognized for many years and has been confirmed by the advent of new technologies. The remarkable temporal and spatial diversities of microglia have been demonstrated in both rodents and humans.
Temporal Heterogeneity
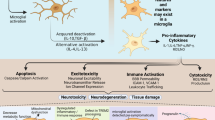
Microglia have specialized functions in different developmental stages of the brain (Fig. 1). RNA sequencing studies have identified a number of new subpopulations of microglia at the embryonic and early postnatal stages, and these subpopulations gradually disappear as the brain continues to grow [7, 19, 20]. The functions of these transient microglia subpopulations remain unknown. Single-cell transcriptomic analyses suggest that microglia go through at least three developmental stages, including early, pre-adult, and adult stages, during mouse brain development [21]. For example, early microglia express genes involved in cell cycling and differentiation, such as Mcm5 and Dab2. Pre-adult microglia express Csf1 and Cxcr2 genes, which are associated with neuronal development. In adult microglia, the main genes expressed are Cd14 and Pmepa1, which are related to immune responses [21]. Another study suggests that postnatal microglia express Tmem119, Selplg, and Slc2a5 genes. In contrast, lysosome-related genes Ctsb, Ctsd, and Lamp1 are highly expressed in embryonic microglia, likely reflecting robust phagocytic capacity in the early stages of brain development when pruning is needed the most [22]. During aging, the diversity of microglia increases even further. The temporal heterogeneity of human microglia at the transcriptional level during aging is incompletely understood, although microglia seem to show a distinct gene signature linked to the age-dependent microenvironment in the CNS [22].
Functional molecular signatures of microglia across developmental stages. Microglia derive from erythromyeloid progenitors in the yolk sac. They harbor specific molecular signatures and play specialized roles in the developing, adult, and aging brain. Microglia in developing brains strongly express Csf1, Cxcr2, Ctsb, Ctsd, and Lamp1 genes, which are associated with synaptic pruning, and synapse generation. In the adult brain, microglia express immune response-related genes, such as Cd14 and Pmepa1. Aging reduces the ability of microglia to be activated and migrate, which increases their pro-inflammatory properties and decrease their capacity to engulf toxic debris and protect neurons
Regional Heterogeneity
The microenvironment is not uniform throughout the CNS, and these variations contribute to the regional heterogeneity of microglia (Fig. 2). First, the density of microglial cells exhibits regional difference in both mouse and human brains [23,24,25]. Using a multicolor fluorescence fate-mapping system, Tay and colleagues found that the turnover rate of microglia differs across brain regions, with the highest proliferation rate in the olfactory bulb [26]. The morphology of microglia also varies regionally, although under physiological conditions they are normally ramified with extended branches in most brain regions [23, 27]. For example, cerebellar microglia contain bigger cytosolic areas and lower ramification complexity than microglia housed in the cortex and striatum [27]. Recent studies using high-throughput RNA sequencing technologies further demonstrate regional heterogeneity of gene expression in microglia. Grabert et al. sorted microglia from several brain regions in 4-month-old mice and found that thousands of genes were differentially expressed in various brain regions [28]. Specifically, microglia in the cerebellum and hippocampus maintain a higher immune-alert state compared with microglia in the striatum and cortex as evidenced by greater expression of immunoregulatory genes and co-regulated genes such as those involved in energy metabolism [28]. Masuda et al. examined at single-cell resolution regional differences in mouse and human microglia under physiological conditions. They detected transcriptionally distinguishable subpopulations of microglia in different brain regions. For example, almost all microglia expressed CST3 and SPARC in the juvenile cortex and these CST3+SPARC+ microglia were slightly diminished in the adult cortex. By contrast, this subpopulation of microglia did not change between the juvenile and adult in the cerebellum [20]. By using single-cell mass cytometry, Böttcher et al. confirmed that human microglia in the subventricular zone (SVZ) are phenotypically distinct from microglia in other brain regions [29]. They exhibit higher expression of CD11c, CD195, and CD45 as well as proliferation markers in SVZ [29]. Although these studies confirm the spatial heterogeneity of transcriptional profiles of microglia, the molecular mechanisms driving these differences remain elusive and need further investigation. We speculate that regional differences in microglial gene expression partly underlie selective neuronal vulnerabilities and that neurons and microglia engage in crosstalk via secreted factors, ligand/receptor signaling, as well as neuron/glia plasma membrane interactions that vary spatially across brain regions as well temporally across the normal lifespan or during the progression of brain disorders.
Regional heterogeneity in density, morphology, molecular signatures, and function of microglia. Microglia exhibit spatial heterogeneity in the brain. Microglial cell density is higher in the hippocampus and olfactory bulb, and lower within fiber tracts, cerebellum, and brain stem. Under homeostatic conditions, microglia are ramified with extended branches in most brain regions, with slight variations. Microglial molecular signatures also vary across brain regions. Higher expression of immune-alert genes has been reported in the cerebellum and hippocampus, and microglial gene expression profiles differ between gray and white matter. Microglia in different brain regions show varying phagocytosis and pro-inflammatory capacities
Diverse Phenotypic Polarization of Microglia after Brain Injury
As part of the innate immune system, microglia are among the first cells to detect microenvironmental changes and respond immediately to brain injuries. Their activation status can persist for quite a long time and changes dynamically during the pathological process. It seems likely that microglial morphology changes cater to their specific roles during brain injury and repair processes.
Phenotypic Polarization of Microglia after Brain Injury
A dichotomous classification has been adopted to characterize microglia polarization. Under this system, classically activated (M1) microglia lead to neuronal damage and brain dysfunction through pro-inflammatory factors, such as IL-1β, IL-6, TNF-α, etc. In contrast, alternatively activated (M2) microglia are beneficial for brain repair as they clear toxic cellular debris through phagocytosis, release neurotropic factors, and resolve cerebral inflammation [6]. The activation status of M1 vs M2 microglia has been detected by surface markers. CD16, CD32, and CD86 are widely used for identification of M1 microglia and CD206 and arginase 1 (Arg1) for M2 microglia. After injury, microglia are not static and can switch their phenotype during the temporal progression of brain recovery, in spatially segregated areas. They may present beneficial phenotypes upon acute activation, but will gradually switch to acting detrimental roles at the later stage. This phenotype switch can also be diverse in different brain lesion locations [30,31,32].
Although it has improved our understanding of the functional status of microglia after brain injuries, the existing M1/M2 classification is increasingly recognized as an oversimplification. The M1 and M2 definitions are mainly based on stimulation of cultured microglia with single cytokines in vitro and represent two rather extreme stages of microglial activation [33]. However, in vivo microglia display more nuanced phenotypes [34]. It is important to note that some microglia show overlapping phenotypes with coexpression of markers of both polarization states, demonstrating even greater phenotypic flexibility in this cell type than expected [33, 35]. In a mouse model of experimental autoimmune encephalomyelitis (EAE), IL-4 induced the expression of M1 marker IL-6 but also resulted in anti-inflammatory effects [36]. New technologies have exposed additional, complex phenotypes of microglia after brain injuries [37], such as specialized microglial subpopulations with distinct molecular signatures in response to brain demyelinating injury [7]. These subpopulations express some genes in common but also exhibit unique transcriptional profiles, indicating that microglia can display multiple forms of activation [7]. There is much discussion of the scientific inadequacy of binary terms such as “M1 vs M2” or even “pro vs anti-inflammatory,” but few alternatives other than sometimes adding “M0 and M1/2 subtypes” are offered in the literature. Rather, most authors default to discussing two polarized states for ease of presentation, even after pointing out the controversy. Wherever appropriate, terms such as “beneficial phenotype”, “detrimental phenotype”, or “neutral phenotype” would better be oriented toward the functional endpoint of these adaptable cells, rather than based on ephemeral expression markers or morphological changes. Alternatively, microglial biology should be viewed along a continuum, with beneficial and destructive at opposite poles, but the polarized view is complicated by RNA seq-based revelations that the continuum is not linear with two extreme poles. Rather, microglial phenotype is multipolar in multiple dimensions, as well as highly plastic. For now, given the current state of the scientific literature, we continue to use the easily-understood terms “M1” and “M2” below, to distinguish phenotype shifts that are based on the expression of protein markers or on pro versus anti-inflammatory effects. Additional research will reveal better ways to distinguish microglial functional status.
Temporal Dynamics of Microglia after Brain Injuries
Microglia change their phenotype over time in response to both acute brain injuries and neurodegenerative diseases. They are activated rapidly after acute brain injury and continue to accumulate at the injury site for over 1 month [38]. In ischemic stroke, microglia are activated and express M2 phenotype markers in the acute stage and then gradually switch toward M1 markers, especially in peri-infarct regions adjacent to ischemic neurons [30]. Likewise, a temporal transition of microglia was also found after experimental traumatic brain injury (TBI), as microglia/macrophages exhibited an immunoregulatory phenotype at early stages of the injury but were gradually replaced by pro-inflammatory phenotypes at the later stages [31]. In preclinical intracerebral hemorrhage (ICH), activation of microglia toward an M1 phenotype occurs mainly in the acute phase after ICH, while M2 responses occur in the subacute and chronic phase and might contribute to clearance of the hematoma and cell debris [39]. Thus, therapies may need to adjust the balance between pro-inflammatory microglia and anti-inflammatory microglia responses at different phases of brain injury.
Microglia also exhibit dynamic phenotype changes and regulate neuroprotection vs neurotoxicity processes in chronic brain injuries and neurodegenerative diseases [40, 41]. In Alzheimer’s disease (AD), Aβ deposition leads to activation of microglia. Acutely activated microglia express cytokines that drive the clearance of Aβ. At the advanced stages of disease, sustained activation of microglia leads to a chronic inflammatory state with exacerbation of neurotoxicity and neurodegeneration [42]. In multiple sclerosis (MS) and EAE, microglia promote both neuroprotective and detrimental responses to injury [43]. The initial response of microglia may be beneficial, aiming to resolve the insult, whereas the chronic activation of microglia might contribute to neurodegeneration [43]. Common microglial signatures have been used to estimate the stages of neurodegenerative disease. For example, a series of genes primarily expressed in microglia, including triggering receptor expressed on myeloid cells 2 (TREM2) [44], ATP-binding cassette subfamily A member 7 [45], and myeloid cell surface antigen CD33 [46], are associated with the risk of developing early-onset AD. In addition, the expression of complement receptor-1 is associated with late-stage AD and may reflect microglial phagocytosis of synapses [47]. These findings suggest that modulating microglia phenotype according to the specific disease stage may halt or slow the neurodegenerative process.
Modulatory Mechanisms Underlying Microglia Phenotype Shifts
Myriad biochemical mediators and signaling pathways are involved in regulating microglia phenotype, as described below (Table 1).
Transcriptional Factors
Transcription factors are proteins that bind to DNA and regulate the transcriptional activity of genes. Several transcription factors are intimately associated with microglia regulation. For example, NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) is associated with M1 polarization, and peroxisome proliferator-activated receptors (e.g., PPARγ) and Nrf2 (nuclear factor erythroid 2–related factor 2) are associated with M2 polarization. NF-κB is part of a ubiquitously expressed protein complex that controls gene transcription, cytokine production, and cell survival. The activation of NF-κB by Toll-like receptor 4 (TLR4) engagement significantly enhances microglial M1 status and impairs M2 responses [60]. Several members of the signal transducer and activator of transcription (STAT) family are also involved in regulating microglia/macrophage functional status [6]. Activation of STAT1 facilitates proinflammatory activities and increases plasma levels of M1 cytokines/chemokines [100]. Activation of STAT6 is observed in microglia/macrophages in the ischemic territory in mice and humans subjected to stroke [53]. Knockout of STAT6 hampers the clearance of dead/dying neurons and increases inflammatory gene signatures in microglia/macrophages after experimental stroke [53]. The functions of STAT3 are diverse, involving both IL10stimulated M2 polarization and IL6stimulated M1 polarization [66, 101].
PPARγ belongs to the nuclear receptor family of ligand-activated transcription factors and is involved in regulating genes for lipid and glucose metabolism, mitochondrial biogenesis, and inflammation [102, 103]. PPARγ agonists, such as troglitazone and pioglitazone, exhibit neuroprotective functions in brain injuries by reducing pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6, as well as increasing anti-inflammatory cytokines such as TGFβ, IL-10, and IL-4, G-CSF, IGF-1 [55, 56, 103]. Furthermore, PPARγ plays a key role in modulating NF-κB and Nrf2/CREB signaling pathways to mediate anti-inflammatory effects [104]. Nrf2 regulates the expression of antioxidant proteins that protect against oxidative damage triggered by injury and inflammation. Activation of Nrf2 by neuroprotectants promotes microglial polarization to the M2 phenotype and reduces oxidative stress and neuroinflammation [57, 58].
Receptors
Toll-Like Receptors
TLRs, a family of transmembrane proteins that act as pattern-recognition receptors, are the key factors in mediating microglial activation in response to inflammation. After brain ischemia/reperfusion injury, the expression of both TLR2 and TLR4 is increased in cortical neurons [105], and knockout of either TLR2 or TLR4 reduces brain infarct volumes by mitigating inflammatory cytokines [106, 107]. Several signaling cascades are associated TLR engagement. TLR4 induction after stroke results in the activation of the NF-κB pro-inflammatory pathway [108]. In addition, inhibition of TLR4 encourages M1 to M2 microglia phenotype transitions in preclinical AD through the MyD88/NF-κB/NLRP3 inflammasome [109].
Purinergic Receptors
The purinergic signaling systems, including adenosine, ATP, other purines, and P1 and P2 receptor subtypes control inflammatory responses in complex ways [110]. Purinergic P2 receptors are composed of two ionotropic receptors, P2X4 and P2X7, and metabotropic receptors. Blockade of P2X4R signaling worsens clinical symptoms in the EAE model and promotes microglia activation to a pro-inflammatory phenotype. Conversely, enhancing P2X4R signaling by the allosteric modulator ivermectin (IVM) encourages microglia to switch to an anti-inflammatory phenotype, which increases myelin phagocytosis and promotes remyelination after EAE [73]. P2X7 is constitutively expressed in mouse and human primary microglia and mediates specific release of IL-1 family cytokines, including IL-1α, IL-1β, and IL-18 in primary microglia cultures [111].
Sphingosine-1-Phosphate Receptors
Sphingosine-1-phosphate receptors (S1PRs) are G protein–coupled receptors expressed in abundance on microglia and include five subtypes (S1Pr1-S1Pr5) [112]. Treatment with a selective S1PR1 agonist, RP101075 significantly reduced pro-inflammatory cytokines IL-1β and TNF-α in the injured brain hemisphere after ICH [76]. FTY720, another S1PR agonist, attenuated microglia-mediated neuroinflammation and promoted oligodendrogenesis by shifting microglia toward M2 polarity in a chronic white matter ischemic injury model [48]. On the contrary, in primary cultured microglia, adding S1P to the culture medium increased expression of IL-17, and exogenous administration of S1P to microglia after oxygen-glucose deprivation (OGD) aggravated neuronal apoptosis [77]. Additional studies are required to determine the role of S1PRs in the regulation of microglia polarization following brain injuries.
Triggering Receptor Expressed on Myeloid Cells-2
TREM2, a member of the innate immune receptor TREM family, is activated when bound to its adaptor DAP12 and promotes several cellular functions such as cell survival, phagocytosis, and cytokine production [113]. However, it remains unclear whether TREM2 is a pro- or anti-inflammatory molecule. Early studies reported that TREM2 on microglia promoted phagocytosis of apoptotic neurons and suppressed expression of pro-inflammatory molecules such as TNF-α [80, 114]. However, in the subacute phase of an experimental stroke model (7-day following stroke), the transcription of pro-inflammatory cytokines TNF-α, IL-1α, and IL-1β as well as chemokines CCL-2 and CCL-3 were decreased in TREM2 knockout mice, suggesting that TREM2 can also contribute to M1 phenotype microglial polarization [81]. Therefore, TREM2 may sustain distinct inflammatory responses at different brain injury stages and in different brain disorders.
The concept of “disease-associated microglia” (DAM) has recently emerged, which defines a special population of microglia in the injured/diseased brain with expression of an array of signature genes (e.g., Lpl, Cst7, Axl, CD11c, etc.). TREM2 was shown to be the principal inducer of this phenotype at late stages in mouse models of AD and amyotrophic lateral sclerosis (ALS) [82, 115]. Therefore, it is likely that dysregulation of TREM2/DAP12 signaling contributes to neurodegenerative diseases.
CD200/CD200R
Transmembrane glycoprotein CD200 is highly expressed in neurons, and its receptor CD200R is mainly present on the surface of microglia [116, 117]. The activation of microglia is therefore regulated by neurons through the interaction of CD200 with CD200R. Once CD200 binds to CD200R, the release of pro-inflammatory factors by microglia is inhibited and microglia are maintained in the resting state [83, 118]. Mechanistically, CD200R-CD200 interactions trigger recruitment of downstream tyrosine kinase and RasGAP, which dampens microglia activation [83]. In mice lacking CD200 or with selective blockade of CD200R, microglia were coaxed toward a detrimental phenotype and the levels of pro-inflammatory cytokines were elevated in the brain [86]. On the contrary, pro-inflammatory markers of microglia were decreased in experimental EAE after administration of a CD200R agonist, CD200Fc [119].
CX3CL1 and CX3CR1
The chemokine CX3CL1 and its receptor CX3CR1 constitute another coupling signal mediating microglial polarization. CX3CL1 is expressed constitutively in neurons and astrocytes, while CX3CR1 is exclusively expressed in microglia in the CNS. CX3CL1/CX3CR1 signaling participates in interactions between neurons/astrocytes and neighboring microglia [120]. In experimental ischemic stroke, CX3CR1 deficiency facilitates microglia polarization toward the M2 phenotype and attenuates synthesis and release of inflammatory cytokines from microglia [88]. Knockdown of CX3CR1 by siRNA prevents the increases in expression of p38MAPK, PKC, TNF-α, IL-1β, and IL-6 after bilateral common carotid artery stenosis (BCAS), which is used to model the slow development of vascular dementia in the brain [89]. Cx3cr1−/− mice subjected to EAE displayed worse symptoms and higher pro-inflammatory cytokines than wild-type animals [91].
MicroRNAs
Numerous microRNAs regulate microglia polarization. For example, overexpression of microRNA-384 significantly attenuates OGD-induced neuroinflammation in primary neonatal microglia [121]. In an in vitro model of subarachnoid hemorrhage (SAH), incubation of primary microglia with microRNA-146a followed by hemoglobin (Hb) induction reduces expression of pro-inflammatory cytokines (TNF-α and IL-1β) and M1 phenotype-related genes (iNOS and CD86) [122]. microRNA-199b was shown to repress pro-inflammatory cytokines via modulation of microglia activation in a rat model of spinal cord injury [123]. MicroRNA-98 could reduce M1 microglia in an experimental stroke model [124]. Among all the microRNAs, microRNA-124 and microRNA-155 are well studied in microglia and will be discussed in detail in the following paragraphs.
microRNA-124
microRNA-124 is the most abundant brain-specific microRNA highly expressed in microglia. microRNA-124 contributes to M2 polarization of microglia in models of CNS disorders [95, 125,126,127]. For example, intracerebral injections of microR-124 at both 2 days and 10 days after experimental stroke shifts pro-inflammatory microglia toward the anti-inflammatory phenotype [95]. Intravenous administration of exosomes packed with microRNA-124 at 24 h after experimental TBI promotes M2 polarization and improves functional recovery [128]. On the other hand, downregulation of microR-124 by cocaine administration increases neuroinflammation by polarizing microglia toward M1 [129]. Mechanistically, microRNA-124 may regulate microglia polarization by inhibiting activation of Krüppel-like factor 4 (KLF4) and TLR4 signaling [128, 129].
microRNA-155
microRNA-155 is well established as a microglia phenotype modulator that promotes M1 polarization. In lipopolysaccharide (LPS)-stimulated microglia cultures, microRNA-155 expression is significantly increased and accompanied by polarization toward the pro-inflammatory phenotype [94]. Inhibition of microR-155 by either gene knockdown or antagomir treatment significantly downregulates expression of pro-inflammatory cytokines, including IL-1β, IL-6, and TNF-α, in injured brain tissues [130, 131]. Intravenous injection of microRNA-155 inhibitor even as late as 48 h after experimental stroke significantly decreases pro-inflammatory cytokine expression at 7 days and increases anti-inflammatory cytokines such as IL-10 and IL-4 at 14 days after stroke, which is likely to influence repair processes [132].
Although modulation of microRNA levels has therapeutic potential against brain disorders, there remain many obstacles. First, there remains the challenge of delivering miRNAs to the CNS across the blood-brain barrier (BBB), and prevention of degradation of exogenous miRNAs by lysosomal and other enzymes. These obstacles may be addressed by the development of new technologies, such as oligonucleotide backbone and sugar modifications (phosphorothioate and 2′-O-methoxyethyl) employed in the antisense therapies milasen and nusinersen [133].
Others
In clinical and experimental studies, a large number of molecules and modulators are able to regulate neuroinflammation and microglia polarization. FAM19A3, a member of a cluster of TAFA family genes that function as brain-specific chemokines or neurokines, is predominantly expressed in the CNS and promotes polarization of microglia toward M2 in brain ischemia [96]. Ion channels such as Hv1 and Kv1.3 have also been associated with microglia polarization. For example, an increase in Hv1 expression induces neuroinflammatory responses through the Hv1/noX/ROS pathway [97]. Kv1.3 modulates Ca2+ signaling and induces neuroinflammation [98]. In addition, infiltrating peripheral immune cells, particularly T lymphocytes, interact with microglia and induce their differentiation toward M1 or M2 phenotypes after brain injury [134]. Overexpression of programmed death protein 1 and programmed death-ligand 1 selectively promotes microglia polarization toward anti-inflammatory phenotypes after intracerebral hemorrhage and inhibits phosphorylation of STAT1 [99]. T cell immunoglobulin- and mucin-domain-containing molecule family-3 (Tim-3), which is mainly expressed microglia, promotes the M1 phenotype in experimental ICH [135]. It is also possible that groups of molecules work synergistically (or differentially at specific times) after CNS injury to regulate microglia phenotypic shifts.
Functional Diversities of Activated Microglia after Brain Injuries
Phagocytosis
As the professional phagocytes of the CNS, microglia are able to recognize and engulf misfolded proteins, cellular debris from apoptotic cells, or invading pathogens [136]. Microglial phagocytosis is a fine-tuned process mediated by the activation of specific membrane receptors, which directly recognize the targets. For example, TLR-4 and scavenger receptors such as CD1 are associated with pathogen recognition after exposure to signaling molecules such as phosphatidylserine and oligosaccharides [137]. Pharmacological or genetic disruption of TLR4 impairs microglial phagocytosis [138]. TAM (Tyro3, Axl, and Mer) receptors recognize mainly apoptotic cells and virus-infected cells exposing phosphatidylserine [139, 140]. TREM2 mediates the internalization of dead cells as well as protein aggregates such as Aβ [115, 141, 142]. In acute CNS injuries, such as TBI, stroke, and spinal cord injury (SCI), phagocytosis is initially performed by resident microglia and subsequently by infiltrating macrophages in an attempt to restore brain homeostasis [143]. Microglia engulf apoptotic cell debris or damaged myelin to prevent the release of cytotoxic and immunogenic intracellular contents [136, 144]. In neurodegenerative disease such as MS, resident microglia and peripheral macrophages are both capable of phagocytosing and degrading large quantities of myelin. Specifically, microglia exhibit a greater ability to engulf degenerating myelin and are more resistant to apoptosis following myelin phagocytosis than peripheral macrophages [145, 146]. In AD, microglia play an important role in preventing the accumulation of Aβ through phagocytosis as well as the production of Aβ-degrading intra- and extracellular enzymes [147]. Genetic defects in neurodegenerative disorders can cause dysregulation of phagocytosis [148, 149]. For example, in prion disease, the pathogenic form of prion protein (PrPsc) is not taken up by microglia, and it even alters the uptake of other particles, resulting in further accumulation of pathology [148]. Live neurons may also be phagocytosed by microglia during brain injury, and this process may contribute to neuronal cell death during pathological states [136]. In sum, precise and context-appropriate modulation of microglial phagocytosis is needed to optimize its beneficial effects (Fig. 3).
Diverse functions of microglia after brain injury. Microglia rapidly activate when the microenvironment changes after brain injury and play diverse roles in brain repair. Activated microglia can engulf misfolded proteins and clear cellular debris and apoptotic cells through the activation of specific membrane receptors. Anti-inflammatory microglia secrete neuroprotective cytokines/chemokines and neurotrophic factors that contribute to improvements in the blood-brain barrier, remyelination, neurogenesis, angiogenesis, and axon regeneration. By contacting the blood-brain barrier, microglia also play a role in direct closure of the injured barrier by P2RY12-mediated chemotaxis of microglial processes. Furthermore, microglial processes make direct contact with neuronal synapses and can rapidly modulate neuroplasticity and neuronal function in response to brain injury
Regulation of BBB Integrity
The phenotype-specific roles of microglia in regulating BBB integrity after brain injury have been demonstrated. At early stages, pro-inflammatory microglia may produce cytokines and chemokines, leading to barrier hyperpermeability in both acute brain injuries and neurodegenerative disorders [84, 150]. Activated microglia also upregulate endothelial cell adhesion molecules and promote leukocyte infiltration. The infiltrated leukocytes further aggravate BBB disruption [150, 151]. In contrast, anti-inflammatory microglia can facilitate long-term neurovascular remodeling and improve neurological functions [152]. Microglia also play a role in rapid closure of the BBB by chemotaxis of microglia processes after brain injury, a process that may be mediated by the purinergic receptor P2Y G protein–coupled 12 (P2RY12) [153].
Axonal Regeneration
Axonal regeneration after injury is very limited in the adult brain. Microglia that are activated by brain injury secrete pro- or anti-inflammatory cytokines and other molecules that are detrimental or beneficial for axonal regeneration. M1 microglia activated by LPS or interferon gamma (IFNγ) inhibit neurite outgrowth and induce axonal retraction in dystrophic neurons in vitro [154, 155]. Pro-inflammatory cytokines secreted by microglia, such as TNF-α, IL-1β, may enhance astrogliosis and glia scar formation, another obstacle to axonal regeneration [156]. In contrast, M2 microglia secrete protective molecules, such as Arg1 and BDNF, and promote axonal regrowth [8]. There are other ways in which microglia can influence axon regeneration. Promoting microglial phagocytosis by deletion of signal regulatory protein-α (SIRPα) also enhances the removal of myelin debris after brain injuries and facilitates axon regeneration from nerve injury [157]. One study disputed the importance of microglia in axonal regeneration by suggesting that microglia depletion failed to change induction of regeneration-associated genes upon optic nerve injury or modify the regenerative potential of retinal ganglion cells (RGCs) after injury [158]. Given the complex roles of microglia in regulating axonal regeneration, timely and controlled microglia activation may be essential for promoting axonal regeneration after brain injury.
Regulation of Synaptic Plasticity
Microglia regulate neuroplasticity not only during brain development but also after brain injuries [159, 160]. Experimental evidence suggests that microglia can rapidly modify neuronal activity and modulate synaptic function in response to injury [161]. Resting microglia processes make brief and direct contacts with neuronal synapses. After cerebral ischemia, however, the duration of these contacts is markedly prolonged and accompanied with the disappearance of presynaptic bouton, suggesting that microglia enhance the turnover of synaptic connections after brain ischemia [160]. Activated microglia can increase neuronal activity by displacing inhibitory presynaptic terminals from cortical neurons in the adult mouse [162]. In a mouse model of spinal cord injury, CX3CR1 deficiency in microglia and macrophages creates a microenvironment that attenuate dendritic pathology and improves synaptic plasticity in ventral horn motor neurons [163]. Whether and how microglia phenotypes differentially affect synaptic plasticity after brain injury remain to be elucidated.
Neurogenesis and Angiogenesis
Microglia regulate neurogenesis mainly through anti-inflammatory effects and the secretion of neuroprotective factors. M2 microglia-conditioned media induced by IL-4 has the ability to promote proliferation and differentiation of neural stem/progenitor cells (NSPCs) in the ipsilateral SVZ of ex vivo ischemic brain sections [164]. Curcumin ameliorates microglia derived brain inflammation and improves neurogenesis in the hippocampus by activating the BDNF/Trkb/PI3K/Akt signaling after TBI [165]. In addition, the phagocytic capacity of microglia also participates in neurogenesis. Neural progenitor cells in the hippocampus are known to give rise to neuroblasts throughout life. However, the majority of these newborn cells undergo death by apoptosis early during the differentiation process. Microglia routinely and rapidly clear out these apoptotic cells through phagocytosis and thereby maintain a non-inflammatory microenvironment in the brain [18]. On the other hand, M1 microglia secrete destructive factors and promote brain inflammation, which can exert detrimental effects on adult neurogenesis after injury [166]. For example, M1 microglia and neuroinflammation driven by IFNγ impair hippocampal neurogenesis in the adult mouse brain [167]. Similarly, microglial shifts toward the anti-inflammatory phenotype may improve angiogenesis after brain injury through production of neuroprotective vascular endothelial growth factor (VEGF) and IL-8 [168, 169]. Knockdown of miR-377 in the ischemic brain suppresses microglial secretion of pro-inflammatory cytokines, promotes angiogenesis, and lessens brain injury [170]. Conversely, knockdown of HIF-1α enhances brain inflammation and attenuates angiogenesis in a rat model of chronic cerebral hypoperfusion [50]. VEGF is a major contributor to the development and patterning of blood vessels. In microglia and endothelial cell co-culture systems, activated microglia increase the release of VEGF-A and platelet-derived growth factor-BB (PDGF-BB) from endothelial cells and enhance angiogenesis [171]. This mechanism may partially underlie the impact of microglia on vascularization.
White Matter Regulation
It is known that microglia clear myelin debris and apoptotic cells during the process of demyelination, which contributes not only to axon regeneration but also to remyelination. Microglia can secrete growth factors and modulate the extracellular matrix to provide an environment that supports oligodendrocyte precursor cell (OPC) recruitment and differentiation [7]. Microglia polarization phenotype strongly influences white matter damage and repair after brain injury. The severity of white matter injury is closely correlated with M1 polarization after brain injuries, such as TBI, ischemic stroke, and ICH [31, 172, 173]. Pro-inflammatory microglia secrete cytokines and reactive oxygen species that can directly damage oligodendrocytes and favor demyelination [37]. Selectively depletion of M1 or M2 microglia has been used to show that oligodendrocyte regeneration is promoted by the M2 phenotype and impaired by the M1 phenotype [174]. Oligodendrocyte differentiation can also be enhanced in vitro with M2 microglia-conditioned media [174]. Recent advances in single-cell techniques have identified additional subpopulations of activated microglia with unique signatures at different stages of white matter disease. The contribution of these microglia subpopulations to the regulation of white matter awaits further elucidation [37]. Thus, precisely timed and spatially segregated modulation of microglia subtypes may yield the greatest improvements in white matter integrity.
Impact of Age and Biological Sex on Microglia Function in Brain Disorders
Aging
Aging is the major risk factor for neurodegenerative diseases and may aggravate the severity of brain injuries such as stroke and TBI. Microglia play pivotal roles in brain immune surveillance and may play a significant role in age-related brain changes. Understanding the temporal changes of microglia in normal or diseased brains over the lifespan may be useful in identifying therapeutic interventions for age-related brain disorders.
Microglia in the Normal Aging Brain
Emerging evidence suggests that microglia undergo aging-related changes, as characterized by the emergence of dystrophic morphologies, dynamic behavior, changes in expression of inflammatory markers, and cytokine production. The number of microglia is quite homogenous across the adult lifespan. Streit et al. identified morphological changes of microglia in the human brain, as demonstrated by abnormalities such as deramification, spheroid formation, gnarling, and fragmentation [175]. In addition, aged microglia have fewer dendritic branches and less process motility, which may be linked to lower migration rates and sustained inflammatory responses in the aged brain [75, 176]. In general, inflammation is increased in the CNS with aging. Accordingly, aged microglia express higher levels of inflammatory markers, such as CD68, TLR, CD11b, CD11c, and MHCII. MHCII was found to be present on microglia predominantly in the aged brain [177]. A recent study observed characteristic lipid droplets in the cytoplasm of aged microglia, but rarely in young microglia. These lipid droplets contribute to deficits in phagocytosis and increased ROS in aged microglia [178]. In 2017, Flowers et al. leveraged mass spectrometry-based proteomics to compare primary cultures of microglia derived from young vs aged animals. They reported that microglia from aged mice show key changes in cellular metabolism and energy regulation, which may underlie alterations in inflammatory signaling [179]. In 2020, Shi et al. reported the genome-wide transcriptional profiles of microglia isolated directly from aged and young mouse brains in vivo, using bulk RNA sequencing [180]. Shi and colleagues discovered that aged microglia upregulate transcriptomic pathways involved in immune-inflammatory responses, suggesting that microglia are in an active inflammatory stage even in the healthy aged brain [180]. Another study using single-cell RNA sequencing found a small age-specific subpopulation of microglia that contributes to age-related brain inflammation [7]. Olah et al. further confirmed the existence of an aging-related microglia phenotype by employing RNA Seq analyses of microglia collected from postmortem human brains [181].
Microglia in Aged and Diseased or Injured Brains
The responses of aged microglia to acute brain injuries or neurodegeneration diseases exhibit features distinct from young microglia. Shi and colleagues compared the morphology and function of microglia in young and aged mice after acute brain ischemia and reported that the somal volumes of Tmem119+ microglia were larger in the latter group [180]. In addition, transcriptomic alterations suggest that aged microglia are less dynamically responsive to acute ischemic insults compared with young microglia, as evidenced by suppressed expression of genes involved in biological functions such as immune cell recruitment, immune responses, cellular homeostasis, and cell-cell interactions [180]. During the brain recovery stage after stroke, genes associated with cell movement, cell-cell interactions, cell viability, and cell homeostasis were more robustly activated in young mice and their expression appeared to be impaired in aged microglia [182]. In the acute phase after TBI, aged animals display robust microglia proliferation but the aged microglia show impairments in phagocytic activity and higher production of IL-1β, which may contribute to the higher mortality and worse neurological outcomes in aged organisms suffering TBI, including humans [183].
The role of senescent microglia in age-associated neurodegenerative diseases has been reviewed extensively. Microglia purified from aged animals secrete molecules that drive brain inflammation, a key contributor to the emergence of brain disorders in the aged brain [184, 185]. Depletion of microglia with PLX3397 in 9-month-old 5xFAD mice can prevent amyloid pathology and rescue synaptic plasticity [186]. Aged microglia display a decrease in the capacity to degrade cellular debris such as β-amyloid, as suggested by reduced expression of β-amyloid degrading enzymes, which may exacerbate neurological deficits in neurodegenerative diseases [187, 188]. In addition, iron-rich dystrophic microglia have been found in most neurodegenerative brain disorders including human and animal models. This glial iron sink could protect the brain from iron toxicity but may damage microglia themselves [189]. Overall, an improvement in our understanding of distinctions between microglia in the young versus aged brain may help us design better therapies for brain disorders.
Sex Differences
Several brain disorders display notable sex differences in incidence, severity, and progression. Women have a lower risk of developing stroke, but display worse outcomes and worse neurological deficits than men after stroke onset [190, 191]. In addition, men and women exhibit different clinical complications, which can impact diagnoses and treatments. Microglia partly regulate sex differences in brain injuries, as discussed below. Further understanding of sex differences in microglia phenotype may help us develop more targeted therapies, especially in diseases where sex differences are prominent.
Microglial Sex Differences in the Healthy Brain
No differences in the number of male versus female microglia have been observed in the fetal brain [192]. After birth, sex differences in the number and morphology of microglia start to emerge [192]. The number of microglial cells in the preoptic area, hippocampus, parietal cortex and amygdala are higher in male than female during postnatal development, whereas females have higher numbers of microglia with activated morphologies later during development [192, 193]. Furthermore, the expression of a large number of cytokines, chemokines and their receptors are highly sex-dependent in both neonatal and adult brains [192]. An ex vivo study using microglia derived from neonatal brains suggests that male versus female microglia exhibit divergent inflammatory signaling responses to LPS and estradiol [194]. The neonatal microglial sex differences may determine sex-specific functions later in life. Sex differences in microglia in the adult brain are incompletely understood. RNA sequencing analyses have recently identified sex distinctions in gene expression profiles of adult murine microglia [195]. Sex hormones in males and females are likely to be a contributing factor to several developmental differences, but the specific roles of sex hormones in the maturation of microglia are not yet fully understood [196, 197]. It is important to recognize immunological differences in the male versus female CNS at various developmental stages, as they may determine susceptibility to neurological conditions later in life.
Microglial Sex Differences in the Injured Brain
Accumulating evidence suggests that microglia dynamics and neuroinflammatory responses are sex-specific under injury conditions. Using RNA sequencing, Villa et al. found that male microglia express more inflammatory genes, while female microglia exhibit a more protective phenotype after stroke. When microglia from female mice were transplanted into male mouse brains, brain infarct volumes were significantly reduced at both 24 h and 48 h after ischemia [195]. Neurodegeneration induced by kainate infusions into the hippocampus is accompanied with microglial activation and this response is more severe in males than females [198]. TBI causes rapid and pronounced cortical microglia activation in male mice and leads to a more aggressive neuroinflammatory profile in males compared with females, in both acute and subacute stages [199]. The blood protein vitronectin promotes stroke-induced microglia activation and leukocyte infiltration in females only [200]. One of the mechanisms underlying sex differences might be sex hormones, which regulate microglia activation and cytokine release in vitro and in vivo [201, 202]. However, as microglia isolated from adult brains maintain sex-specific features when grown in culture or transplanted into the brains of the opposite sex, hormone-independent mechanisms may also exist [195].
Potential Opportunities and Challenges in Targeting Microglia for Rationally Designed Therapies against Brain Disorders
Due to the long-lasting influence of microglia on brain injuries, targeting this cell type offers promise in the development of therapies against brain injuries. However, the complicated and dynamic features of microglial responses make it challenging to develop microglia-targeting treatments.
An enormous number of pharmacological or molecular modulators have been tested in experimental models and/or clinical trials. Fingolimod, an FDA-approved drug for the treatment of MS, polarizes microglia toward the M2 state and promotes angiogenesis by suppressing STAT3 signaling in experimental models [48, 203]. Rosiglitazone treatment increases M2 microglia responses following experimental ischemic stroke and improves white matter integrity [204]. Other pharmaceutical agents and factors such as ion channels and transcription factors might be able to regulate microglia polarization toward anti-inflammatory phenotypes and have therapeutic potential for brain protection after experimental injury [205,206,207,208,209,210]. Cellular therapies that target microglia-mediated neuroinflammation have also been tested in preclinical studies. Intrathecal transplantation of autologous M2 macrophages in nonacute stroke patients elicited an improvement in neurological scores and increased spontaneous production of IL-10, FGF-β, PDGF and VEGF [211]. Despite the beneficial effects of therapies that regulate microglia polarization in experimental or preclinical studies, the clinical translation of these factors is in its infancy.
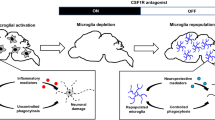
The process of microglial depletion and subsequent repopulation is emerging as a potential therapy against brain injuries. Microglia can be depleted from the brain by pharmacological as well as genetical approaches [212]. Depletion of microglia results in few to no behavioral consequences in the normal brain, and elicits varying outcomes in models of brain diseases [213, 214]. Depleted microglia are able to repopulate in vivo, but the origin of the repopulated microglia is still debated [215,216,217,218]. Freshly repopulated microglia in the mammalian brain tend to adopt a neuroprotective and pro-regenerative phenotype, which facilitates brain repair and alleviates cognitive deficits after brain injury. In 24-month-old aged mice, acute and noninvasive depletion and repopulation of microglia with the CSF1 receptor (CSF1R) inhibitor PLX5622 significantly improved spatial memories and reversed age-related changes in neuronal gene expression [219]. Sustained microglial depletion after experimental TBI with continuous PLX5622 treatment (i.e., before and during TBI) does not affect injury-induced cognitive deficits. However, the repopulation of microglia after both pharmacological or genetical depletion successfully promotes functional recovery and improves neurogenesis after experimental TBI in an IL-6 dependent manner [220]. Even in the chronic stages after TBI, short-term elimination of microglia followed by repopulation results in long-term improvements in neurological function and a reduction in persistent neurodegenerative processes, thereby dramatically extending the therapeutic window for TBI [221]. Furthermore, microglia depletion and repopulation by PLX3397 has the potential to attenuate chronic immune activation in primary organotypic hippocampal slice cultures, as demonstrated by elevated anti-inflammatory cytokine and growth factor expression in repopulated microglia [222]. Although microglial depletion and replacement has therapeutic potential against brain injury, there remain limitations, as with all clinical treatments. First, pharmacological approaches to deplete microglia by oral drug intake may have equivalent effects on peripheral macrophages or other immune cells that express the targeted receptors, which may elicit unwanted effects. In addition, microglia depletion at different disease stages may have distinct outcomes. Further investigations are essential before we deploy the power of microglia depletion as a clinical intervention in patients.
Given the lack of effective therapeutic options for many neurological diseases, further understanding of microglial function is essential [223, 224]. Technological advances in molecular biology, imaging, and single-cell analysis have provided considerable insights into dynamic microglial responses to brain injuries and boosted the potential of microglia-based treatments against brain injury. A superior understanding of crosstalk between microglia and other peripheral immune cells might allow us to modulate microglial function indirectly without forced ingress into the CNS. However, there are still obstacles that need to be overcome before microglial weaponry can be exploited against CNS disorders. In particular, it is difficult but necessary to develop drugs that target disease-associated microglia specifically without negatively affecting normal microglia. Future studies are warranted to further distinguish differences between microglia phenotypes and shift the equilibrium toward the beneficial microglial responses.
Conclusion
The present review provides an overview of the activation status and functional diversities of microglia following brain injury. As resident myeloid cells and professional phagocytes, microglia shape neural circuits and neuroplasticity during brain development. In the postnatal and adult brain, microglia serve as the primary arm of the innate immune system of the brain, monitoring its dynamic microenvironment and maintaining homeostasis. The morphological changes and physiological functions of microglia exhibit temporal and spatial heterogeneity, allowing them to assume diverse roles in different brain regions and across the lifespan. In the injured brain, microglia undergo rapid activation and phenotypic change, functioning as dual-edged swords in brain damage and restorative processes. Age and sex differences significantly influence microglia function in both physiological and pathological conditions, and a variety of modulators have the capacity to regulate microglia phenotype polarization. Thus, recent insights into microglia biology open a new horizon in the battle against brain disorders.
References
R-H P. Estudios sobre la neuroglía. La microglía y su transformación en células en bastoncito y cuerpos gránulo-adiposos. Trab Lab Invest Biol Univ Madrid. 1920;18:37–82.
Mittelbronn M, Dietz K, Schluesener HJ, Meyermann R. Local distribution of microglia in the normal adult human central nervous system differs by up to one order of magnitude. Acta Neuropathol. 2001;101(3):249–55.
Lawson LJ, Perry VH, Gordon S. Turnover of resident microglia in the normal adult mouse brain. Neuroscience. 1992;48(2):405–15.
Paolicelli RC, Bolasco G, Pagani F, Maggi L, Scianni M, Panzanelli P, et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–8.
Schafer DP, Stevens B. Phagocytic glial cells: sculpting synaptic circuits in the developing nervous system. Curr Opin Neurobiol. 2013;23(6):1034–40.
Hu X, Leak RK, Shi Y, Suenaga J, Gao Y, Zheng P, et al. Microglial and macrophage polarization-new prospects for brain repair. Nat Rev Neurol. 2015;11(1):56–64.
Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A, et al. Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity. 2019;50(1):253–71 e6.
Louveau A, Nerriere-Daguin V, Vanhove B, Naveilhan P, Neunlist M, Nicot A, et al. Targeting the CD80/CD86 costimulatory pathway with CTLA4-Ig directs microglia toward a repair phenotype and promotes axonal outgrowth. Glia. 2015;63(12):2298–312.
Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10(11):1387–94.
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330(6005):841–5.
Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FM. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat Neurosci. 2007;10(12):1538–43.
Hashimoto D, Chow A, Noizat C, Teo P, Beasley MB, Leboeuf M, et al. Tissue-resident macrophages self-maintain locally throughout adult life with minimal contribution from circulating monocytes. Immunity. 2013;38(4):792–804.
Bennett ML, Bennett FC, Liddelow SA, Ajami B, Zamanian JL, Fernhoff NB, et al. New tools for studying microglia in the mouse and human CNS. Proc Natl Acad Sci U S A. 2016;113(12):E1738–46.
Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, et al. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nat Neurosci. 2014;17(1):131–43.
Chiu IM, Morimoto ET, Goodarzi H, Liao JT, O'Keeffe S, Phatnani HP, et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 2013;4(2):385–401.
Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR 3rd, Lafaille JJ, et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–609.
Shigemoto-Mogami Y, Hoshikawa K, Goldman JE, Sekino Y, Sato K. Microglia enhance neurogenesis and oligodendrogenesis in the early postnatal subventricular zone. J Neurosci. 2014;34(6):2231–43.
Sierra A, Encinas JM, Deudero JJ, Chancey JH, Enikolopov G, Overstreet-Wadiche LS, et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell. 2010;7(4):483–95.
Li Q, Cheng Z, Zhou L, Darmanis S, Neff NF, Okamoto J, et al. Developmental heterogeneity of microglia and brain myeloid cells revealed by deep single-cell RNA sequencing. Neuron. 2019;101(2):207–23 e10.
Masuda T, Sankowski R, Staszewski O, Bottcher C, Amann L. Sagar, et al. spatial and temporal heterogeneity of mouse and human microglia at single-cell resolution. Nature. 2019;566(7744):388–92.
Matcovitch-Natan O, Winter DR, Giladi A, Vargas Aguilar S, Spinrad A, Sarrazin S, et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science. 2016;353(6301):aad8670.
Galatro TF, Holtman IR, Lerario AM, Vainchtein ID, Brouwer N, Sola PR, et al. Transcriptomic analysis of purified human cortical microglia reveals age-associated changes. Nat Neurosci. 2017;20(8):1162–71.
Lawson LJ, Perry VH, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal adult mouse brain. Neuroscience. 1990;39(1):151–70.
Savchenko VL, McKanna JA, Nikonenko IR, Skibo GG. Microglia and astrocytes in the adult rat brain: comparative immunocytochemical analysis demonstrates the efficacy of lipocortin 1 immunoreactivity. Neuroscience. 2000;96(1):195–203.
Tan YL, Yuan Y, Tian L. Microglial regional heterogeneity and its role in the brain. Mol Psychiatry. 2020;25(2):351–67.
Tay TL, Mai D, Dautzenberg J, Fernandez-Klett F, Lin G. Sagar, et al. a new fate mapping system reveals context-dependent random or clonal expansion of microglia. Nat Neurosci. 2017;20(6):793–803.
Verdonk F, Roux P, Flamant P, Fiette L, Bozza FA, Simard S, et al. Phenotypic clustering: a novel method for microglial morphology analysis. J Neuroinflammation. 2016;13(1):153.
Grabert K, Michoel T, Karavolos MH, Clohisey S, Baillie JK, Stevens MP, et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nat Neurosci. 2016;19(3):504–16.
Bottcher C, Schlickeiser S, Sneeboer MAM, Kunkel D, Knop A, Paza E, et al. Human microglia regional heterogeneity and phenotypes determined by multiplexed single-cell mass cytometry. Nat Neurosci. 2019;22(1):78–90.
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, et al. Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke. 2012;43(11):3063–70.
Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 2013;33(12):1864–74.
Perego C, Fumagalli S, De Simoni MG. Temporal pattern of expression and colocalization of microglia/macrophage phenotype markers following brain ischemic injury in mice. J Neuroinflammation. 2011;8:174.
Ransohoff RM. A polarizing question: do M1 and M2 microglia exist? Nat Neurosci. 2016;19(8):987–91.
Jassam YN, Izzy S, Whalen M, McGavern DB, El Khoury J. Neuroimmunology of traumatic brain injury: time for a paradigm shift. Neuron. 2017;95(6):1246–65.
Morganti JM, Riparip LK, Rosi S. Call off the dog(ma): M1/M2 polarization is concurrent following traumatic brain injury. PLoS One. 2016;11(1):e0148001.
Casella G, Garzetti L, Gatta AT, Finardi A, Maiorino C, Ruffini F, et al. IL4 induces IL6-producing M2 macrophages associated to inhibition of neuroinflammation in vitro and in vivo. J Neuroinflammation. 2016;13(1):139.
Lee J, Hamanaka G, Lo EH, Arai K. Heterogeneity of microglia and their differential roles in white matter pathology. CNS Neurosci Ther. 2019;25(12):1290–8.
Thiel A, Heiss WD. Imaging of microglia activation in stroke. Stroke. 2011;42(2):507–12.
Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13(7):420–33.
Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7(4):354–65.
Kluge MG, Abdolhoseini M, Zalewska K, Ong LK, Johnson SJ, Nilsson M, et al. Spatiotemporal analysis of impaired microglia process movement at sites of secondary neurodegeneration post-stroke. J Cereb Blood Flow Metab. 2019;39(12):2456–70.
Sarlus H, Heneka MT. Microglia in Alzheimer's disease. J Clin Invest. 2017;127(9):3240–9.
Voet S, Prinz M, van Loo G. Microglia in central nervous system inflammation and multiple sclerosis pathology. Trends Mol Med. 2019;25(2):112–23.
Jonsson T, Stefansson H, Steinberg S, Jonsdottir I, Jonsson PV, Snaedal J, et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med. 2013;368(2):107–16.
Hollingworth P, Harold D, Sims R, Gerrish A, Lambert JC, Carrasquillo MM, et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer's disease. Nat Genet. 2011;43(5):429–35.
Bradshaw EM, Chibnik LB, Keenan BT, Ottoboni L, Raj T, Tang A, et al. CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat Neurosci. 2013;16(7):848–50.
Thambisetty M, An Y, Nalls M, Sojkova J, Swaminathan S, Zhou Y, et al. Effect of complement CR1 on brain amyloid burden during aging and its modification by APOE genotype. Biol Psychiatry. 2013;73(5):422–8.
Qin C, Fan WH, Liu Q, Shang K, Murugan M, Wu LJ, et al. Fingolimod protects against ischemic White matter damage by modulating microglia toward M2 polarization via STAT3 pathway. Stroke. 2017;48(12):3336–46.
Chen S, Ye J, Chen X, Shi J, Wu W, Lin W, Lin W, Li Y, Fu H, Li S. Valproic acid attenuates traumatic spinal cord injury-induced inflammation via STAT1 and NF-κB pathway dependent of HDAC3. J Neuroinflammation. 2018;15(1):150.
Yang Y, Ju J, Deng M, Wang J, Liu H, Xiong L, et al. Hypoxia inducible factor 1alpha promotes endogenous adaptive response in rat model of chronic cerebral hypoperfusion. Int J Mol Sci. 2017;18(1):3.
Zhou S, Guo X, Chen S, Xu Z, Duan W, Zeng B. Apelin-13 regulates LPS-induced N9 microglia polarization involving STAT3 signaling pathway. Nuropeptides. 2019;76:101938. https://doi.org/10.1016/j.npep.2019.101938.
Li R, Liu W, Yin J, Chen Y, Guo S, Fan H, Li X, Zhang X, He X, Duan C. TSG-6 attenuates inflammation-induced brain injury via modulation of microglial polarization in SAH rats through the SOCS3/STAT3 pathway. J Neuroinflammation. 2018;15(1):231.
Cai W, Dai X, Chen J, Zhao J, Xu M, Zhang L, et al. STAT6/Arg1 promotes microglia/macrophage efferocytosis and inflammation resolution in stroke mice. JCI Insight. 2019;4(20):e131355.
Corona JC, Duchen MR. PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Radic Biol Med. 2016;100:153–63.
Ji J, Xue TF, Guo XD, Yang J, Guo RB, Wang J, et al. Antagonizing peroxisome proliferator-activated receptor gamma facilitates M1-to-M2 shift of microglia by enhancing autophagy via the LKB1-AMPK signaling pathway. Aging Cell. 2018;17(4):e12774.
Pisanu A, Lecca D, Mulas G, Wardas J, Simbula G, Spiga S, et al. Dynamic changes in pro- and anti-inflammatory cytokines in microglia after PPAR-gamma agonist neuroprotective treatment in the MPTPp mouse model of progressive Parkinson's disease. Neurobiol Dis. 2014;71:280–91.
Wang Y, Huang Y, Xu Y, Ruan W, Wang H, Zhang Y, et al. A dual AMPK/Nrf2 activator reduces brain inflammation after stroke by enhancing microglia M2 polarization. Antioxid Redox Signal. 2018;28(2):141–63.
Pilipović I, Stojić-Vukanić Z, Prijić I, Jasnić N, Leposavić G. Propranolol diminished severity of rat EAE by enhancing immunoregulatory/protective properties of spinal cord microglia. Neurobiol Dis. 2020;134:104665.
Park SY, Jin ML, Ko MJ, Park G, Choi Y-W. Anti-neuroinflammatory effect of Emodin in LPS-Stimulated Microglia: Involvement of AMPK/Nrf2 Activation. Neurochem Res. 2016;41(11):2981–92.
Yao L, Kan EM, Lu J, Hao A, Dheen ST, Kaur C, Ling E-A. Toll-like receptor 4 mediates microglial activation and production of inflammatory mediators in neonatal rat brain following hypoxia: role of TLR4 in hypoxic microglia. J Neuroinflammation. 2013;10:785.
Stivers NS, Pelisch N, Orem BC, Williams J, Nally JM, Stirling DP. The toll-like receptor 2 agonist Pam3CSK4 is neuroprotective after spinal cord injury. Exp Neurol. 2017;294:1–11.
Drouin-Ouellet J, St-Amour I, Saint-Pierre M, Lamontagne-Proulx J, Kriz J, Barker RA, Cicchetti F. Toll-like receptor expression in the blood and brain of patients and a mouse model of Parkinson´s disease. Int J Neuropsychopharmacol. 2014;18(6):pyu103.
Doorn KJ, Moors T, Drukarch B, van de Berg WD, Lucassen PJ, van Dam A-M. Microglial phenotypes and tolllike receptor 2 in the substantia nigra and hippocampus of incidental Lewy body disease cases and Parkinson´s disease patients. Acta Neuropathol Commun. 2014;2:90.
Stirling DP, Cummins K, Mishra M, Teo W, Yong VW, Stys P. Toll-like receptor 2-mediated alternative activation of microglia is protective after spinal cord injury. Brain. 2014;137(Pt 3):707–23.
Daniele SG, Béraud D, Davenport C, Cheng K, Yin H, Maguire-Zeiss KA. Activation of MyD88-dependent TLR1/2 signaling by misfolded α-synuclein, a protein linked to neurodegenerative disorders. Sci Signal. 2015;8(376):ra45.
Liu ZJ, Ran YY, Qie SY, Gong WJ, Gao FH, Ding ZT, et al. Melatonin protects against ischemic stroke by modulating microglia/macrophage polarization toward anti-inflammatory phenotype through STAT3 pathway. CNS Neurosci Ther. 2019;25(12):1353–62.
Go M, Kou J, Lim J-E, Yang J, Fukuchi K-I. Microglial response to LPS increases in wild-type mice during aging but diminishes in an Alzheimer’s mouse model: Implication of TLR4 signaling in disease progression. Biochem Biophys Res Commun. 2016;479(2):331–7.
Qin Y, Liu Y, Hao W, Decker Y, Tomic I, Menger MD, Liu C, Fassbender K. Stimulation of TLR4 attenuates Alzheimer’s disease-related symptoms and pathology in Tau-Transgenic Mice. J Immunol. 2016;197(8):3281–92.
Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31(1):33–40.
Ros-Bernal F, Hunot S, Herrero MT, Parnadeau S, Corvol J-C, Lu L, Alvarez-Fischer D, Carrillo-de Sauvage MA, Saurini F, Coussieu C, Kinugawa K, Prigent A, Höglinger G, Hamon M, Tronche F, Hirsch EC, Vyas S. Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc Natl Acad Sci U S A. 2011;108(16):6632–7.
Shao Q-H, Yan W-F, Zhang Z, Ma K-L, Peng S-Y, Cao Y-L, Yuan Y-H, Chen N-H. Nurr1: A vital participant in the TLR4-NF-κB signal pathway stimulated by α-synuclein in BV-2 cells. Neuropharmacology. 2019;144:388–99.
De Paola M, Sestito SE, Mariani A, Memo C, Fanelli R, Freschi M, Bendotti C, Calabrese V, Peri F. Synthetic and natural small molecule TLR4 antagonists inhibit motoneuron death in cultures from ALS mouse model. Pharmacol Res. 2016;103:180–7.
Zabala A, Vazquez-Villoldo N, Rissiek B, Gejo J, Martin A, Palomino A, et al. P2X4 receptor controls microglia activation and favors remyelination in autoimmune encephalitis. EMBO Mol Med. 2018;10(8):e8743.
Masuch A, Shieh C-H, van Rooijen N, van Calker D, Biber K. Mechanism of microglia neuroprotection: involvement of P2X7, TNFα, and valproic acid. Glia. 2016;64(1):76–89.
Choi JH, Lee CH, Hwang IK, Won MH, Seong JK, Yoon YS, et al. Age-related changes in ionized calcium-binding adapter molecule 1 immunoreactivity and protein level in the gerbil hippocampal CA1 region. J Vet Med Sci. 2007;69(11):1131–6.
Sun N, Shen Y, Han W, Shi K, Wood K, Fu Y, et al. Selective Sphingosine-1-phosphate receptor 1 modulation attenuates experimental intracerebral hemorrhage. Stroke. 2016;47(7):1899–906.
Lv M, Zhang D, Dai D, Zhang W, Zhang L. Sphingosine kinase 1/sphingosine-1-phosphate regulates the expression of interleukin-17A in activated microglia in cerebral ischemia/reperfusion. Inflamm Res. 2016;65(7):551–62.
Su D, Cheng Y, Li S, Dai D, Zhang W, Lv M. Sphk1 mediates neuroinflammation and neuronal injury via TRAF2/NF-κB pathways in activated microglia in cerebral ischemia reperfusion. J Neuroimmunol. 2017;305:35–41.
Nayak D, Huo Y, Kwang WXT, Pushparaj PN, Kumar SD, Ling E-A, Dheen ST. Sphingosine kinase 1 regulates the expression of proinflammatory cytokines and nitric oxide in activated microglia. Neuroscience. 2010;166(1):132–44.
Takahashi K, Prinz M, Stagi M, Chechneva O, Neumann H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 2007;4(4):e124.
Sieber MW, Jaenisch N, Brehm M, Guenther M, Linnartz-Gerlach B, Neumann H, et al. Attenuated inflammatory response in triggering receptor expressed on myeloid cells 2 (TREM2) knock-out mice following stroke. PLoS One. 2013;8(1):e52982.
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK, et al. A unique microglia type associated with restricting development of Alzheimer's disease. Cell. 2017;169(7):1276–90 e17.
Manich G, Recasens M, Valente T, Almolda B, Gonzalez B, Castellano B. Role of the CD200-CD200R Axis during homeostasis and Neuroinflammation. Neuroscience. 2019;405:118–36.
Zhao J, Mu H, Liu L, Jiang X, Wu D, Shi Y, et al. Transient selective brain cooling confers neurovascular and functional protection from acute to chronic stages of ischemia/reperfusion brain injury. J Cereb Blood Flow Metab. 2019;39(7):1215–31.
Wang X-J, Zhang S, Yan Z-Q, Zhao Y-X, Zhou H-Y, Wang Y, Lu G-Q, Zhang J-D. Impaired CD200-CD200R-mediated microglia silencing enhances midbrain dopaminergic neurodegeneration: roles of aging, superoxide, NADPH oxidase, and p38 MAPK. Free Radic Biol Med. 2011;50(9):1094–106.
Lago N, Pannunzio B, Amo-Aparicio J, Lopez-Vales R, Peluffo H. CD200 modulates spinal cord injury neuroinflammation and outcome through CD200R1. Brain Behav Immun. 2018;73:416–26.
Tang Z, Gan Y, Liu Q, Yin J-X, Liu Q, Shi J, Shi F-D. CX3CR1 deficiency suppresses activation and neurotoxicity of microglia/macrophage in experimental ischemic stroke. J Neuroinflammation. 2014;11:26.
Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nature Medicine. 2011;17(7):796–808.
Liu Y, Wu XM, Luo QQ, Huang S, Yang QW, Wang FX, et al. CX3CL1/CX3CR1-mediated microglia activation plays a detrimental role in ischemic mice brain via p38MAPK/PKC pathway. J Cereb Blood Flow Metab. 2015;35(10):1623–31.
Donnelly DJ, Longbrake EE, Shawler TM, Kigerl KA, Lai W, Tovar CA, Ransohoff RM, Popovich PG. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. J Neurosci. 2011;31(27):9910–22.
Garcia JA, Pino PA, Mizutani M, Cardona SM, Charo IF, Ransohoff RM, et al. Regulation of adaptive immunity by the fractalkine receptor during autoimmune inflammation. J Immunol. 2013;191(3):1063–72.
Stojković L, Djurić T, Stanković A, Dinčić E, Stančić O, Veljković N, Alavantić D, Zivković M. The association of V249I and T280M fractalkine receptor haplotypes with disease course of multiple sclerosis. J Neuroimmunol. 2012;245:87–92.
Lukiw WJ, Pogue AI. Vesicular transport of encapsulated microRNA between glial and neuronal cells. Int J Mol Sci. 2020;21(14):5078.
Zheng X, Huang H, Liu J, Li M, Liu M, Luo T. Propofol attenuates inflammatory response in LPS-activated microglia by regulating the miR-155/SOCS1 pathway. Inflammation. 2018;41(1):11–9.
Hamzei Taj S, Kho W, Aswendt M, Collmann FM, Green C, Adamczak J, et al. Dynamic modulation of microglia/macrophage polarization by miR-124 after focal cerebral ischemia. J NeuroImmune Pharmacol. 2016;11(4):733–48.
Shao Y, Deng T, Zhang T, Li P, Wang Y. FAM19A3, a novel secreted protein, modulates the microglia/macrophage polarization dynamics and ameliorates cerebral ischemia. FEBS Lett. 2015;589(4):467–75.
Wu LJ, Wu G, Akhavan Sharif MR, Baker A, Jia Y, Fahey FH, et al. The voltage-gated proton channel Hv1 enhances brain damage from ischemic stroke. Nat Neurosci. 2012;15(4):565–73.
Nguyen HM, Grossinger EM, Horiuchi M, Davis KW, Jin LW, Maezawa I, et al. Differential Kv1.3, KCa3.1, and Kir2.1 expression in "classically" and "alternatively" activated microglia. Glia. 2017;65(1):106–21.
Wu J, Sun L, Li H, Shen H, Zhai W, Yu Z, et al. Roles of programmed death protein 1/programmed death-ligand 1 in secondary brain injury after intracerebral hemorrhage in rats: selective modulation of microglia polarization to anti-inflammatory phenotype. J Neuroinflammation. 2017;14(1):36.
Hongwei Qin ATH, Liu Y, Reynolds SL, Yanagisawa LL, Benveniste EN. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J Immunol. 2012;189(7):3439–48.
Koscso B, Csoka B, Kokai E, Nemeth ZH, Pacher P, Virag L, et al. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J Leukoc Biol. 2013;94(6):1309–15.
Guo M, Li C, Lei Y, Xu S, Zhao D, Lu XY. Role of the adipose PPARgamma-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol Psychiatry. 2017;22(7):1056–68.
Zhao Q, Wu X, Yan S, Xie X, Fan Y, Zhang J, et al. The antidepressant-like effects of pioglitazone in a chronic mild stress mouse model are associated with PPARgamma-mediated alteration of microglial activation phenotypes. J Neuroinflammation. 2016;13(1):259.
Choi MJ, Lee EJ, Park JS, Kim SN, Park EM, Kim HS. Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: critical role of PPAR-gamma signaling pathway. Biochem Pharmacol. 2017;144:120–31.
Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, et al. Pivotal role for neuronal toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104(34):13798–803.
Cao CX, Yang QW, Lv FL, Cui J, Fu HB, Wang JZ. Reduced cerebral ischemia-reperfusion injury in toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353(2):509–14.
Hua F, Ma J, Ha T, Kelley JL, Kao RL, Schweitzer JB, et al. Differential roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion injury in mice. Brain Res. 2009;1262:100–8.
Qiao H, Zhang X, Zhu C, Dong L, Wang L, Zhang X, et al. Luteolin downregulates TLR4, TLR5, NF-kappaB and p-p38MAPK expression, upregulates the p-ERK expression, and protects rat brains against focal ischemia. Brain Res. 2012;1448:71–81.
Cui W, Sun C, Ma Y, Wang S, Wang X, Zhang Y. Inhibition of TLR4 induces M2 microglial polarization and provides neuroprotection via the NLRP3 Inflammasome in Alzheimer's disease. Front Neurosci. 2020;14:444.
Beamer E, Goloncser F, Horvath G, Beko K, Otrokocsi L, Kovanyi B, et al. Purinergic mechanisms in neuroinflammation: An update from molecules to behavior. Neuropharmacology. 2016;104:94–104.
He Y, Taylor N, Fourgeaud L, Bhattacharya A. The role of microglial P2X7: modulation of cell death and cytokine release. J Neuroinflammation. 2017;14(1):135.
Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K. Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediat Inflamm. 2016;2016:8606878.
Konishi H, Kiyama H. Microglial TREM2/DAP12 signaling: a double-edged sword in neural diseases. Front Cell Neurosci. 2018;12:206.
Takahashi K, Rochford CD, Neumann H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. J Exp Med. 2005;201(4):647–57.
Krasemann S, Madore C, Cialic R, Baufeld C, Calcagno N, El Fatimy R, et al. The TREM2-APOE pathway drives the transcriptional phenotype of dysfunctional microglia in neurodegenerative diseases. Immunity. 2017;47(3):566–81 e9.
Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001;102(2):173–9.
Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol. 2003;171(6):3034–46.
Feng Z, Ye L, Klebe D, Ding Y, Guo ZN, Flores JJ, et al. Anti-inflammation conferred by stimulation of CD200R1 via Dok1 pathway in rat microglia after germinal matrix hemorrhage. J Cereb Blood Flow Metab. 2019;39(1):97–107.
Zhao X, Li J, Sun H. CD200-CD200R interaction: An important regulator after stroke. Front Neurosci. 2019;13:840.
Bazan JF, Bacon KB, Hardiman G, Wang W, Soo K, Rossi D, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385(6617):640–4.
Zhang G, Ye M, Li M. Deregulated miR-384 serves as a biomarker in neonatal hypoxic-ischemic encephalopathy and alleviates microglia-mediated neuroinflammation. Mol Biol Rep. 2020;47(7):5411–20.
Liu GJ, Zhang QR, Gao X, Wang H, Tao T, Gao YY, et al. MiR-146a ameliorates hemoglobin-induced microglial inflammatory response via TLR4/IRAK1/TRAF6 associated pathways. Front Neurosci. 2020;14:311.
Zhou HJ, Wang LQ, Wang DB, Yu JB, Zhu Y, Xu QS, et al. Long noncoding RNA MALAT1 contributes to inflammatory response of microglia following spinal cord injury via the modulation of a miR-199b/IKKbeta/NF-kappaB signaling pathway. Am J Phys Cell Physiol. 2018;315(1):C52–61.
Bernstein DL, Zuluaga-Ramirez V, Gajghate S, Reichenbach NL, Polyak B, Persidsky Y, et al. miR-98 reduces endothelial dysfunction by protecting blood-brain barrier (BBB) and improves neurological outcomes in mouse ischemia/reperfusion stroke model. J Cereb Blood Flow Metab. 2020;40(10):1953–65.
Sun Y, Luo ZM, Guo XM, Su DF, Liu X. An updated role of microRNA-124 in central nervous system disorders: a review. Front Cell Neurosci. 2015;9:193.
Yao L, Zhu Z, Wu J, Zhang Y, Zhang H, Sun X, et al. MicroRNA-124 regulates the expression of p62/p38 and promotes autophagy in the inflammatory pathogenesis of Parkinson's disease. FASEB J. 2019;33(7):8648–65.
Yao L, Ye Y, Mao H, Lu F, He X, Lu G, et al. MicroRNA-124 regulates the expression of MEKK3 in the inflammatory pathogenesis of Parkinson's disease. J Neuroinflammation. 2018;15(1):13.
Yang Y, Ye Y, Kong C, Su X, Zhang X, Bai W, et al. MiR-124 enriched exosomes promoted the M2 polarization of microglia and enhanced Hippocampus neurogenesis after traumatic brain injury by inhibiting TLR4 pathway. Neurochem Res. 2019;44(4):811–28.
Periyasamy P, Liao K, Kook YH, Niu F, Callen SE, Guo ML, et al. Cocaine-mediated downregulation of miR-124 activates microglia by targeting KLF4 and TLR4 signaling. Mol Neurobiol. 2018;55(4):3196–210.
Zhou X, Chen J, Tao H, Cai Y, Huang L, Zhou H, et al. Intranasal delivery of miR-155-5p Antagomir alleviates acute seizures likely by inhibiting hippocampal inflammation. Neuropsychiatr Dis Treat. 2020;16:1295–307.
Zhang L, Liu C, Huang C, Xu X, Teng J. miR-155 knockdown protects against cerebral ischemia and reperfusion Injury by targeting MafB. Biomed Res Int. 2020;2020:6458204. https://doi.org/10.1155/2020/6458204.
Pena-Philippides JC, Caballero-Garrido E, Lordkipanidze T, Roitbak T. In vivo inhibition of miR-155 significantly alters post-stroke inflammatory response. J Neuroinflammation. 2016;13(1):287.
Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, et al. Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med. 2019;381(17):1644–52.
Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11(10):889–96.
Chen ZQ, Yu H, Li HY, Shen HT, Li X, Zhang JY, et al. Negative regulation of glial Tim-3 inhibits the secretion of inflammatory factors and modulates microglia to antiinflammatory phenotype after experimental intracerebral hemorrhage in rats. CNS Neurosci Ther. 2019;25(6):674–84.
Galloway DA, Phillips AEM, Owen DRJ, Moore CS. Phagocytosis in the brain: homeostasis and disease. Front Immunol. 2019;10:790.
Venezia S, Refolo V, Polissidis A, Stefanis L, Wenning GK, Stefanova N. Toll-like receptor 4 stimulation with monophosphoryl lipid a ameliorates motor deficits and nigral neurodegeneration triggered by extraneuronal alpha-synucleinopathy. Mol Neurodegener. 2017;12(1):52.
Rajbhandari L, Tegenge MA, Shrestha S, Ganesh Kumar N, Malik A, Mithal A, et al. Toll-like receptor 4 deficiency impairs microglial phagocytosis of degenerating axons. Glia. 2014;62(12):1982–91.
Fourgeaud L, Traves PG, Tufail Y, Leal-Bailey H, Lew ED, Burrola PG, et al. TAM receptors regulate multiple features of microglial physiology. Nature. 2016;532(7598):240–4.
Tufail Y, Cook D, Fourgeaud L, Powers CJ, Merten K, Clark CL, et al. Phosphatidylserine exposure controls viral innate immune responses by microglia. Neuron. 2017;93(3):574–86 e8.
Han J, Wang M, Ren M, Lou H. Contributions of triggering-receptor-expressed-on-myeloid-cells-2 to neurological diseases. Int J Neurosci. 2017;127(4):368–75.
Kurisu K, Zheng Z, Kim JY, Shi J, Kanoke A, Liu J, et al. Triggering receptor expressed on myeloid cells-2 expression in the brain is required for maximal phagocytic activity and improved neurological outcomes following experimental stroke. J Cereb Blood Flow Metab. 2019;39(10):1906–18.
Arandjelovic S, Ravichandran KS. Phagocytosis of apoptotic cells in homeostasis. Nat Immunol. 2015;16(9):907–17.
Fukumoto Y, Tanaka KF, Parajuli B, Shibata K, Yoshioka H, Kanemaru K, et al. Neuroprotective effects of microglial P2Y1 receptors against ischemic neuronal injury. J Cereb Blood Flow Metab. 2019;39(11):2144–56.
Fricker M, Neher JJ, Zhao JW, Thery C, Tolkovsky AM, Brown GC. MFG-E8 mediates primary phagocytosis of viable neurons during neuroinflammation. J Neurosci. 2012;32(8):2657–66.
Durafourt BA, Moore CS, Zammit DA, Johnson TA, Zaguia F, Guiot MC, et al. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia. 2012;60(5):717–27.
Greenhalgh AD, David S. Differences in the phagocytic response of microglia and peripheral macrophages after spinal cord injury and its effects on cell death. J Neurosci. 2014;34(18):6316–22.
Hughes MM, Field RH, Perry VH, Murray CL, Cunningham C. Microglia in the degenerating brain are capable of phagocytosis of beads and of apoptotic cells, but do not efficiently remove PrPSc, even upon LPS stimulation. Glia. 2010;58(16):2017–30.
Janda E, Boi L, Carta AR. Microglial phagocytosis and its regulation: a therapeutic target in Parkinson's disease? Front Mol Neurosci. 2018;11:144.
da Fonseca AC, Matias D, Garcia C, Amaral R, Geraldo LH, Freitas C, et al. The impact of microglial activation on blood-brain barrier in brain diseases. Front Cell Neurosci. 2014;8:362.
Faustino J, Chip S, Derugin N, Jullienne A, Hamer M, Haddad E, et al. CX3CR1-CCR2-dependent monocyte-microglial signaling modulates neurovascular leakage and acute injury in a mouse model of childhood stroke. J Cereb Blood Flow Metab. 2019;39(10):1919–35.
Yang Y, Salayandia VM, Thompson JF, Yang LY, Estrada EY, Yang Y. Attenuation of acute stroke injury in rat brain by minocycline promotes blood-brain barrier remodeling and alternative microglia/macrophage activation during recovery. J Neuroinflammation. 2015;12:26.
Lou N, Takano T, Pei Y, Xavier AL, Goldman SA, Nedergaard M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A. 2016;113(4):1074–9.
Kitayama M, Ueno M, Itakura T, Yamashita T. Activated microglia inhibit axonal growth through RGMa. PLoS One. 2011;6(9):e25234.
Horn KP, Busch SA, Hawthorne AL, van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J Neurosci. 2008;28(38):9330–41.
Cai Z, Pang Y, Lin S, Rhodes PG. Differential roles of tumor necrosis factor-alpha and interleukin-1 beta in lipopolysaccharide-induced brain injury in the neonatal rat. Brain Res. 2003;975(1–2):37–47.
Elberg G, Liraz-Zaltsman S, Reichert F, Matozaki T, Tal M, Rotshenker S. Deletion of SIRPalpha (signal regulatory protein-alpha) promotes phagocytic clearance of myelin debris in Wallerian degeneration, axon regeneration, and recovery from nerve injury. J Neuroinflammation. 2019;16(1):277.
Hilla AM, Diekmann H, Fischer D. Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. J Neurosci. 2017;37(25):6113–24.
Eyo UB, Wu LJ. Bidirectional microglia-neuron communication in the healthy brain. Neural Plast. 2013;2013:456857.
Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29(13):3974–80.
Sandvig I, Augestad IL, Haberg AK, Sandvig A. Neuroplasticity in stroke recovery. The role of microglia in engaging and modifying synapses and networks. Eur J Neurosci. 2018;47(12):1414–28.
Chen Z, Jalabi W, Hu W, Park HJ, Gale JT, Kidd GJ, et al. Microglial displacement of inhibitory synapses provides neuroprotection in the adult brain. Nat Commun. 2014;5:4486.
Freria CM, Hall JC, Wei P, Guan Z, McTigue DM, Popovich PG. Deletion of the fractalkine receptor, CX3CR1, improves endogenous repair, axon sprouting, and synaptogenesis after spinal cord injury in mice. J Neurosci. 2017;37(13):3568–87.
Choi JY, Kim JY, Kim JY, Park J, Lee WT, Lee JE. M2 phenotype microglia-derived cytokine stimulates proliferation and neuronal differentiation of endogenous stem cells in ischemic brain. Exp Neurobiol. 2017;26(1):33–41.
Sun G, Miao Z, Ye Y, Zhao P, Fan L, Bao Z, et al. Curcumin alleviates neuroinflammation, enhances hippocampal neurogenesis, and improves spatial memory after traumatic brain injury. Brain Res Bull. 2020;162:84–93.
Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci U S A. 2003;100(23):13632–7.
Zhang J, He H, Qiao Y, Zhou T, He H, Yi S, et al. Priming of microglia with IFN-gamma impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia. 2020. https://doi.org/10.1002/glia.23878.
Medina RJ, O'Neill CL, O'Doherty TM, Knott H, Guduric-Fuchs J, Gardiner TA, et al. Myeloid angiogenic cells act as alternative M2 macrophages and modulate angiogenesis through interleukin-8. Mol Med. 2011;17(9–10):1045–55.
Jiang X, Andjelkovic AV, Zhu L, Yang T, Bennett MVL, Chen J, et al. Blood-brain barrier dysfunction and recovery after ischemic stroke. Prog Neurobiol. 2018;163–164:144–71.
Fan Y, Ding S, Sun Y, Zhao B, Pan Y, Wan J. MiR-377 regulates inflammation and angiogenesis in rats after cerebral ischemic injury. J Cell Biochem. 2018;119(1):327–37.
Ding X, Gu R, Zhang M, Ren H, Shu Q, Xu G, et al. Microglia enhanced the angiogenesis, migration and proliferation of co-cultured RMECs. BMC Ophthalmol. 2018;18(1):249.
Jiang X, Pu H, Hu X, Wei Z, Hong D, Zhang W, et al. A post-stroke therapeutic regimen with omega-3 polyunsaturated fatty acids that promotes white matter integrity and beneficial microglial responses after cerebral ischemia. Transl Stroke Res. 2016;7(6):548–61.
Fowler JH, McQueen J, Holland PR, Manso Y, Marangoni M, Scott F, et al. Dimethyl fumarate improves white matter function following severe hypoperfusion: involvement of microglia/macrophages and inflammatory mediators. J Cereb Blood Flow Metab. 2018;38(8):1354–70.
Miron VE, Boyd A, Zhao JW, Yuen TJ, Ruckh JM, Shadrach JL, et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16(9):1211–8.
Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45(2):208–12.
Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011;10(2):263–76.
Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain Behav Immun. 2009;23(3):309–17.
Marschallinger J, Iram T, Zardeneta M, Lee SE, Lehallier B, Haney MS, et al. Lipid-droplet-accumulating microglia represent a dysfunctional and proinflammatory state in the aging brain. Nat Neurosci. 2020;23(2):194–208.
Flowers A, Bell-Temin H, Jalloh A, Stevens SM Jr, Bickford PC. Proteomic anaysis of aged microglia: shifts in transcription, bioenergetics, and nutrient response. J Neuroinflammation. 2017;14(1):96.
Shi L, Rocha M, Zhang W, Jiang M, Li S, Ye Q, et al. Genome-wide transcriptomic analysis of microglia reveals impaired responses in aged mice after cerebral ischemia. J Cereb Blood Flow Metab. 2020:271678X20925655. https://doi.org/10.1177/0271678X20925655.
Olah M, Patrick E, Villani AC, Xu J, White CC, Ryan KJ, et al. A transcriptomic atlas of aged human microglia. Nat Commun. 2018;9(1):539.
Jiang L, Mu H, Xu F, Xie D, Su W, Xu J, et al. Transcriptomic and functional studies reveal undermined chemotactic and angiostimulatory properties of aged microglia during stroke recovery. J Cereb Blood Flow Metab. 2020:271678X20902542. https://doi.org/10.1177/0271678X20902542.
Ritzel RM, Doran SJ, Glaser EP, Meadows VE, Faden AI, Stoica BA, et al. Old age increases microglial senescence, exacerbates secondary neuroinflammation, and worsens neurological outcomes after acute traumatic brain injury in mice. Neurobiol Aging. 2019;77:194–206.
Koellhoffer EC, McCullough LD, Ritzel RM. Old maids: aging and its impact on microglia function. Int J Mol Sci. 2017;18(4):769.
Hu MY, Lin YY, Zhang BJ, Lu DL, Lu ZQ, Cai W. Update of inflammasome activation in microglia/macrophage in aging and aging-related disease. CNS Neurosci Ther. 2019;25(12):1299–307.
Son Y, Jeong YJ, Shin NR, Oh SJ, Nam KR, Choi HD, et al. Inhibition of colony-stimulating factor 1 Receptor by PLX3397 prevents amyloid beta pathology and rescues dopaminergic signaling in aging 5xFAD mice. Int J Mol Sci. 2020;21(15):5553.
Hickman SE, Allison EK, El Khoury J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer's disease mice. J Neurosci. 2008;28(33):8354–60.
Ries M, Sastre M. Mechanisms of abeta clearance and degradation by glial cells. Front Aging Neurosci. 2016;8:160.
Krabbe G, Halle A, Matyash V, Rinnenthal JL, Eom GD, Bernhardt U, et al. Functional impairment of microglia coincides with Beta-amyloid deposition in mice with Alzheimer-like pathology. PLoS One. 2013;8(4):e60921.
Roy-O'Reilly M, McCullough LD. Sex differences in stroke: the contribution of coagulation. Exp Neurol. 2014;259:16–27.
Kerr N, Dietrich DW, Bramlett HM, Raval AP. Sexually dimorphic microglia and ischemic stroke. CNS Neurosci Ther. 2019;25(12):1308–17.
Schwarz JM, Sholar PW, Bilbo SD. Sex differences in microglial colonization of the developing rat brain. J Neurochem. 2012;120(6):948–63.
Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J Neurosci. 2013;33(7):2761–72.
Loram LC, Sholar PW, Taylor FR, Wiesler JL, Babb JA, Strand KA, et al. Sex and estradiol influence glial pro-inflammatory responses to lipopolysaccharide in rats. Psychoneuroendocrinology. 2012;37(10):1688–99.
Villa A, Gelosa P, Castiglioni L, Cimino M, Rizzi N, Pepe G, et al. Sex-specific features of microglia from adult mice. Cell Rep. 2018;23(12):3501–11.
Villa A, Vegeto E, Poletti A, Maggi A. Estrogens, neuroinflammation, and neurodegeneration. Endocr Rev. 2016;37(4):372–402.
Hanamsagar R, Bilbo SD. Sex differences in neurodevelopmental and neurodegenerative disorders: focus on microglial function and neuroinflammation during development. J Steroid Biochem Mol Biol. 2016;160:127–33.
Li F, Liu L. Comparison of kainate-induced seizures, cognitive impairment and hippocampal damage in male and female mice. Life Sci. 2019;232:116621.
Villapol S, Loane DJ, Burns MP. Sexual dimorphism in the inflammatory response to traumatic brain injury. Glia. 2017;65(9):1423–38.
Jia C, Malone HM, Keasey MP, Lovins C, Elam J, Hagg T. Blood Vitronectin induces detrimental brain Interleukin-6 and correlates with outcomes after stroke only in female mice. Stroke. 2020;51(5):1587–95.
Habib P, Beyer C. Regulation of brain microglia by female gonadal steroids. J Steroid Biochem Mol Biol. 2015;146:3–14.
de Rivero Vaccari JP, Bramlett HM, Perez-Pinzon MA, Raval AP. Estrogen preconditioning: a promising strategy to reduce inflammation in the ischemic brain. Cond Med. 2019;2(3):106–13.
Shang K, He J, Zou J, Qin C, Lin L, Zhou LQ, et al. Fingolimod promotes angiogenesis and attenuates ischemic brain damage via modulating microglial polarization. Brain Res. 1726;2020:146509.
Han L, Cai W, Mao L, Liu J, Li P, Leak RK, et al. Rosiglitazone promotes white matter integrity and long-term functional recovery after focal cerebral ischemia. Stroke. 2015;46(9):2628–36.
Liu R, Diao J, He S, Li B, Fei Y, Li Y, et al. XQ-1H protects against ischemic stroke by regulating microglia polarization through PPARgamma pathway in mice. Int Immunopharmacol. 2018;57:72–81.
Tian DS, Li CY, Qin C, Murugan M, Wu LJ, Liu JL. Deficiency in the voltage-gated proton channel Hv1 increases M2 polarization of microglia and attenuates brain damage from photothrombotic ischemic stroke. J Neurochem. 2016;139(1):96–105.
Chen F, Weng Z, Xia Q, Cao C, Leak RK, Han L, et al. Intracerebroventricular delivery of recombinant NAMPT deters inflammation and protects against cerebral ischemia. Transl Stroke Res. 2019;10(6):719–28.
Cai W, Liu S, Hu M, Sun X, Qiu W, Zheng S, et al. Post-stroke DHA treatment protects against acute ischemic brain injury by skewing macrophage polarity toward the M2 phenotype. Transl Stroke Res. 2018;9(6):669–80.
Haber M, James J, Kim J, Sangobowale M, Irizarry R, Ho J, et al. Minocycline plus N-acteylcysteine induces remyelination, synergistically protects oligodendrocytes and modifies neuroinflammation in a rat model of mild traumatic brain injury. J Cereb Blood Flow Metab. 2018;38(8):1312–26.
Shi H, Chen D, Gao Y. Potential role of omega-3 polyunsaturated fatty acids in ischemic stroke: review on clinical and preclinical studies. Cond Med. 2019;2(4):152–63.
Chernykh ER, Shevela EY, Starostina NM, Morozov SA, Davydova MN, Menyaeva EV, et al. Safety and therapeutic potential of M2 macrophages in stroke treatment. Cell Transplant. 2016;25(8):1461–71.
Waisman A, Ginhoux F, Greter M, Bruttger J. Homeostasis of microglia in the adult brain: review of novel microglia depletion systems. Trends Immunol. 2015;36(10):625–36.
Han J, Harris RA, Zhang XM. An updated assessment of microglia depletion: current concepts and future directions. Mol Brain. 2017;10(1):25.
Wu W, Li Y, Wei Y, Bosco DB, Xie M, Zhao MG, et al. Microglial depletion aggravates the severity of acute and chronic seizures in mice. Brain Behav Immun. 2020;S0889–1591(20):30264–6.
Jin WN, Shi SX, Li Z, Li M, Wood K, Gonzales RJ, et al. Depletion of microglia exacerbates postischemic inflammation and brain injury. J Cereb Blood Flow Metab. 2017;37(6):2224–36.
Huang Y, Xu Z, Xiong S, Sun F, Qin G, Hu G, et al. Repopulated microglia are solely derived from the proliferation of residual microglia after acute depletion. Nat Neurosci. 2018;21(4):530–40.
Varvel NH, Grathwohl SA, Baumann F, Liebig C, Bosch A, Brawek B, et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc Natl Acad Sci U S A. 2012;109(44):18150–5.
Lund H, Pieber M, Parsa R, Han J, Grommisch D, Ewing E, et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat Commun. 2018;9(1):4845.
Elmore MRP, Hohsfield LA, Kramar EA, Soreq L, Lee RJ, Pham ST, et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell. 2018;17(6):e12832.
Willis EF, MacDonald KPA, Nguyen QH, Garrido AL, Gillespie ER, Harley SBR, et al. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell. 2020;180(5):833–46 e16.
Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB, et al. Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. J Neurosci. 2020;40(14):2960–74.
Coleman LG Jr, Zou J, Crews FT. Microglial depletion and repopulation in brain slice culture normalizes sensitized proinflammatory signaling. J Neuroinflammation. 2020;17(1):27.
Priller J, Prinz M. Targeting microglia in brain disorders. Science. 2019;365(6448):32–3.
Lan X, Han X, Liu X, Wang J. Inflammatory responses after intracerebral hemorrhage: from cellular function to therapeutic targets. J Cereb Blood Flow Metab. 2019;39(1):184–6.
Acknowledgments
The authors thank Patricia Strickler for administrative support.
Funding
This project was supported by the University of Pittsburgh School of Medicine. X.J. is supported by the American Heart Association Postdoctoral Fellowship 18POST33960201. Y.S. is supported by the American Heart Association grant 17SDG33630130, the Competitive Medical Research Fund of the University of Pittsburgh Medical Center Health System, and the Pittsburgh Institute of Brain Disorders & Recovery startup funds. J.C. is the Richard King Mellon Professor of Neurology and a recipient of a VA Senior Research Career Scientist Award. J.C. and X.H. are also supported by VA Merit Review grants I01BX002495, I01BX003377, and I01BX003651.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lyu, J., Jiang, X., Leak, R.K. et al. Microglial Responses to Brain Injury and Disease: Functional Diversity and New Opportunities. Transl. Stroke Res. 12, 474–495 (2021). https://doi.org/10.1007/s12975-020-00857-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-020-00857-2