Abstract
It has recently emerged the concept of “obesity paradox,” a term used to describe the unexpected improved prognosis and lower mortality rates found in several diseases in patients with higher body weight. Concerning stroke, few clinical studies have assessed this obesity paradox showing contradictory results. Therefore, our aim was to compare clinical evolution and inflammatory balance of obese and non-obese patients after ischemic stroke. We designed a prospective case-control study in patients with acute ischemic stroke categorized into obese (body mass index, BMI ≥ 30 kg/m2) and non-obese (BMI < 30 kg/m2). We compared clinical, anthropometric, radiological, and laboratory variables. The main outcome variable was the functional outcome at 3 months. We included 98 patients (48 non-obese and 50 obese). No differences in functional outcome at 3 months were found (p = 0.882) although a tendency of a greater recovery on neurological impairments was seen in obese subjects. Importantly, obese patients (p = 0.007) and patients who experienced poor outcome (p = 0.006) exhibited a higher reduction in body weight at 3 months after stroke. Moreover, pro-inflammatory IL-6 levels (p = 0.002) were higher in the obese group. However, IL-6 levels decreased over the first week in obese while increased in non-obese. On the contrary, levels of the anti-inflammatory IL-10 rose over the first week in obese patients, whereas remained stable in non-obese. In summary, despite exhibiting several factors associated with poor outcome, obese patients do not evolve worse than non-obese after ischemic stroke. Obesity may counterbalance the inflammatory reaction through an anti-inflammatory stream enhanced in the first moments of stroke.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a rising public health problem that has reached pandemic proportions in the last decades [1]. It has traditionally been considered an indicator of poor health. However, it has recently emerged the concept of “obesity paradox,” a term used to describe the unexpected improved prognosis and lower mortality rates found in several diseases in patients with higher body weight [2,3,4,5,6,7]. Concerning stroke, few clinical studies have assessed this obesity paradox showing contradictory results [8,9,10,11,12]. Therefore, whether this obesity paradox actually exists is unclear and very little data is known on potential mechanisms that could explain such association. In this respect, inflammation could be an issue of special importance. Obesity generates a low-grade inflammatory response known as “metainflammation” [13], where immune cells, cytokines, chemokines, or adipokines are involved. This immune activation might exert a determinant role since elements of both innate and adaptive immunity participate in all phases of the ischemic cascade during stroke [14]. The way obesity influences the inflammatory response in ischemic stroke is not known and might provide answers on how excess body weight affects the outcome. In this sense, we hypothesize that obese patients counteract their morbidity through the increased expression of anti-inflammatory cytokines. To evaluate this, we compared the functional outcome and the pro- and anti-inflammatory cytokines expression in obese and non-obese patients with ischemic stroke.
Materials and Methods
Study Design and Patients’ Characteristics
We performed a prospective case-control study in patients with a first-ever ischemic stroke within 24 h from symptom onset. Patients were admitted to the Stroke Unit of a single center from January 2014 to September 2016. Participants were categorized into two groups: (a) cases, obese patients, subjects with a body mass index (BMI) ≥ 30 kg/m2; and (b) controls, non-obese patients, subjects with a BMI ≤30 kg/m2. The inclusion was consecutive with a 1:1 proportion. The exclusion criteria were as follows: previous disability (defined as the modified Rankin scale score (mRS) ≥ 2), chronic inflammatory disease, cancer, severe systemic disease which determines a life expectancy lower than 6 months, infectious disease in the last 15 days, and continuous anti-inflammatory drugs intake in the last 15 days.
Standard Protocol Approvals, Registrations, and Patient Consents
This research was carried out in accordance with the Declaration of Helsinki of the World Medical Association (2008) and approved by the Ethics Committee of the Servizo Galego de Saúde. Informed consent was obtained from all individual participants included in the study. If patients were not able to sign, relatives gave signed informed consent.
Clinical Variables
All patients were admitted to an acute Stroke Unit and treated according to the European Stroke Organization and the Spanish Neurological Society guidelines [15, 16]. The patients were evaluated during hospital stay and at 3 months after stroke onset. In accordance with the protocol of our Stroke Unit, every patient with alimentation difficulties was evaluated and followed by the Nutrition Unit from Endocrinology Department to maintain and adequate nutritional status during their stay at Hospital and at discharge. We collected demographic variables, previous medical history, vital signs, anthropometric variables, presence of infections during hospitalization, severity of neurological impairments, and functional disability. Axillary temperature was registered every 6 h during the first 48 h with a monitoring probe. Height and basal body weight (dressed with underwear or completely nude) were measured during hospitalization if patient was able to stand upright; if not, were obtained from Primary Care registries; if not present, the patient or his family were asked for it; if not known, were estimated using the Chumlea et al. [17, 18] formulas for men and women based in body measurements. Body weight was also recorded at 3 months after stroke. BMI was calculated according to the formula, weight (kg)/height (m2), and classified according to World Health Organization cut-off points [19]. Stroke subtype was classified according to the TOAST criteria [20]. To assess the severity of neurological impairments and the functional disability, the NIH Stroke Scale (NIHSS) and the mRS were respectively measured on admission, at discharge, and at 3 months of follow-up by a certified neurologist.
Neuroimaging Variables
Brain CT studies were performed at admission and between 4th to 7th day. We registered the presence of hemorrhagic transformation and infarct volumes in 4th- to 7th-day follow-up CT. Lesion volume was calculated by using the formula 0.5 × A × B × C, where A and B represent the largest perpendicular diameters and C the thickness of the cut [21]. The same neurologist, blinded to clinical or laboratory results, evaluated all neuroimaging tests.
Laboratory Test
Blood samples for routine laboratory tests were extracted on admission and were assessed in the central laboratory of the hospital. For the molecular determinations, venous blood samples were collected in Vacutainer tubes (Becton Dickinson, San Jose, CA, USA) at admission, 72 ± 24 h and 7 ± 1 days from stroke onset. After allowing to clot for 60 min, the blood samples were centrifuged at 3000g for 10 min, and the serum was immediately aliquoted, frozen, and stored at − 80 °C until analysis. With the aim to assess the inflammatory profile, we analyzed the serum levels of the pro-inflammatory cytokine IL-6 and the anti-inflammatory cytokine IL-10. We selected these cytokines because their role in ischemic stroke has been widely studied and is known in depth [22,23,24,25], in addition to being markers with which we have an extensive experience in our laboratory [26,27,28,29,30,31]. Cytokines determinations were performed by using the immunodiagnostic IMMULITE 1000 System (Siemens Healthcare Global, Los Angeles, CA, USA). The intra-assay and inter-assay coefficients of variation were < 5%. Determinations were performed in a laboratory blinded to clinical and neuroimaging data.
Outcome Variables
The first primary endpoint was the functional outcome at 3 months, dichotomized into good outcome (mRS ≤ 2) and poor outcome (mRS > 2). The second primary objective was to determine the inflammatory balance in obese and non-obese ischemic stroke patients and its influence in the functional outcome. With this aim, after the analysis of the serum levels of IL-6 and IL-10, we defined an index named as “anti/pro-inflammatory index” in every patient on admission, at 72 h and at 7th day, according to the following formula: the decile of IL-10 divided by the decile of IL-6.
Among the secondary objectives, we analyzed the clinical improvement at 3 months defined as follows: NIHSS on admission minus NIHSS at 3 months, divided by NIHSS on admission, and multiplied by 100. If the patient was dead at 3 months after stroke, a clinical improvement of − 100% was established. In addition, the body weight evolution after stroke and its influence in functional outcome were also assessed. We defined the body weight difference at 3 months as basal body weight minus body weight at 3 months after stroke. Finally, since hyperthermia is one of the most potent predictors of poor outcome in acute ischemic stroke [32], we also decided to compare axillary temperatures between obese and non-obese patients in the acute phase and their role in prognosis depending on BMI. For this analysis, we selected the mean temperature over the first 48 h, baseline temperature, maximum temperature within the first 24 h, maximum temperature within 24 to 48 h, and hyperthermia within the first 24 h (defined by an axillary temperature higher than 37.5 °C) as variables.
Statistical Analysis
The statistical analysis was conducted by an investigator who was blinded to the randomization using IBM®SPSS® statistics version 20 for Mac (SPSS Inc. Chicago, IL, USA). Results were expressed as percentages for categorical variables. To identify the continuous variables that followed a normal distribution, the Kolmogorov-Smirnov test was used. The continuous variables with normal distribution were expressed as mean (SD), and those variables not normally distributed were expressed as median [quartiles]. Proportions between groups were compared by chi-square test. Student’s t test was used to compare continuous variables with normal distribution between two groups. In case of variables with non-normal distribution, the Mann-Whitney U test was used to compare the two groups. The influence of the anti/pro-inflammatory index, body weight evolution, and body temperature on functional outcome was evaluated using logistic regression analysis. The results were expressed as adjusted odds ratios (OR) with the corresponding 95% confidence intervals (95% CI). To analyze the association between body weight evolution and neurological impairments assessed by NIHSS, the Pearson correlation coefficient (r) was performed. Values of p < 0.05 were considered to be statistically significant in all tests.
Results
Descriptive Analysis of the Sample
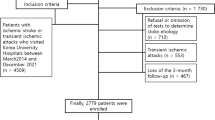
A total of 616 patients with a first-ever ischemic stroke within 24 h from symptom onset were evaluated. According to previously established exclusion criteria and the consecutive character of the inclusion, 516 patients were excluded. After the initial inclusion, two patients in the control group were later excluded at the request of their families, leaving 98 patients who qualified for the study (women 45.9%; mean age 69.3 ± 14.6 years), 48 non-obese and 50 obese. Body weight and height were obtained from direct measures during hospitalization in 62 patients (63.3%), 31 non-obese (64.6%) and 31 obese (62%) subjects; in the rest of patients, previously detailed methods were necessary.
Baseline characteristics were comparable among groups (Table 1). No significant differences were observed with respect to age, gender, previous functional disability, severity of neurological impairments at admission, recanalization treatment, etiology of stroke, or infarct volume. Regarding medical history variables, the most noticeable finding was that atrial fibrillation was more frequent in the obese group (46% vs. 22.9%, p = 0.016). Concerning the routine laboratory inflammatory markers, we found that leukocyte count (10.4 ± 2.1 × 103 vs. 8.1 ± 2.9 × 103/mL, p < 0.001) and fibrinogen (461.5 ± 86.9 vs. 405.9 ± 89.8 mg/dL, p = 0.005) were higher in obese patients. During hospitalization, the obese group showed a trend to suffer more infections (28.0% vs. 16.7%, p = 0.179) and a significant higher risk for hemorrhagic transformations (30.0% vs. 12.5%, p = 0.035).
Primary Endpoint: Functional Outcome at 3 Months
Two patients were lost to follow-up for functional outcome assessment at 3 months. The results did not show any significant difference in good functional outcome at 3 months between obese and non-obese patients (57.1% vs. 53.2%, p = 0.697), nor in mRS scores at discharge (3 [1, 5] vs. 3 [1, 4], p = 0.882) or at 3 months (2 [1, 3] vs. 2 [1, 4], p = 0.508) (Fig. 1a), nor in mortality at 3 months (6.1% vs. 10.6%, p = 0.424). However, a higher proportion of obese patients who were not able to walk unassisted (mRS ≥ 4) at discharge recovered their independence to walk (mRS ≤ 3) at 3 months compared with non-obese (50.0% vs. 16.7%, p = 0.034).
a Comparison in mRS at 3 months between non-obese and obese patients, showing no significant difference in good functional outcome (mRS ≤ 2) at 3 months (p = 0.508). b Comparison in clinical improvement (% of NIHSS) at 3 months between non-obese and obese patients, showing a trend to be higher in obese patients (p = 0.141)
Primary Endpoint: Inflammatory Balance
To assess the inflammatory profile in both groups of patients, we first analyzed the serum levels of IL-6 and IL-10. IL-6 levels at admission were significantly lower in non-obese compared with obese patients (10.4 ± 9.3 vs. 14.1 ± 6.7 pg/mL, p = 0.002), no significant differences were found at 72 h (12.9 ± 16.4 vs. 12.2 ± 8.1 pg/mL, p = 0.394), and a non-significant trend towards higher levels at 7th day was observed in non-obese ones (15.2 ± 14.8 vs. 10.9 ± 5.2 pg/mL, p = 0.115) (Fig. 2a). Importantly, at 72 h and at 7th day, the levels of IL-6 increased in non-obese while decreased in obese. On the other side, levels of IL-10 were significantly lower in non-obese compared with obese individuals at the three sampling moments, and raised over the first week in this latter group (Fig. 2b) (at admission 2.6 ± 1.4 vs. 7.1 ± 2.1 pg/mL, p < 0.0001; at 72 h 2.1 ± 0.9 vs. 7.8 ± 2.1 pg/mL, p < 0.0001; at 7th day 2.3 ± 1.1 vs. 8.4 ± 1.6 pg/mL, p < 0.0001).
Temporal profile of IL-6 (a) and IL-10 (b) in obese and non-obese ischemic stroke patients. a IL-6 levels at admission were significantly lower in non-obese compared with obese patients (10.4 ± 9.3 vs. 14.1 ± 6.7 pg/mL, p = 0.002), no significant differences were found at 72 h (12.9 ± 16.4 vs. 12.2 ± 8.1 pg/mL, p = 0.394), and a non-significant trend towards higher levels at 7th day was observed in non-obese ones (15.2 ± 14.8 vs. 10.9 ± 5.2 pg/mL, p = 0.115). Importantly, at 72 h and at 7th day, the levels of IL-6 increased in non-obese while decreased in obese. b Levels of IL-10 were significantly lower in non-obese compared with obese individuals at the three sampling moments, and raised over the first week in the obese group (at admission 2.6 ± 1.4 vs. 7.1 ± 2.1 pg/mL, p < 0.0001; at 72 h 2.1 ± 0.9 vs. 7.8 ± 2.1 pg/mL, p < 0.0001; at 7th day 2.3 ± 1.1 vs. 8.4 ± 1.6 pg/mL, p < 0.0001)
To determine the balance between cytokines, the previously defined anti/pro-inflammatory index was used. The analysis showed that the values of this index were higher in obese compared with non-obese patients at 72 h (2.0 ± 1.7 vs. 0.8 ± 0.4, p < 0.0001) and at 7th day (2.0 ± 0.9 vs. 1.0 ± 1.0, p = 0.001) but not at admission (1.3 ± 0.5 vs. 1.1 ± 0.9, p = 0.203) (Fig. 3a). We also analyzed the association of this anti/pro-inflammatory index with functional outcome at 3 months showing significant higher values at admission in those patients who had good outcome, but not at 72 h or at day 7 (at admission 1.3 ± 0.8 vs. 1.0 ± 0.6, p = 0.037; at 72 h 1.7 ± 1.6 vs. 1.2 ± 1.3, p = 0.122; at 7th day 1.6 ± 1.1 vs. 1.3 ± 1.1, p = 0.295) (Fig. 3b).
a Temporal profile (admission 72 h and day 7) of anti/pro-inflammatory index in obese and non-obese, showing higher values of this index in obese compared with non-obese patients at 72 h (2.0 ± 1.7 vs. 0.8 ± 0.4, p < 0.0001) and at 7th day (2.0 ± 0.9 vs. 1.0 ± 1.0, p = 0.001) but not at admission (1.3 ± 0.5 vs. 1.1 ± 0.9, p = 0.203). b Temporal profile (admission 72 h and day 7) of anti/pro-inflammatory index regarding patient outcome at 3 months, showing significant higher values at admission in those patients who had good outcome, but not at 72 h or at day 7 (at admission 1.3 ± 0.8 vs. 1.0 ± 0.6, p = 0.037; at 72 h 1.7 ± 1.6 vs. 1.2 ± 1.3, p = 0.122; at 7th day 1.6 ± 1.1 vs. 1.3 ± 1.1, p = 0.295)
Finally, in the logistic regression analysis, only the anti/pro-inflammatory index at 72 h (OR 0.03; 95% CI 0.01–0.09) was independently associated with good functional outcome at 3 months and just for obese patients in the non-adjusted analysis (Table 2).
Secondary Endpoints
With respect to clinical improvement, three patients were lost to follow-up for NIHSS assessment at 3 months. Clinical improvement at 3 months showed a trend to be higher in obese patients (62.4 ± 54.1% vs.41.4 ± 79.6%, p = 0.141) (Fig. 1b).
For the analysis of the evolution of body weight after stroke, 19 patients were lost to follow-up. After 3 months, there was a reduction in body weight in both groups, which was higher in obese compared with non-obese patients (5.7 ± 9.1 vs. 0.9 ± 5.6 kg, p = 0.007). Importantly, we also found that reductions in body weight were statistically higher in the poor outcome group at 3 months (6.8 ± 9.1% vs. 1.7 ± 6.7%, p = 0.006). When performing this analysis in non-obese and obese patients separately, we found that the loss was significantly higher in the bad outcome group but only for obese patients (table shown in Online Resource 1). Therefore, the next step was to assess the prognostic value of the reduction in body weight through a logistic regression model. In obese patients, the non-adjusted model showed that reductions in body weight were significantly associated with poor outcome at 3 months (OR 1.12; 95% CI 1.02–1.22). By contrast, no significant associations were found for non-obese patients (OR 1.04; 95% CI 0.92–1.18). Finally, to analyze the influence of the severity of neurological impairments, we adjusted the model by the NIHSS at 48 h (as it represents a reliable marker of the actual stabilized neurological deficit after the hyperacute phase), and the association disappeared (adjusted OR 0.95; 95% CI 0.83–1.09).
Moreover, we analyzed the influence of body weight in body temperature during the acute phase of stroke. Firstly, we found that mean temperatures were significantly higher in obese patients during the first 48 h (36.5 ± 0.4 vs. 36.3 ± 0.4 °C, p = 0.027) (Fig. 4). Baseline axillary temperature (36.0 [35.6–36.0] vs. 35.7 [35.2–36.0]°C, p = 0.033) and maximum temperature within the first 24 h (37.0 ± 0.6 vs. 36.7 ± 0.6 °C, p = 0.016) were also higher in obese patients, although no significant differences were found in the maximum temperature within 24 to 48 h (37.4 ± 0.6 vs. 36.8 ± 0.6 °C, p = 0.233) and in the percentage of patients who experience hyperthermia within the first 24 h (28% vs. 12.5%, p = 0.057). To assess the prognostic value of temperature in both groups, a non-adjusted logistic regression analysis was performed. The mean temperature within the first 48 h (obese patients OR 12.04; 95% CI 2.04–71.03; non-obese patients OR 22.6; 95% CI 3.18–160.94) and maximum temperature during the first 24 h (obese patients OR 2.94; 95% CI 1.06–8.20; non-obese patients OR 6.78; 95% CI 1.82–25.23) were independently associated to poor outcome in both groups of patients, although greater significant associations were found for non-obese ones. No significant associations were found for baseline temperature in both groups (obese patients OR 1.87; 95% CI 0.52–6.76; non-obese patients OR 1.57; 95% CI 0.55–4.52). Finally, we conducted a multivariate analysis adjusted by the presence of infections during hospitalization which showed that mean temperature within the first 48 h was independently associated with poor outcome in both groups (obese patients OR 9.85; 95% CI 1.48–65.57; non-obese patients OR 11.02; 95% CI 1.30–93.44). However, for maximum temperature during the first 24 h, the association remained significant only in non-obese patients (obese patients OR 2.36; 95% CI 0.80–7.02; non-obese patients OR 7.62; 95% CI 1.42–40.96).
Discussion
In this prospective study, even though they have several factors strongly associated with poor outcome (higher serum levels of inflammation markers at admission, a higher body temperature in the acute phase, a higher proportion of atrial fibrillation, and an increased frequency of hemorrhagic transformations), obese patients do not evolve worse than non-obese. In fact, there was a trend in favor of a greater recovery of neurological impairments and a significant improvement of disability in obese subjects. To the best of our knowledge, our study is the first to analyze and show that trend by using a scale specifically designed for this neurological deficit evaluation (NIHSS). A key consideration is that, contrary to previously proposed justifications [12, 33], these differences in neurological improvement are not justified by the severity of stroke on admission or by the age of the patients, since the median NIHSS on admission, the mean infarct volumes, and the mean age were similar for obese and non-obese patients in our sample. Another justification for the obesity paradox subscribed by some authors [12, 34] was that obese subjects show a higher relative proportion of lacunar strokes, which are typically associated with a better prognosis than cardioembolic strokes. Such hypothesis is discarded with present results as obese subjects showed not only a similar percentage of lacunar strokes than non-obese ones but also a non-significant higher proportion of cardioembolic strokes. This could be explained by the presence of a higher proportion of atrial fibrillation in that group of patients, which agrees with recent literature [35].
Interestingly, obese ischemic stroke patients had significantly more hemorrhagic transformations than non-obese. Although Kim et al. [36] found that the risk of hemorrhagic transformation decreased significantly with obesity, our finding is in line with that previously observed in animal models [37], which suggests that obesity intensifies inflammatory damage of cerebral microvasculature. As we will discuss next, obese patients may develop a more intense inflammatory response in the first moments after stroke onset, so it is very likely that such response will contribute to the blood-brain barrier damage resulting in an increased risk of hemorrhagic transformation.
Laboratory findings suggest that inflammation could play a key role in the evolution of obese patients with ischemic stroke. Excessive white adipose tissue results in a chronic systemic elevation of acute phase reactants and pro-inflammatory cytokines [13, 38, 39]. Accordingly, some experimental studies have previously shown that post-stroke peripheral immune response is increased in obesity models [40]. In this line, we found higher levels of leukocytes, fibrinogen, hsCRP, and IL-6 in blood samples of obese patients compared with non-obese on admission, which suggests the presence of a more powerful inflammatory response in this group of patients during the very first moments of the acute phase of stroke. In addition, many studies have shown that the release of pro-inflammatory cues has potentially harmful effects that contribute to tissue damage and negatively influence post-stroke outcome [22, 23, 41,42,43,44]. Thus, it could be expected a negative influence in the evolution and outcome of obese patients; however, our data show the opposite. We propose that obese subjects, chronically exposed to low-grade inflammation, exhibit a certain tolerance and counterbalance the inflammatory response and, to some extent, the harmful action of pro-inflammatory cues in acute ischemic stroke. The temporal profile of cytokines IL-6 and IL-10, pro-inflammatory and anti-inflammatory, respectively, supports such hypothesis. Even though obese patients exhibited higher serum levels of IL-6 at the hyperacute phase of stroke, over the following 7 days, they decreased at the same time as the levels of IL-10 increased, phenomenon not observed in non-obese ones. The values of the anti/pro-inflammatory index, which relates serum levels of both cytokines and which could represent the “battle” that takes place between both antagonistic responses, were higher in obese patients, and logistic regression models showed their influence in prognosis in this group of patients. All this suggests that during the first week after stroke, an anti-inflammatory stream which counteracts the deleterious effects of pro-inflammatory signals is boosted in obese patients. This may be one of the reasons why such patients do not evolve worse and experience a higher clinical improvement despite the fact that they probably have a more powerful baseline and hyperacute inflammatory response.
Another interesting finding was that 3 months after stroke, there was a reduction of body weight that was higher in obese patients. The first and simplest explanation is that control of vascular risk factors with diet results in a higher loss of adiposity in those patients who arise from more extreme situations and would show more remarkable changes. On the other hand, since ischemic stroke stress results in sympathetically induced catabolic responses [45], it is likely that this response may be higher in obese subjects due to the chronic low-grade inflammation and a higher inflammatory reaction in the hyperacute phase of stroke, leading to higher lipolysis and, subsequently, greater reduction in adipose tissue depots. In accordance with previous studies [46,47,48], we also observed that there was a higher reduction in body weight in patients with poor outcome. In those patients, the significant correlation between the severity of neurological impairments (NIHSS at 48 h) and body weight reduction (r = 0.495; p < 0.0001) suggests that weight loss could probably be the consequence of prolonged immobilization or difficulties for alimentation and malnutrition, but even sarcopenia secondary to occult diseases or more marked catabolic/anabolic imbalance [45, 49] could exert a role.
Our temperature analysis showed the presence of higher temperatures in the early phases of stroke in obese patients when compared with controls. This finding is not justified by the higher frequency of infections in those patients. An analysis exclusively selecting those patients who did not suffer infections showed that the trends were maintained (figure shown in Online Resource 1). Moreover, unlike late hyperthermia which is related to infection, earliest hyperthermia (the one registered in our patients) is usually associated with a neurogenic response as a consequence of acute phase reaction [32]. Our hypothesis is that the metainflammation and the potent inflammatory response in the very early phases of stroke of obese patients are responsible for this temperature increase in the first hours after onset. This increased hyperacute inflammatory cascade with pyrogenic activity leads to increased pro-inflammatory cytokines (IL-6 is an endogenous pyrogen [50]) and leukocytes around the infarcted tissue, leading to hypothalamic stimulation and finally to neurogenic hyperthermia [51,52,53,54,55]. In the analysis of the prognostic value, it seems that obese patients, despite having higher axillary temperatures, they better tolerate it. Although we do not know the exact reasons for this phenomenon and that other specific designed studies would be necessary, we suspect that, at least in part, the anti-inflammatory counterpart associated to this type of patients could be involved, as levels of the pyrogen IL-6 decrease over the first week.
We must recognize some limitations. The main one is that, despite this was a prospective study in which the leading characteristics of both groups were comparable, the size of the sample was small. Another limitation is the use of BMI to assess excess body fat and to classify patients, since this index does not distinguish between weight associated with muscle or with fat [19] and is dependent on age and sex [55]. In addition, we did not register aspects such as immobilization, alimentation difficulties, or nutritional status which might have provided more data to explain the variations in body weight. However, as we previously mentioned, every patient with alimentation difficulties was evaluated and followed by the Nutrition Unit from the Endocrinology Department to maintain and adequate nutritional status during their stay at the Hospital and at discharge. On the other hand, to assess the inflammatory balance we employed an index that relates IL-6 and IL-10 levels, which has not been previously validated, and we do not discard that the use of other cytokines or even a combination of some of them would have been more useful or precise. However, the results showed a clear trend to better recovery for obese ischemic stroke patients that should be confirmed in further larger clinical studies.
In the present work, we have tried to elucidate new insights regarding the potential connection between obesity and ischemic stroke, two major global health problems with severe economic and clinical consequences. In summary, we have shown that, despite exhibiting several factors associated with poor prognosis, obese patients do not evolve worse than non-obese after ischemic stroke but seem to experience a greater recovery of neurological impairments. We postulate that obesity can counterbalance the inflammatory reaction responsible for the deleterious effects of the mentioned factors of poor prognosis, through an anti-inflammatory stream enhanced in the first moments of stroke.
References
Roth J, Qiang X, Marbán SL, Redelt H, Lowell BC. The obesity pandemic: where have we been and where are we going? Obes Res. 2004;12(Suppl 2):88S–101S.
Gruberg L, Weissman NJ, Waksman R, Fuchs S, Deible R, Pinnow EE, et al. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39:578–84.
Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int. 1999;55:1560–7.
Curtis JP, Selter JG, Wang Y, Rathore SS, Jovin IS, Jadbabaie F, et al. The obesity paradox: body mass index and outcomes in patients with heart failure. Arch Intern Med. 2005;165:55–61.
Senoo K, Lip GYH. Body mass index and adverse outcomes in elderly patients with atrial fibrillation: the AMADEUS trial. Stroke. 2016;47:523–6.
Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:1856–61.
Escalante A, Haas RW, del Rincón I. Paradoxical effect of body mass index on survival in rheumatoid arthritis. Arch Intern Med. 2005;165:1624–9.
Olsen TS, Dehlendorff C, Petersen HG, Andersen KK. Body mass index and poststroke mortality. Neuroepidemiology. 2008;30:93–100.
Kim BJ, Lee SH, Jung KH, Yu KH, Lee BC, Roh JK. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology. 2012;79:856–63.
Kazemi-Bajestani SMR, Ghayour-Mobarhan M, Thrift AG, Ferns GA, Frazadfard MT, Mokhber N, et al. Obesity paradox versus frailty syndrome in first-ever ischemic stroke survivors. Int J Stroke. 2015;10:E75.
Jang SY, Shin Y, Kim DY, Sohn MK, Lee J, Lee S, et al. Effect of obesity on functional outcomes at 6 months post-stroke among elderly Koreans: a prospective multicentre study. BMJ Open. 2015;5:e008712.
Ryu W-S, Lee S-H, Kim CK, Kim BJ, Yoon B-W. Body mass index, initial neurological severity and long-term mortality in ischemic stroke. Cerebrovasc Dis. 2011;32:170–6.
Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–7.
Iadecola C, Anrathner J. The immunology of stroke: from mechanism to translation. Nat Med. 2012;17:796–808.
European Stroke Organisation (ESO) Executive Committee, ESO Writing Committee. Guidelines for Management of Ischaemic Stroke and Transient Ischaemic Attack 2008. Cerebrovasc Dis. 2008;25:457–507.
Alonso de Leciñana M, Egido JA, Casado I, Ribó M, Dávalos A, Masjuan J, et al. Guía para el tratamiento del infarto cerebral agudo. Neurología. 2014;29:102–22.
Chumlea WC, Guo SS, Wholihan K, Cockram D, Kuczmarski RJ, Johnson CL. Stature prediction equations for elderly non-Hispanic white, non-Hispanic black, and Mexican-American persons developed from NHANES III data. J Am Diet Assoc. 1998;98:137–42.
Chumlea WC, Guo S, Roche AF, Steinbaugh ML. Prediction of body weight for the nonambulatory elderly from anthropometry. J Am Diet Assoc. 1988;88:564–8.
WHO. Obesity. Preventing and managing the global epidemic. Report of a WHO consultation. WHO technical report series 894. Geneva: World Health Organization; 2000.
Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41.
Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, et al. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–10.
Castillo J, Rodríguez I. Biochemical changes and inflammatory response as markers for brain ischaemia: molecular markers of diagnostic utility and prognosis in human clinical practice. Cerebrovasc Dis. 2004;17(Suppl 1):7–18.
Smith CJ, Emsley HCA, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2.
Spera PA, Ellison JA, Feuerstein GZ, Barone FC. IL-10 reduces rat brain injury following focal stroke. Neurosci Lett. 1998;251:189–92.
Strle K, Zhou JH, Shen WH, Broussard SR, Johnson RW, Freund GG, et al. Interleukin-10 in the brain. Crit Rev Immunol. 2001;21:427–49.
Blanco M, Castellanos M, Rodríguez-Yáñez M, Sobrino T, Leira R, Vivancos J, et al. High blood pressure and inflammation are associated with poor prognosis in lacunar infarctions. Cerebrovasc Dis. 2006;22:123–9.
Leira R, Rodríguez-Yáñez M, Castellanos M, Blanco M, Nombela F, Sobrino T, et al. Hyperthermia is a surrogate marker of inflammation-mediated cause of brain damage in acute ischaemic stroke. J Intern Med. 2006;260:343–9.
Castellanos M, Sobrino T, Pedraza S. High plasma glutamate concentrations are associated with growth in acute ischemic stroke. Neurology. 2008;71:1862–8.
Castillo J, Alvarez-Sabín J, Martínez-Vila E, Montaner J, Sobrino T, Vivancos J. Inflammation markers and prediction of post-stroke vascular disease recurrence: the MITICO study. J Neurol. 2009;256:217–24.
Rodríguez-Yáñez M, Sobrino T, Arias S, Vázquez-Herrero F, Brea D, Blanco M, et al. Early biomarkers of clinical-diffusion mismatch in acute ischemic stroke. Stroke. 2011;42:2813–8.
Rodríguez-Yáñez M, Castellanos M, Sobrino T, Brea D, Ramos-Cabrer P, Pedraza S, et al. Interleukin-10 facilitates the selection of patients for systemic thrombolysis. BMC Neurol. 2013;13:62.
Campos F, Blanco M, Barral D, Agulla J, Ramos-Cabrer P, Castillo J. Influence of temperature on ischemic brain: basic and clinical principles. Neurochem Int. 2012;60:495–505.
Towfighi A, Ovbiagele B. The impact of body mass index on mortality after stroke. Stroke. 2009;40:2704–8.
Kim Y, Kim CK, Jung S, Yoon B, Lee S. Obesity-stroke paradox and initial neurological severity. J Neurol Neurosurg Psychiatry. 2015;86:743–7.
Nalliah CJ, Sanders P, Kottkamp H, Kalman JM. The role of obesity in atrial fibrillation. Eur Heart J. 2016;37:1565–72.
Kim CK, Ryu W-S, Kim BJ, Lee S-H. Paradoxical effect of obesity on hemorrhagic transformation after acute ischemic stroke. BMC Neurol. 2013;13:123.
Haley MJ, Lawrence CB. Obesity and stroke: can we translate from rodents to patients? J Cereb Blood Flow Metab. 2016;36:2007–21.
Maachi M, Piéroni L, Bruckert E, Jardel C, Fellahi S, Hainque B, et al. Systemic low-grade inflammation is related to both circulating and adipose tissue TNFα, leptin and IL-6 levels in obese women. Int J Obes. 2004;28:993–7.
Khaodhiar L, Ling P-R, Blackburn GL, Bistrian BR. Serum levels of interleukin-6 and c-reactive protein correlate with body mass index across the broad range of obesity. J Parenter Enter Nutr. 2004;28:410–5.
Terao S, Yilmaz G, Stokes KY, Ishikawa M, Kawase T, Granger DN. Inflammatory and injury responses to ischemic stroke in obese mice. Stroke. 2008;39:943–50.
Bowes MP, Rothlein R, Fagan SC, Zivin JA. Monoclonal antibodies preventing leukocyte activation reduce experimental neurologic injury and enhance efficacy of thrombolytic therapy. Neurology. 1995;45:815–9.
Härtl R, Schürer L, Schmid-Schönbein GW, del Zoppo GJ. Experimental antileukocyte interventions in cerebral ischemia. J Cereb Blood Flow Metab. 1996;16:1108–19.
Vila N, Castillo J, Dávalos A, Chamorro A. Proinflammatory cytokines and early neurological worsening in ischemic stroke. Stroke. 2000;31:2325–9.
Castellanos M, Castillo J, García MM, Leira R, Serena J, Chamorro A, et al. Inflammation-mediated damage in progressing lacunar infarctions. Stroke. 2002;33:982–7.
Scherbakov N, Dirnagl U, Doehner W. Body weight after stroke: lessons from the obesity paradox. Stroke. 2011;42:3646–50.
Wohlfahrt P, Lopez-jimenez F, Krajcoviechova A, Jozifova M, Mayer O, Vanek J, et al. The obesity paradox and survivors of ischemic stroke. J Stroke Cerebrovasc Dis. 2015;24:1443–50.
Kim Y, Kim CK, Jung S, Ko S-B, Lee S-H, Yoon B-W. Prognostic importance of weight change on short-term functional outcome in acute ischemic stroke. Int J Stroke. 2015;10:62–8.
Jonsson A-C, Lindgren I, Norrving B, Lindgren A. Weight loss after stroke: a population-based study from the Lund Stroke Register. Stroke. 2008;39:918–23.
Myers J, Lata K, Chowdhury S, McAuley P, Jain N, Froelicher V. The obesity paradox and weight loss. Am J Med. 2011;124:924–30.
Dinarello CA, Cannon JG, Mancilla J, Bishai I, Lees J, Coceani F. Interleukin-6 as an endogenous pyrogen: induction of prostaglandin E2 in brain but not in peripheral blood mononuclear cells. Brain Res. 1991;562:199–206.
Kinoshita K, Chatzipanteli i K, Vitarbo E, Truettner JS, Alonso OF, Dietrich WD. Interleukin-1beta messenger ribonucleic acid and protein levels after fluid-percussion brain injury in rats: importance of injury severity and brain temperature. Neurosurgery. 2002;51:195–203.
Dietrich WD, Chatzipanteli K, Vitarbo E, Wada K, Kinoshita K. The role of inflammatory processes in the pathophysiology and treatment of brain and spinal cord trauma. Acta Neurochir Suppl. 2004;89:69–74.
Badjatia N. Hyperthermia and fever control in brain injury. Crit Care Med. 2009;37:S250–7.
Contreras C, González F, Fernø J, Diéguez C, Rahmouni K, Nogueiras R, et al. The brain and brown fat. Ann Med. 2005;47:150–68.
Gallagher D, Visser M, Sepúlveda D, Pierson RN, Harris T, Heymsfield SB. How useful is body mass index for comparison of body fatness across age, sex, and ethnic groups? Am J Epidemiol. 1996;143:228–39.
Funding
This project was partially supported by grants from the Spanish Ministry of Economy and Competitiveness (SAF2014-56336-R, SAF2015-71026-R and SAF2017-84267-R), Xunta de Galicia (Consellería Educación: GRC2014/027, 2016-PG068 and IN607A2018/3), Instituto de Salud Carlos III (Proyecto de Excelencia dentro de los Institutos de Investigación Sanitaria (PIE13/00024) and PI17/01103), Spanish Research Network on Cerebrovascular Diseases RETICS-INVICTUS PLUS (RD16/0019), and by the European Union FEDER program. Furthermore, T. Sobrino (CPII17/00027) and F. Campos (CP14/00154) are recipients of research contracts from the Miguel Servet Program of Instituto de Salud Carlos III. The sponsors did not participate in study design, collection, analysis, or interpretation of the data, in writing the report, or in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethical Approval and Patient Consent
This research was carried out in accordance with the Declaration of Helsinki of the World Medical Association (2008) and approved by the Ethics Committee of the Servizo Galego de Saúde. Informed consent was obtained from all individual participants included in the study. If patients were not able to sign, relatives gave signed informed consent.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Online Resource 1
(DOCX 287 kb)
Rights and permissions
About this article
Cite this article
Rodríguez-Castro, E., Rodríguez-Yáñez, M., Arias-Rivas, S. et al. Obesity Paradox in Ischemic Stroke: Clinical and Molecular Insights. Transl. Stroke Res. 10, 639–649 (2019). https://doi.org/10.1007/s12975-019-00695-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-019-00695-x