Abstract
Asymptomatic small vessel disease (SVD), including white matter hyperintensities (WMHIs), periventricular hyperintensities (PVHIs), silent stroke (SS), and cerebral microbleeds (CMBs), increases the risk of stroke. There are limited studies of SVD in subjects from the Middle East and Southeast Asia (SA). All patients admitted to stroke service between 2014 and 2015 were reviewed for presence of “pre-existing” SVD. Stroke mimics with no previous history of stroke were used as controls. There were 1727 patients admitted with stroke. Analysis was done on 988 subjects (914 strokes and 74 controls) who had MRI scan done. Pre-existing SVD was seen in 642 (64.9%) patients (WMHIs 19.6%, PVHIs 33.2%, SS 51.4%, and CMBs 22%). Silent strokes were significantly more common with ischemic stroke (IS) compared to intracranial hemorrhage (ICH) (62.0 vs 34.3%, p < 0.001). CMBs were more in ICH compared to IS (42.9 vs 23.1%, p < 0.001). The risk of developing CMBs among Far Eastern (FE) patients was 1.58 times more (p = 0.07), while 1.48 times more in Arabs (AR) (p = 0.026) compared to SA after adjusting for age. The risk of developing PVHIs was significantly higher in Arab compared to SA (odds ratio (OR) 1.43; p value = 0.021). Similarly, the risk of developing WMHIs was also significantly higher in AR patients (OR 1.6; p value = 0.009) compared to SA. The majority of ethnic AR, SA, and FE populations show pre-existing SVD on MRI. The advanced changes at a young age may be related to high prevalence of untreated risk factors and possibly as yet defined genetic and environmental factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Advances in brain imaging technology have resulted in improvements in the detection of asymptomatic cerebrovascular disease [1, 2]. Long-term observational follow-up studies in communities, for example Framingham [3] and Rotterdam [4], have shown that the presence of silent stroke (SI), small vessel disease (SVD), and cerebral microbleeds (CMBs) can lead to cognitive deficits and increase the risk of dementia and stroke [1, 5,6,7]. The American Heart Association/American Stroke Association recently published their scientific statement on the importance of the diagnosis and management of silent stroke. Until recently, most of the research on silent stroke has been done in Europe and North America and there is very little information on its prevalence in patients from Southeast Asia or the Middle East [8,9,10,11,12,13].
Uncontrolled hypertension and diabetes increase the risk of small vessel disease, including silent stroke (SS), periventricular hyperintensities (PVHIs), white matter hyperintensities (WMHIs), and cerebral microbleeds (CMBs) [1, 5]. In low-/middle-income countries, stroke is common and presents at a younger age and the disease frequently manifests with intracranial atherosclerosis and intracranial hemorrhage (ICH) [8,9,10,11,12,13]. A lack of awareness, poor control of risk factors for vascular diseases, and possibly environmental pollution may be important contributing factors [14].
We recently reported on the risk factors and clinical characteristics of stroke in Qatar [15, 16]. Our studies reveal that stroke manifests at a younger age and risk factors are common and poorly managed. This current study reports on the MRI abnormalities in this cohort. We had two major objectives. Firstly, using 3T-MRI, we investigated for the presence of “pre-existing” cerebral abnormalities. Secondly, we investigated if there were any links between the pre-existing subclinical disease to ethnicity, vascular risk factors, and the type of MRI abnormalities.
Materials and Methods
Data from all patients admitted with a diagnosis of stroke or transient ischemic attack (TIA) to the Hamad General Hospital, Qatar, from January 1, 2014, through December 31, 2015, was analyzed from a hospital-based prospective stroke database. The study center and the stroke service are both Joint Commission International accredited. The hospital is a 600-bed facility, with 200 beds reserved for medical patients, the only tertiary care facility in the country where the stroke service is located. Almost 95% of all strokes requiring admission to a hospital are admitted at Hamad General Hospital. Annually, the hospital admits approximately 1500 patients with acute stroke from diverse ethnicities [15, 17]. The study has received IRB approval from the Medical Research Centre at Hamad Medical Corporation.
Patient Characteristics
Demographics included age, sex, ethnicity, medical comorbidities, and prior medication use. Data from emergency medical service, immediate emergency department care, NIHSS score, length of stay, type and timing of investigations completed, post-stroke complications, in-hospital mortality, and disposition were collected as part of the hospital stroke database. Stroke diagnosis was made using the Trial of Org 10172 in Acute Stroke Treatment classifications [18]. Outcome measures at time of discharge and 90 days with the mRS and stroke classification were also collected.
Subjects included individuals diagnosed as having ischemic stroke (IS) or TIA and ICH. Patients with IS were evaluated in the following categories: large vessel disease, small vessel disease, and cardio-embolic stroke. Embolic strokes of undetermined source were categorized according to the location of the lesion on MRI. For this study, we only included patients in whom brain MRI was completed during their stay in the hospital (n = 914). We excluded stroke patients who had uncommon causes of stroke such as connective tissue disease, migraine-related stroke, and moyamoya disease. For controls, we included subjects who were evaluated for suspected stroke but did not have a vascular mechanism for their symptoms and were diagnosed as “stroke mimics.” This group included 89 subjects. Control subjects with previous history of stroke were not included. There were very few Caucasian and African subjects in the data set and these were excluded from further analysis.
Brain MR Imaging Analysis
Diffusion-weighted imaging, apparent diffusion coefficient, axial T1, T2, fluid-attenuated inversion recovery, sagittal T1, coronal T2 susceptibility-weighted imaging, contrast-enhanced sagittal T1 three-dimensional magnetization prepared rapid acquisition gradient echo (MPRAGE) with axial and coronal reformats, and three-dimensional time of flight intracranial and post-contrast cervical MR angiography were obtained for each subject with a 3.0-T superconducting magnet (MAGNETOM Skyra, Siemens, Germany).
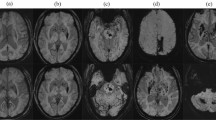
For diagnosis of SVD, we included SS, PVHIs, WMHIs, and CMBs [1, 5]. Silent stroke was defined as focal hyperintensities on T2-weighted images, 3 mm in size or larger. Cerebral infarction was defined as “silent” if there was no corresponding history of a stroke or TIA. Combination of T1, T2, and fluid-attenuated inversion recovery (FLAIR) scans was used to distinguish infarcts from dilated perivascular spaces. Lesions in the white matter also required corresponding prominent hypointensities on T1-weighted images, in order to distinguish them from other cerebral white matter abnormalities. Lacunes were defined as focal lesions between 3 and < 15 mm seen on FLAIR, T1-weighted, and T2-weighted sequences. If lacunes were more than 15 mm, they were defined as subcortical infarcts, and if cortical gray matter was affected, they were classified as cortical infarcts. A modification of previously published scales was used to describe the different types of hyperintense signal abnormalities surrounding the ventricles and in the deep white matter [19, 20]. This modification has been used in earlier studies [21]. PVHI was graded as 0 = absence, 1 = “caps” or pencil-thin lining, 2 = smooth “halo,” and 3 = irregular PVHI extending into the deep white matter. Separate deep WMHIs were rated as 0 = absence, 1 = punctate foci, 2 = beginning confluence of foci, and 3 = large confluent areas (Fig. 1). For the current analysis, a score of 2 or more was considered significant for WMHIs and PVHIs.
Cerebral microbleeds were defined as round-shaped homogenous foci of low signal intensity lesions less than 5 mm on SWI [22, 23]. The foci located in the subarachnoid spaces or symmetrically in the globus pallidus were not included because these may be confused as vessel markings heavy mineral deposits or calcifications. The locations of CMBs were classified as lobar, central gray matter (basal ganglia and thalamus), white matter (periventricular and white matter), and infra-tentorial area (brainstem and cerebellum) [6]. The effects of age, vascular risk factors, different ethnic groups, and severity on the degree and nature of the abnormalities were noted.
The MR studies were performed under the direct supervision of the neuroradiologists in our team, who officially report the studies. Five stroke neurologists blindly reviewed the MRI images of the subjects. Inter-rater evaluation (n = 60 scans) for detecting infarcts showed excellent agreement among the five raters. The investigators were provided MRI anonymized to the diagnosis and clinical presentation of the patients. They were also provided with a data sheet and offered training on how to interpret the imaging. Each investigator reviewed 200 MRI scans.
Data Analysis and Statistics
Descriptive results for all continuous variables were reported as mean ± standard deviation and numbers (percentage) for all categorical variables. The distribution of continuous variables was assessed before using statistical tools. Mean-level comparisons between patients among different subtypes of stroke were assessed using the ANOVA test and multiple comparison analysis was performed using the Scheffe test. If assumption of an ANOVA test was violated, then an alternative non-parametric Kruskal Wallis test was performed. The Pearson chi-square test or Fisher exact test whenever appropriate was used to compare the proportion of all categorical variables (for example: ethnicity, hypertension (HTN)) among different subtypes of stroke. Multinomial logistic regression analyses were performed for objective 1 [impact of MRI abnormalities (CMBs, PVHIs, WMHIs, and SS) on developing the risk of IS, TIA, and ICH compared to non-stroke]. Odds ratio and 95% confidence interval were reported. Objective 2 [impact of demographics including age, gender, and ethnicity, and risk factors such as diabetes mellitus (DM), HTN, dyslipidemia, prior stroke, coronary artery disease (CAD), atrial fibrillation (AF), and smoking on the risk of developing of MRI abnormalities] was assessed using univariate and multiple logistic regression analysis. Odds ratio (OR) and 95% CI were reported. A “p” value < 0.05 (two tailed) was considered significant. SPSS 21·0 statistical package was used for the analysis.
Results
Patient Characteristics
A total of 1727 patients were admitted to the hospital with a diagnosis of acute stroke, TIA, or mimic between January 2014 and December 2015. Out of 1727, there were 988 subjects available for analysis (see Table 1 for demographics, risk factors, and clinical characteristics for patients with SVD). A total of 914 patients with the diagnosis of stroke/TIA underwent MRI as part of their investigations—718 (78.5%) had ischemic stroke, 126 (13.8%) TIA, and 70 (7.7%). ICH was selected for further analysis for the current study. In addition, we reviewed the MRIs in 74 patients with stroke mimics (most common diagnoses were psychogenic weakness, migraine, Todd’s paresis, and dizziness).
The age, gender, risk factors, and the localization of the vascular lesions on MRI are shown in Tables 2 and 3. The majority of patients were men (81.8%). This reflects the demographics of the country with a large male expatriate community [15]. The mean age of patients presenting with stroke was 54.2 (± 13.7) years. Overall DM, smoking, HbA1c levels, random blood sugar on admission, and serum LDL levels were found to be significantly higher in patients with IS, while HTN was found to be more predominant in ICH patients (Table 2). Patients presenting with ICH were significantly younger compared to patients presenting with acute ischemic stroke, TIA, or stroke mimics (48.5 ± 11.4 vs 54.7 ± 14 vs 54.3 ± 13.8 vs 54.3 ± 12.0, p = 0.004). The ICHs were predominantly located in the brainstem, cerebellum, thalamus, and basal ganglion region. None of the patients had a clinical diagnosis of Alzheimer’s disease and there were no ICHs where cerebral amyloid angiopathy (CAA) was suspected.
CMBs were found to be more frequent in patients with ICH as compared to IS, TIA, or mimic (42.9 vs 23.1 vs 7.1 vs 16.2%, p < 0.001; Table 3). In patients with ICH, CMB was found to be mainly in subcortical areas (21.4%) as compared to cortical areas (1.4%, p < 0.001), while 20.0% had CMBs present in both cortical and subcortical areas. In patients older than 55 years, CMBs were found to be more common in Far Easterns as compared to Arabs and South Asians (34.8 vs 29.3 vs 20.6%, p = 0.007; Fig. 2a–d).
Objective 1: “Pre-existing” 3T-MRI Abnormalities in Patients with Acute Stroke
Pre-existing abnormalities on the MRI were evaluated for the presence of SS, deep WMHIs, PVHIs, and CMBs. Overall, SSs were evident in 51.4% of patients and these were significantly more common in patients with IS compared to ICH (62.0 vs 34.3%, p < 0.001). SS was more commonly seen in patients with lacunar stroke (64.7%) compared to large vessel disease (58.6%) with p value = 0.16. Significantly fewer SSs were seen in patients with TIAs and stroke mimics compared to IS and ICH [6.8 and 27%, respectively] (p < 0.001). CMBs were evident in 22.0% of patients and were significantly more common in patients with ICH compared to ischemic stroke (42.9 vs 23.1%, p < 0.001). Whereas CMBs were significantly more in the subcortical areas in patients with ICH, a similar relationship for localization of CMBs was also evident in patients with IS. Cerebral microbleeds were significantly more common in patients with subcortical lacunar strokes compared to cortical strokes (14 vs 1.5%, p < 0.001) and 7.7% at both locations. WMHIs were evident in 19.6% of patients and PVHIs were evident in 33.2% of patients. These were significantly more common in patients with IS and ICH compared to TIAs and stroke mimics (p < 0.001). There were no significant differences in the presence of WMHIs or PVHIs between the patients with IS or ICH. However, the presence of PVHIs was significantly different (p = 0.049) across the various types of IS, while WMHIs showed a similar trend (p = 0.061; see Table 3). The lesions however increased with advancing age and in the presence of HTN and DM (Table 4).
The OR and 95% CI for the impact of pre-existing MR imaging abnormalities on subsequent risk of the stroke type are shown in Table 5. Patient with ICH were associated with higher prevalence of CMBs [OR 3.9 (1.8–8.4)], while patients with ischemic stroke were associated with the higher prevalence of silent stroke [OR 22.5 (8.9–56.4)].
Objective #2: Relationship of MRI Abnormalities to Age, Ethnicity, and the Type of Stroke
All pre-existing vascular abnormalities on MRI were evident at all ages, including in patients presenting with a stroke at less than 30 years of age (Fig. 3). Overall, at least one or more lesions of SVD were present in 50% (14/28) of patients with age of less than 30, while 44.1% (93/211) patients with age 30–45 years, 65.9% (286/434) in ages 45–60 years, and 79.0% (249/315) in ages greater than 60 years.
Overall, pre-existing SVD was seen in 642 (64.9%) patients (WMHIs 19.6%, PVHIs 33.2%, SS 51.4%, and CMBs 22.0%). Pre-existing SVD increased with age. The OR for developing SVD was 1.53 times more among AR (p = 0.004), while it was 20% less in FE (p = 0.296) compared SA. The risk of development of CMBs [OR 1.56 (1.15–2.10)], SS [1.55 (1.2–1.99)], PVHIs [2.68 (2.04–3.52)], and WMHIs [2.81 (2.02–3.91)] increased significantly after the age of 55 years (Fig. 2 and Table 4). Compared to South Asians, CMBs are more likely in Arab [1.68 (1.22–2.32)] and Far Eastern [1.49 (0.92–2.4)] populations. SSs are more common in Arabs when compared to South Asians and Far Eastern patients (Table 4).
On bivariate logistic regression analysis, microbleeds were more likely in patients with the following risk factors: HTN [OR 1.9 (1.4–2.9)], prior stroke [2.3 (1.5–3.6)], and dyslipidemia [0.66 (0.48–0.91)]. For SS, the presence of DM [1.6 (1.24–2.05)], HTN [1.8 (1.38–2.4)], dyslipidemia [0.64 (0.49–0.84)], and prior stroke [2.9 (1.77–4.56)] was a significant risk factor (Table 4).
The relationship between ethnicity and MRI abnormalities (CMBs, PVHIs, WMHIs, and SS) after adjusting for age has been shown in Fig. 2a–d and Table 6. The risk of development of CMBs [OR 1.43 (1.03–1.98)], SS [OR 1.49 (1.14–1.97)], PVHIs [OR 2.38 (1.77–3.19)], and WMHIs [OR 2.4 (1.69–3.42)] increased significantly among patient above 55 years after adjusting for ethnicity (Table 6). The adjusted odds ratio for developing CMBs was 1.58 times more among Far Eastern (p = 0.07), while 1.48 times more in Arabs (p = 0.026) compared to South Asian after adjusting for age (Table 6). The adjusted odds ratio for developing SS was slightly higher in patients from South Asia (OR 1.13) and Arab (OR 1.19) compared to patients from Far East after adjusting for age, but it was not statistically significant. However, the adjusted odds ratio for developing PVHIs was significantly higher in patients from Arab (OR 1.43; p value = 0.021) but it was not significantly different among Far Eastern (OR 1.1; p value = 0.845) compared to patients from South Asia after adjusting for age. Similarly, the adjusted odds ratio for developing WMHIs was significantly higher in patients from Arab (OR 1.6; p value = 0.009) but it was not significantly different among Far Eastern (OR 1.09; p value = 0.767) compared to patients from South Asia after adjusting for age (Table 6).
Discussion
To the best of our knowledge, this is the first comprehensive prospective study reviewing the relationship between risk factor profiles, clinical features, and 3T–MRI abnormalities in a large ethnically diverse population of stroke patients from the Middle East, South Asia, and the Far East. The most striking finding in this MR imaging study is the presence of extensive pre-existing SVD at a younger age in patients with acute stroke. The combination of untreated HTN and DM (especially in Arab and South Asian patients) was associated with a higher frequency of silent strokes, CMBs, PVHIs, and WMHIs. The ICH patients are under-represented here, as most patients did not have MRI studies because of the severity of illness or difficult to perform or medically unstable.
The first objective of our study was to evaluate if pre-existing abnormalities evident on MRI were distinguished between patients at risk for ischemic stroke versus ICH. In our study, patients with ischemic stroke were more likely to harbor prior silent strokes on imaging (SSs were evident in more than 50% of patients). SS is known to double the risk of recurrent stroke and dementia [7]. Patients with ICH had higher rates of CMBs. HTN, prior stroke, and low LDL cholesterol were significantly more common in patients with CMBs. Compared to previous studies [3, 24], patients in our series with ICH were significantly younger and none had CAA.
Another observation in our study was the MRI findings of advanced WMHIs and CMBs in patients at a young age. Previous work from Europe and USA has shown that such abnormalities are infrequent in individuals less than 50 years of age and are seen in fewer than 5% of subjects [4, 6, 25, 26]. In our study, 16.2% of control subjects had CMBs noted. The numbers increased to over 24.9% in patients with acute stroke (ICH 42.9% and ischemic stroke 23.1%). A recent study from Rotterdam showed that the presence of such abnormalities on MRI significantly increases the risk of subsequent stroke and dementia [4]. A higher risk of stroke and dementia has also been reported previously from Framingham [3] and in the Asymptomatic Risk in Communities (ARIC) studies in the USA [27].
There are a number of strengths in our study. We are the first to report on the magnitude of cerebrovascular disease in an ethnically diverse population. Cerebrovascular disease manifests at a younger age, especially in patients from South Asia and Far East. We report on high rates of undiagnosed or poorly controlled DM and HTN, which was likely a major contributor to the disease at an early age. The higher rate of subcortical strokes evident in Arabic and South Asian ethnic groups is likely related to the higher rates of dysglycemia and HTN. The higher rates of ICHs seen in patients from the Far East are also likely related to poorly controlled HTN. Imaging identified advanced vascular changes in the majority of patients at all ages. In addition to PVHIs and WMHIs seen in equal frequency in patients with IS and ICH, silent stroke was seen in higher frequency in patients with IS and CMBs in patients with ICH.
We acknowledge several limitations of this study. Our study comprised patients with an acute stroke. SVD are seen in higher frequency in patients with an acute stroke compared to similar age-matched population with no history of cerebrovascular disease [28]. While the data was collected prospectively, we do not know the duration of DM and HTN prior to the acute stroke or how well these conditions were treated. Such data is however difficult to collect and has inherent inaccuracies. Although we report on the imaging abnormalities in over 914 subjects, the sample is under representative of severe strokes. The control subjects had presented to the hospital with symptoms suggestive of an acute stroke. It is possible that we may have missed an actual ischemic stroke in these “mimics” but we believe that this is highly unlikely given the extensive evaluation of the subjects prior to discharge from hospital. In addition, in our study, we did not quantify the extent of changes noted on imaging. Previous studies using scoring scales have shown that there is an increase in the risk of recurrent stroke with increasing severity of white matter changes.
Conclusion
We report that stroke develops at a young age in our country of study. The high rates of subcortical stroke are likely related to the risk factor profile of the study population. There is a very high prevalence of subclinical advanced MRI abnormalities that are evident in all patients, including those who are younger than 30 years of age.
Change history
13 January 2018
The author names “Dr. Pablo Garcia Bermejo” and “Dr. Muhammad Faisal Wadiwala” needed to be added as the 6th and 7th authors, respectively. The authors regret this error.
References
Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010;9:689–701.
Lan KK, Li L, Schulz U, Simoni M, Chan KH, Ho SL, et al. Total small vessel disease score and the risk of recurrent stroke. Neurology. 2017;88:2260–7.
Romero JR, Preis SR, Beiser A, DeCarli C, Viswanathan A, Martinez-Ramirez S, et al. Risk factors, stroke prevention treatments, and prevalence of cerebral microbleeds in the Framingham Heart Study. Stroke. 2014;45:1492–4.
Portegies MLP, Wolters FJ, Hofman A, Ikram MK, Koudstaal PJ, Ikram MA. Prestroke vascular pathology and the risk of recurrent stroke and poststroke dementia. Stroke. 2016;47:2119–22.
Kuller LH, Longstreth WT, Arnold AM, Bernick C, Bryan RN, Beauchamp NJ. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–5.
Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Salman RA, Warach S, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet Neurol. 2009;8:165–74.
Gupta A, Giambrone AE, Gialdini G, Finn C, Delgado D, Gutierrez J, et al. Silent brain infarction and the risk of future stroke. A systemic meta-analysis. Stroke. 2016;47:719–25.
Feigin VL. Stroke in developing countries: can the epidemic be stopped and outcomes improved. Lancet Neurol. 2007;6:94–7.
O’Donnell M, Yousef S. Tracking the global burden of stroke: the need for large scale international studies. Lancet Neurol. 2009;8:306–7.
Wasay M, Khatri IA, Kaul S. Stroke in South Asian countries. Nat Rev Neurol. 2014;10:135–43.
Johnson SC, Mendis S, Mathers CD. Global variations in stroke burden and mortality: estimates from monitoring, surveillance and modelling. Lancet Neurol. 2009;8:345–54.
O’Donnell MJ, Xavier D, Liu L, Zhang H, Chin SL, A-Melacini P, et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case-control study. Lancet. 2010;376:112–23.
Mehndiratta MM, Khan M, Mehndiratta P, Wasay M. Stroke in Asia: geographical variations and temporal trends. J Neurol Neurosurg Psychiatry. 2014;85:1308–12.
Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, et al. Global burden of stroke and risk factors in 188 countries during 1988-2013. Lancet Neurol. 2016;15:913–24.
Akhtar N, Salam A, Kamran S, Bourke P, Joseph S, Santos M, et al. Ethnic variation in acute cerebrovascular disease: analysis from the Qatar stroke registry. Eur Stroke Jour. 2016;1:231–41.
Akhtar N, Kamran S, Singh R, Cameron P, D’Souza A, Imam Y, et al. Beneficial effects of implementing stroke protocols require establishment of a geographically distinct unit. Stroke. 2015;46:3494–501.
Population Chapter 2015. Ministry of Development Planning and Statistics. http://www.mdps.gov.qa/en/Statistics1. Accessed 3 Aug 2016.
Adam HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke. 1993;24:35–41.
Brant-Zawadzki M, Fein G, Van Dyke C, Kiernan R, Davenport L, de Groot J, et al. MR imaging of the aging brain: patchy white-matter lesions and dementia. AJNR. 1985;6:675–82.
Zimmerman RD, Fleming CA, Lee BCP, Saint-Louis LA, Deck MD. Peri ventricular hyperintensity as seen by magnetic resonance: prevalence and significance. AJR. 1986;146:443–50. AJNR .1986;7: 13–20
Fazekas F, Chawlak JB, Alavi A, Hurtig HI, Zimmerman RA. MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol. 1987;149:351–6.
Kakar P, Charidimou A, Werring DJ. Cerebral microbleeds: new dilemma in stroke medicine. JRSM Cardiovasc Dis. 2012;1:22.
Charidimou A, Jäger HR, Werring DJ. Cerebral microbleed detection and mapping: principles, methodological aspects and rationale in vascular dementia. Exp Gerontol. 2012;47:843–52.
Pasquini M, Bebedictus MR, Boulouis G, Rossi C, Dequatre-Ponchelle N, Cordonnier C. Incident cerebral microbleeds in a cohort of intracerebral hemorrhage. Stroke. 2016;47:689–94.
Wang Y, Liu G, Hong D, Chen F, Ji X, Cao G. White matter injury and ischemic stroke. Prog Neurobiology. 2016;141:45–60.
Kleining TJ. Associations and implications of cerebral microbleeds. J Clin Neurosci. 2013;20:919–27.
Wong T, Klein R, Sharrett AR, Couper DJ, Klein BE, Liao DP, et al. Cerebral white matter lesions, retinopathy and incident clinical stroke. JAMA. 2002;288:67–74.
Smith EE, Saposnik G, Biessels GJ, Doubal FN, Fornage M, Gorelick PB, et al. Prevention of stroke in patients with silent cerebrovascular disease. Stroke. 2017;48:e44–71.
Acknowledgments
The team is grateful to Ms. Reny Francis from the Neuroscience Institute, Hamad Medical Corporation, for providing formatting support and reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study has received IRB approval at Hamad Medical Corporation.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
For this type of study, formal consent is not required.
Rights and permissions
About this article
Cite this article
Akhtar, N., Salam, A., Kamran, S. et al. Pre-existing Small Vessel Disease in Patients with Acute Stroke from the Middle East, Southeast Asia, and Philippines. Transl. Stroke Res. 9, 274–282 (2018). https://doi.org/10.1007/s12975-017-0578-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-017-0578-7