Abstract
Vascular smooth muscle cells (SMC) maintain significant plasticity. Following environmental stimulation, SMC can alter their phenotype from one primarily concerned with contraction to a pro-inflammatory and matrix remodeling phenotype. This is a critical process behind peripheral vascular disease and atherosclerosis, a key element of cerebral aneurysm pathology. Evolving evidence demonstrates that SMCs and phenotypic modulation play a significant role in cerebral aneurysm formation and rupture. Pharmacological alteration of smooth muscle cell function and phenotypic modulation could provide a promising medical therapy to inhibit cerebral aneurysm progression. This study reviews vascular SMC function and its contribution to cerebral aneurysm pathophysiology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Unlike terminally differentiated skeletal and cardiac muscle cells, vascular smooth muscle cells (SMC) are able to undergo significant changes in their phenotype depending on their necessary changing function [1]. In their most common state, SMC are primarily concerned with contraction, regulation of blood flow, and maintenance of pressure. Following a number of stimuli, SMC can differentiate into a phenotype concerned with inflammation and matrix remodeling—deemed phenotypic modulation [2, 3]. This process contributes to peripheral vascular disease and atherosclerosis as well as plaque stability [4, 5]. Recent studies have provided evidence that both SMCs and phenotypic modulation contribute to cerebral aneurysm formation, progression, and rupture.
Cerebral aneurysms occur in 2–3 % of the present human population [6, 7]. Aneurysmal subarachnoid hemorrhage ranges from 6.9 to 10.5/100,000 per year with mortality rates following rupture from 11 to 70 % [8–14]. Approximately, 30 % of survivors remain dependant, and only 30–45 % are able to recover and maintain previous or comparable professions [15–17]. Treatment with both endovascular therapy and microsurgery is effective but can also result in significant morbidity and mortality [18, 19]. Currently, there are no pharmacological alternatives, which could be particularly beneficial, especially in patients that are high risk for intervention. The pathophysiology of aneurysm formation and rupture has been incompletely elucidated, but SMCs are known to play a key role [20]. Therapeutic alteration of SMC function and phenotypic modulation could be a beneficial medical target. This study reviews vascular SMC function and its potential contributions to cerebral aneurysm pathophysiology.
Smooth Muscle Cell Plasticity
In contrast to skeletal and cardiac muscle cells which are terminally differentiated, vascular SMC have been demonstrated to exhibit a remarkable degree of plasticity in response to injury and inflammation [5]. SMCs display significant plasticity throughout development and angiogenesis during which an initially primordial vascular network is gradually expanded into functional vessels [21]. The intricacies of SMC plasticity are dependent on a host of external factors. However, the critical role of SMCs in vascular disease is based not on its plasticity during embryogenesis, but rather on its ability to maintain the same potential throughout maturation of the organism into adulthood. Markers of mature, fully differentiated SMCs include smooth muscle 22 alpha (SM22α), smooth muscle alpha actin (SM-α-actin), SM myosin heavy chain (MHC), h1-calponin, and smoothelin [22–25]. Since markers, such as SM22α and SM-α-actin, may be expressed in cells other than SMCs during development and following external insults, expression of these markers alone is inadequate for identifying SMCs [26, 27]. Therefore, further testing, including identification of marker genes in cells and localization to the tunica media in vascular tissue, is necessary for proper identification of mature SMCs [5].
In order to better understand the influence of SMCs on vascular physiology and disease, it is important to be able to delineate the origins of cells expressing SMC-specific markers whether they are genuine SMCs or cells of other origins masquerading as SMCs. In vitro studies have demonstrated induction of SM-α-actin expression in macrophages by thrombin and transforming growth factor β (TGFβ) [28, 29]. Caplice et al. studied coronary artery specimens from patients who underwent bone marrow transplantation and were found to have atherosclerosis at autopsy [27]. The authors found that approximately 10 % of SM α-actin-expressing cells were of myeloid rather than SMC lineage. These studies suggest the inadequacy of using marker genes and proteins alone to label cells as differentiated SMCs.
Further contributing to the difficulty in elucidating the role of SMCs in vascular pathology is their ability to transdifferentiate into myeloid lineage cells, most notably macrophages. Andreeva et al. showed co-localization of SM α-actin and CD68, a macrophage marker, in cells cultured from human aortic intima; the degree of co-localization was greatest in lipid-rich areas such as fatty streaks and atherosclerotic plaques, leading the authors to conclude that phagocytic stimulators led to expression of macrophage markers in the vascular subendothelial intima [30]. Rong et al. demonstrated transdifferentiation of cultured mouse aortic SMCs into macrophage-like cells after cholesterol loading [31]. These changes included decreased expression of SM-α-actin, α-tropomyosin, MHC, and h1-calponin with concomitant increases in the expression of CD68 and Mac-2, another macrophage marker, as well as increased phagocytic activity. It should be noted that both of these studies were performed in vitro on cultured SMCs and in vivo evidence of SMC transdifferentiation in to macrophage-like cells has yet to be demonstrated [32].
It has become increasingly apparent that identification of SMCs by markers alone is frequently inadequate in the setting of vascular disease. SMCs clearly wield an incredible degree of versatility in the face of external pathobiological processes. In these altered environments, the distinction between SMCs and inflammatory cells, such as macrophages, is blurred. Due to the lack of rigorous lineage tracing studies that allow definitive identification of cell origins, the relative contribution of SMC versus myeloid lineage cells to the pathogenesis, evolution, and repair of vascular injury remains incompletely understood.
Smooth Muscle Cell Phenotypic Modulation in Atherosclerosis and Vascular Injury
Atherosclerosis is pervasively implicated in myocardial infarction and stroke which are leading causes of mortality in developed countries [33]. The pathogenesis of atherosclerosis begins with the intracellular lipid accumulation resulting in macrophage infiltration of the intima which subsequently develops into fatty streaks, atheromatous plaque formation, and eventual plaque rupture [34]. The stability of atherosclerotic lesions depends on multiple factors, one of which is the balance of SMC-like cells to macrophage-like cells. A ratio favoring SMCs will result in a relatively stable lesion compared to a ratio favoring macrophages which results in a relatively unstable lesion. Following vascular injury, SMCs have the unique ability to drastically alter their structure and function, a phenomenon known as phenotypic modulation or phenotypic switching [35]. SMCs undergoing phenotypic modulation lose expression of mature SMC markers and demonstrate increased migratory, proliferative, and synthetic capabilities [2]. Dimethylation of lysine 4 of histone H3 at the myosin heavy chain locus has been shown to be a SMC-specific epigenetic signature which is preserved in SMCs undergoing phenotypic modulation [36]. Depending on local, external, and environmental signals, a dedifferentiated SMC may alter its original function as a contractile cell in the tunica media to one of several different phenotypically distinct cell types [2].
Stimulation by platelet-derived growth factor (PDGF) results in a migroproliferative SMC which contributes to fibrous cap formation [37]. This may be a potential mechanism by which SMCs stabilize ruptured plaques following localization of platelets to the lesion with concomitant release with PDGF [38]. TGFβ stimulation results in SMC differentiation in a matrigenic phenotype which is proficient in the production and deposition of extracellular matrix [39]. Oxidized phospholipids promote a phenotype which is an intermediate between migroproliferative and matrigenic SMCs [40]. Inflammatory cytokines, such as tumor necrosis factor α (TNF-α) and interleukin-1 (IL-1), promote the development of inflammatory SMCs which further propagate the inflammatory cascade by secreting cytokines such as IL-6 and proteolytic enzymes, such as matrix metalloproteinases (MMP), and adhesion molecules such as intercellular adhesion molecule-1 (ICAM-1) [41–43]. The combined effect of expression of these molecules by inflammatory SMCs is recruitment of monocytes, macrophages, and T cells to ruptured plaques. An osteochondrogenic SMC, which deposits calcium in vessel walls, forms after stimulation with inorganic phosphate [44]. These various phenotypes are summarized by Alexander and Owens [2]. It is important to keep in mind that while we have presented these SMC subtypes as distinct phenotypes, there is currently no evidence that any of the aforementioned phenotypically modulated SMCs is completely unique from the others. Future studies using lineage tracing methods are necessary to evaluate the genetic, structural, and functional differences between the different SMC phenotypes.
The mechanisms of SMC phenotypic modulation have yet to be fully elucidated, but the current understanding involves activation of the transcription factor kruppel-like factor 4 (KLF4) [45]. KLF4, along with other transcription factors mediates the reprogramming of differentiated somatic cells, such as SMCs, into embryonic stem cell-like cells, or induced pluripotential stem cells [46]. Yoshida et al. demonstrated a transient delay in downregulation of mature SMC markers as well as increased neointimal formation in a KLF4 conditional knockout mouse model [47]. The same study reported diminished proliferation of cultured SMCs overexpressing KLF4 due to increased activation of the cell cycle inhibitor p21 by KLF4. Wamhoff et al. showed that a highly conserved G/C repressor element of the SM22α gene promoter is necessary for SMC phenotypic modulation [48]. In a subsequent study, Salmon et al. used chromatin immunoprecipitation assays to further elucidate the epigenetic mechanisms underlying KL4-dependent SMC phenotypic switching [49]. The authors determined that a complex comprised of KLF4 and pELK-1 binds to the G/C repressor element of the SM22α gene resulting in recruitment of histone deacetylase 2 which in turn facilitates epigenetic silencing of the SMC protein SM22α.
While it is clear that SMCs undergo phenotypic modulation in response to vascular injury, it remains to be determined how the versatility of SMCs affects the finely tuned balance of repair and inflammation which occurs after injury. It is unknown whether the overall impact of KLF4-dependent phenotypic modulation is beneficial or harmful to the development and eventual destabilization of atherosclerotic plaques. Additionally, the role of other transcription factors in SMC phenotypic modulation has yet to be defined.
Potential Role of Smooth Muscle Cells in Cerebral Aneurysms
SMC phenotypic modulation to an inflammatory state may promote cerebral aneurysm formation. Inflammation is known to play a critical role in cerebral aneurysm pathogenesis [20]. Oxidative damage by reactive oxygen species may directly damage vessel walls via generation of unstable free radicals or indirectly via recruitment of pro-inflammatory cells [50]. Cytokine secretion by recruited cells, such as macrophages and T cells, propagate the inflammatory cascade and induce phenotypic switching by the SMCs which reside in the media of the vasculature. Cerebral aneurysms are known to develop at vessel branch points or along artery curvatures which are localized areas of relatively high hemodynamic stress [51]. Kolega et al. studied the basilar artery of rabbits after bilateral carotid artery ligation and demonstrated loss of the internal elastic lamina (IEL), which separates the intima from the media, in portions of the vessel subjected to the highest hemodynamic shear stress [52]. Apoptosis and increased MMP expression was identified at the basilar bifurcation where IEL loss was the greatest. Loss of the IEL results in increased exposure of the media, where SMCs reside, to hemodynamic stress although the effect of high shearing forces on SMC phenotype has not been studied in detail.
Aoki et al. demonstrated SMC expression of the transcription factor Ets-1, a regulator of vascular remodeling in response to inflammation, in cerebral aneurysms in rats and humans [53]. Ets-1 induces expression of monocyte chemoattractant protein-1 (MCP-1) which then results in the recruitment of pro-inflammatory cells such as monocytes and macrophages. Ali et al. induced phenotypic modulation of cerebral vascular SMCs into an inflammatory state by treatment with TNF-α which was shown to exert its effects through KLF4-dependent pathways both in cultured cerebral vascular SMCs and in an early in vivo model prior to aneurysm formation [3].
Bygglin et al. successfully cultured SMCs from human cerebral aneurysms which were resected during microsurgical clipping [54]. Obviously, studies on cultured human SMCs are limited to in vitro models. Since in vivo data is necessary before potential novel therapies may proceed to early phase human clinical trials and there persists a lack of correlation between in vitro and in vivo findings in regard to SMC physiology, the value of performing tests on cultured SMCs from human cerebral aneurysms when an animal model will inevitably need to be utilized remains unknown. Nevertheless, the ability to obtain mechanistic data from human SMCs will likely prove valuable in the future. Additional studies are necessary in order to determine how changing SMC function via induction of phenotypic modulation will affect aneurysm formation, progression, and rupture.
The Smooth Muscle Cell in Cerebral Aneurysm Pathogenesis
There is compelling support for the role of SMCs in cerebral aneurysm formation, growth, and rupture, and a number of molecular mediators have been implicated (Table 1). As discussed above, the media where SMCs are concentrated provides structural integrity to the arterial wall, and its degeneration invariably causes vessel weakening. Collagen fibers in arterial walls are repaired and maintained by continuous synthesis of new collagen to counter the effect of hemodynamic stress and degradation by matrix metalloproteinases. Under physiologic conditions, SMCs are the predominant source of collagen fibers and play a central role in maintaining the integrity of vessel wall. Alterations in the differentiation and synthetic capacity of SMCs coupled with an increased release of matrix metalloproteinases in part by SMCs are major events leading to cerebral aneurysm rupture [20, 50, 55].
Evidence suggests that SMCs migrate, proliferate, and produce extracellular matrix components early in cerebral aneurysm formation, a process that may be analogous to myointimal hyperplasia in atherosclerosis [20]. In an immunocytochemical analysis of human saccular aneurysms, Kosierkiewicz et al. [56] noted atherosclerotic lesions in the smallest aneurysms characterized by diffuse intimal thickening and composed predominantly of SMCs. These observations were further corroborated by Frosen et al. [57] who found that experimental saccular aneurysms developed luminal pads of neointimal hyperplasia deriving primarily from the aneurysm wall rather than from bone marrow neointimal cells.
As discussed above, SMCs are highly dynamic cells, and their phenotype depends on the combined influences of multiple factors in the perivascular environment. In the setting of endothelial cell injury and arterial wall inflammation, these properties can result in dedifferentiation of SMCs. Specifically, SMCs undergo phenotypic modulation from a differentiated phenotype concerned mainly with contraction to an undifferentiated, pro-inflammatory, promatrix-remodeling phenotype [3, 58]. This is a crucial event because the structural integrity of the arterial wall is dependent on SMC differentiation and extracellular matrix organization. Using immunohistochemistry, Nakajima et al. [59] were the first to demonstrate that phenotypic modulation of SMCs is present in cerebral aneurysm walls. The authors found that the expression of contractile genes/proteins such as smooth muscle myosin heavy chain and smooth muscle-α-actin were significantly decreased in cerebral aneurysm samples as compared to control cerebral arteries. Other authors have confirmed SMC phenotypic modulation in cerebral aneurysms [60–62]. Kilic et al. [60] noted progressive decrease in staining intensity of contractile proteins (α-actin) from normal vessel wall to unruptured aneurysms to ruptured aneurysms. From a morphological standpoint, they observed angioarchitectural disorganization in aneurysm walls where SMCs were no longer arranged in tightly compacted bands but were loose and formed diffuse patchy zones of staining, a likely reflection of SMC phenotypic modulation. Indeed, Merei and Gallyas [63] reported that spindle-like SMCs, arranged in a parallel manner, may dissociate from each other to be transformed into spider-like cells in aneurysm walls [63]. Sibon et al. [62] demonstrated that there was a dramatic reduction in semicarbazide-sensitive amine oxidase (SSAO), an enzyme associated with SMC differentiation, as well as smooth muscle myosin heavy chain during the development of cerebral aneurysms. Furthermore, SMC dedifferentiation was closely associated with elastic lamellae thinning, a hallmark of aneurysm formation. The authors concluded that SMC dedifferentiation could impair elastic lamellae organization through a decrease in elastin concentration and a possible SSAO-related elastin cross-link deficiency. A genetic study showing an association between aneurysm formation and the mutation of GLUTs, which is regulated by SSAO, provides further support for the role of SMC dedifferentiation/phenotypic modulation in aneurysm pathogenesis [64].
The phenotypic switching of SMCs into an inflammatory, promatrix-remodeling phenotype has recently been the subject of intense investigation by our group. Despite the significant contribution of SMCs to cerebral aneurysm biology, the mechanisms underlying SMC phenotypic modulation were poorly understood. Our lab has recently demonstrated that the phenotypic modulation of SMCs is driven by TNF-α and is mediated at least in part by KLF4 [3]. The novel observation was initially made in vitro on cultured SMCs and subsequently confirmed in vivo early on in an animal model of cerebral aneurysm formation. Both in vitro and in vivo experiments showed that TNF-α induces downregulation of contractile genes namely myocardin, SM-α-actin, smooth muscle myosin heavy chain (SM-MHC), and SM22α and upregulation of pro-inflammatory genes namely KLF4, MMP-3, MMP-9, MCP-1, vascular cell adhesion molecule-1 (VCAM-1), and IL-1β in a dose-dependent manner. These changes appeared to be reversible with selective inhibition of TNF-α with 3,6-dithiothalidomide. Importantly, inhibition of KLF4 prevented TNF-α activation of pro-inflammatory genes and suppression of contractile genes, suggesting that the effects of TNF-α on SMCs are mediated by KLF4. Accordingly, chromatin immunoprecipitation assays in vivo and in vitro showed that TNF-α induces epigenetic changes with enhancement of the binding of KLF4 to the promoter regions of SMC marker genes and recruitment of histone deacetylase, thus resulting in histone modifications and altered gene expression.
In a rodent model of cerebral aneurysm formation, we provided the first direct evidence that there is marked SMC phenotypic modulation in cerebral aneurysm walls characterized by decreased expression of SMC contractile genes and increased expression of pro-inflammatory, promatrix-remodeling genes namely chemokines (MCP-1 and VCAM-1), transcription factors (NF-kB and KLF4), matrix remodeling proteins (MMP-2 and 3), IL-1β, and nitric oxide synthase (NOS) [65]. These inflammatory molecules have been previously shown to be implicated in the pathogenesis of cerebral aneurysms. Specifically, chemokines including MCP-1 have been recently found to be expressed at high levels in the lumen of human cerebral aneurysms [66]. Additionally, MCP-1 knockout mice demonstrated inhibition of aneurysmal growth [67]. Nuclear factor kB (NF-kB) was upregulated in the early stage of aneurysm formation in mice, and NF-kB p50 subunit-deficient mice demonstrated a decreased incidence of cerebral aneurysms along with decreased inflammatory changes [68]. Moreover, NF-kB blockade efficiently prevented aneurysm formation and macrophage infiltration [69]. Increases in MMPs expression have been demonstrated in humans as well as in animal experiments where inhibition of MMPs by tolylsam blocked aneurysm progression [70, 71]. IL-1β is induced early during aneurysm formation in mice and functions to inhibit collagen production while promoting SMC apoptosis and aneurysm progression [72]. Lastly, NOS was found to parallel the development of cerebral aneurysms in rats, and its blockade attenuated the incidence of induced aneurysms [73]. Collectively, these data show that SMCs initiate or at least promote the inflammatory and degenerative response that drives cerebral aneurysm formation, progression, and rupture.
Using the same aneurysm model, we also demonstrated that infliximab significantly suppressed TNF-α activity and reversed phenotypic modulation of SMCs following aneurysm induction [65]. The inhibition of SMCs by infliximab was accompanied by decreased expression of inflammatory markers and leukocytes. Thus, the phenotypic modulation of SMCs appears to be reversible at least in the early stages of aneurysm formation and could potentially represent a legitimate therapeutic target.
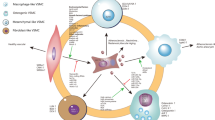
Following phenotypic modulation during aneurysm formation, SMCs may ultimately lose their phenotype altogether and undergo apoptosis leading to aneurysm rupture (Fig. 1). The loss of SMCs is the last step in the cascade of events leading to aneurysm rupture [20]. This results in decreased collagen production, which in addition to matrix degradation by SMC and macrophage-derived proteinases, leads to thinning of the media with aneurysm weakening/rupture. Indeed, Nakajima et al.[59] reported that following phenotype modulation both SMC phenotypes (contractile and modulated) were lost in ruptured aneurysms [74]. A Japanese study also reported that SMCs in the wall of ruptured aneurysms were much more degenerated than those in the wall of unruptured aneurysms and exhibited a higher number of apoptotic bodies [75]. Likewise, Hara et al. [76] reported increased apoptotic cells in cerebral aneurysms, especially in the thin wall close to the rupture point within the dome. Guo et al. [77] found decreased SMC density and more than sixfold increase in the activity of caspases in ruptured aneurysms compared with control vessels, thus bringing further confirmation that SMC apoptosis is associated with aneurysm rupture.
Potential role of SMC phenotypic modulation in cerebral aneurysm formation and rupture. In normal vascular SMCs, there is increased expression of SMC marker genes (SM = α-actin, SMC-MHC, and SMC22α) (a). During cerebral aneurysm formation, SMC undergo phenotypic modulation which contributes to cerebral aneurysm growth and progression (b). Eventually, SMC undergo apoptosis increasing the risk of rupture (c)
The loss of SMCs prior to aneurysm rupture may be related to TNF-α as well. In fact, TNF-α and its proapoptotic downstream target, Fas-associated death domain protein, were found to be increased in human cerebral aneurysms. Our experiments have also revealed increased apoptosis of SMCs after high-dose TNF-α exposure [3]. The underlying mechanism may be related to TNF-α upregulation of KLF4, which has a key role in apoptosis and phagocytosis of SMCs [78]. Although apoptosis plays a definite role in cerebral aneurysm pathogenesis, the degree of apoptosis is relatively low compared to the degree of mural cell loss, which indicates that SMC necrosis may also be involved in the process [79]. Scattered debris in the extracellular matrix and areas of fibrinoid necrosis in aneurysm walls support a role for necrotic cell death in cerebral aneurysm biology [79].
Smoking is a known risk factor for cerebral aneurysm formation [55, 80, 81]. Furthermore, it is believed to be one of the strongest risk factors for aneurysm rupture [55, 80, 81]. The effect of cigarette smoke on cerebral SMCs has been investigated by our group. In a rodent model, we demonstrated that cigarette smoke induces profound phenotypic modulation of SMCs with decreased expression of SMC marker genes and myocardin and increased expression of inflammatory/matrix remodeling genes namely MMP, MCP-1, IL-1, and TNF-α [58]. Importantly, cigarette smoke-induced modulation of SMCs was mediated by KLF4, as selective inhibition of this factor reversed these changes. Elsewhere, cigarette smoke was shown to induce injury and death of SMCs from upregulation of calcium channels [82]. We also note that smokers are known to have high levels of inflammatory cytokines including TNF-α, which as discussed above plays a key role in driving phenotypic modulation of SMCs [55]. Altogether, these data indicate that the early and late changes exhibited by SMCs during cerebral aneurysm formation, progression, and rupture may be initiated or aggravated by cigarette smoke exposure.
Future Directions and Therapeutic Implications
Although studies in cellular, animal, and human studies support the role of SMCs in the pathogenesis of cerebral aneurysm formation and rupture, there have not been any human clinical trials to determine the potential benefit of therapies targeting SMCs. The potential pathways that may be targeted are the phenotypic modulation of SMCs, the associated inflammatory response, the degenerative reaction implicating MMPs, and the SMC loss. Such therapies would be a major advance in aneurysm treatment as current endovascular and microsurgery therapies carry a significant risk of morbidity and mortality even in the most experienced hands. Therapies that alter SMC function and phenotypic modulation may be beneficial in inhibition of cerebral aneurysm progression. TNF-α inhibition has shown to have efficacy in preventing aneurysm rupture in mice [83], and there are currently many potential therapeutic TNF inhibitors currently in use for the treatment of inflammatory disease in humans [84, 85]. Therefore, this might be a promising therapeutic agent, although there are concerns that cerebral aneurysms are a focal disease that might not respond well to systemic therapies.
Targeted molecular imaging for identifying rupture-prone aneurysms is another desirable strategy that would optimize the management and outcomes of patients harboring cerebral aneurysms. Promising data have been reported by Hasan et al. [86–89] with ferumoxytol-enhanced MRI, a technique for imaging macrophages in the wall of human cerebral aneurysms. Preliminary findings suggest that aneurysms exhibiting early (within 24 h) signal change on MRI may be unstable and require urgent intervention [86]. We hope that a similar technique can be developed to identify unstable lesions with a high density of modulated/dedifferentiated SMCs.
Conclusion
Although there is a coordinated effort amongst many inflammatory mediators, SMCs play a critical role in cerebral aneurysm formation, progression, and rupture. This is in part characterized by phenotypic switching characterized by a pro-inflammatory, matrix remodeling phenotype. Further studies are indicated to determine key molecular determinates of this process in cerebral aneurysm pathogenesis as well assess how SMC can not only alter their phenotype but also change into alternative cell lines following exposure to pluripotency factors. Therapeutics targeted at altering SMC function, the ability to image these cellular alterations, and the ability to deliver targeted therapeutics to SMCs in cerebral aneurysms provide a promising potential medical alternative.
Abbreviations
- ICAM-1:
-
Intercellular adhesion molecule-1
- IL-1β:
-
Interleukin 1β
- MMP:
-
Matrix metalloproteinases
- NF-kB:
-
Nuclear factor kB
- NOS:
-
Nitric oxide synthase
- PDGF:
-
Platelet-derived growth factor
- SM-MHC:
-
Smooth muscle myosin heavy chain
- SM-α-actin:
-
Smooth muscle alpha actin
- SM22α:
-
Smooth muscle 22 alpha
- SMC:
-
Smooth muscle cells
- SSAO:
-
Semicarbazide-sensitive amine oxidase
- TNF-α:
-
Tumor necrosis factor alpha
- TGFβ:
-
Transforming growth factor β
- VCAM:
-
Vascular cell adhesion molecule-1
References
Owens GK. Molecular control of vascular smooth muscle cell differentiation and phenotypic plasticity. Novartis Found Symp. 2007;283:174–91. discussion 91–3, 238–41.
Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi:10.1146/annurev-physiol-012110-142315.
Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. TNF-alpha induces phenotypic modulation in cerebral vascular smooth muscle cells: implications for cerebral aneurysm pathology. J Cereb Blood Flow Metab. 2013. doi:10.1038/jcbfm.2013.109.
Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012;95(2):156–64. doi:10.1093/cvr/cvs115.
Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84(3):767–801. doi:10.1152/physrev.00041.2003.
Rinkel GJ. Natural history, epidemiology and screening of unruptured intracranial aneurysms. J Neuroradiol. 2008;35(2):99–103.
Komotar RJ, Zacharia BE, Mocco J, Connolly Jr ES. Controversies in the surgical treatment of ruptured intracranial aneurysms: the First Annual J. Lawrence Pool Memorial Research Symposium—controversies in the management of cerebral aneurysms. Neurosurgery. 2008;62(2):396–407.
van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306–18.
Kassell NF, Torner JC. The International Cooperative Study on Timing of Aneurysm Surgery—an update. Stroke. 1984;15(3):566–70.
Schievink WI. Intracranial aneurysms. N Engl J Med. 1997;336(1):28–40.
Ronkainen A, Miettinen H, Karkola K, Papinaho S, Vanninen R, Puranen M, et al. Risk of harboring an unruptured intracranial aneurysm. Stroke. 1998;29(2):359–62.
Laidlaw JD, Siu KH. Ultra-early surgery for aneurysmal subarachnoid hemorrhage: outcomes for a consecutive series of 391 patients not selected by grade or age. J Neurosurg. 2002;97(2):250–8. doi:10.3171/jns.2002.97.2.0250. discussion 47–9.
Hop JW, Rinkel GJ, Algra A, van Gijn J. Quality of life in patients and partners after aneurysmal subarachnoid hemorrhage. Stroke. 1998;29(4):798–804.
Ropper AH, Zervas NT. Outcome 1 year after SAH from cerebral aneurysm. Management morbidity, mortality, and functional status in 112 consecutive good-risk patients. J Neurosurg. 1984;60(5):909–15.
Nieuwkamp DJ, Setz LE, Algra A, Linn FH, de Rooij NK, Rinkel GJ. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: a meta-analysis. Lancet Neurol. 2009;8(7):635–42. doi:10.1016/S1474-4422(09)70126-7.
Rosenorn J, Eskesen V, Schmidt K, Espersen JO, Haase J, Harmsen A, et al. Clinical features and outcome in 1076 patients with ruptured intracranial saccular aneurysms: a prospective consecutive study. Br J Neurosurg. 1987;1(1):33–45.
Saveland H, Sonesson B, Ljunggren B, Brandt L, Uski T, Zygmunt S, et al. Outcome evaluation following subarachnoid hemorrhage. J Neurosurg. 1986;64(2):191–6.
Molyneux AJ, Kerr RS, Yu LM, Clarke M, Sneade M, Yarnold JA, et al. International subarachnoid aneurysm trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: a randomised comparison of effects on survival, dependency, seizures, rebleeding, subgroups, and aneurysm occlusion. Lancet. 2005;366(9488):809–17. doi:10.1016/S0140-6736(05)67214-5.
Wiebers DO, Whisnant JP, Huston 3rd J, Meissner I, Brown Jr RD, Piepgras DG, et al. Unruptured intracranial aneurysms: natural history, clinical outcome, and risks of surgical and endovascular treatment. Lancet. 2003;362(9378):103–10.
Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. Biology of intracranial aneurysms: role of inflammation. J Cereb Blood Flow Metab. 2012;32(9):1659–76. doi:10.1038/jcbfm.2012.84.
Hungerford JE, Little CD. Developmental biology of the vascular smooth muscle cell: building a multilayered vessel wall. J Vasc Res. 1999;36(1):2–27.
Gabbiani G, Schmid E, Winter S, Chaponnier C, de Ckhastonay C, Vandekerckhove J, et al. Vascular smooth muscle cells differ from other smooth muscle cells: predominance of vimentin filaments and a specific alpha-type actin. Proc Natl Acad Sci U S A. 1981;78(1):298–302.
Babij P, Kelly C, Periasamy M. Characterization of a mammalian smooth muscle myosin heavy-chain gene: complete nucleotide and protein coding sequence and analysis of the 5′ end of the gene. Proc Natl Acad Sci U S A. 1991;88(23):10676–80.
Duband JL, Gimona M, Scatena M, Sartore S, Small JV. Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation. 1993;55(1):1–11.
van der Loop FT, Schaart G, Timmer ED, Ramaekers FC, van Eys GJ. Smoothelin, a novel cytoskeletal protein specific for smooth muscle cells. J Cell Biol. 1996;134(2):401–11.
Li L, Miano JM, Cserjesi P, Olson EN. SM22 alpha, a marker of adult smooth muscle, is expressed in multiple myogenic lineages during embryogenesis. Circ Res. 1996;78(2):188–95.
Caplice NM, Bunch TJ, Stalboerger PG, Wang S, Simper D, Miller DV, et al. Smooth muscle cells in human coronary atherosclerosis can originate from cells administered at marrow transplantation. Proc Natl Acad Sci U S A. 2003;100(8):4754–9. doi:10.1073/pnas.0730743100.
Stewart HJ, Guildford AL, Lawrence-Watt DJ, Santin M. Substrate-induced phenotypical change of monocytes/macrophages into myofibroblast-like cells: a new insight into the mechanism of in-stent restenosis. J Biomed Mater Res A. 2009;90(2):465–71. doi:10.1002/jbm.a.32100.
Martin K, Weiss S, Metharom P, Schmeckpeper J, Hynes B, O’Sullivan J, et al. Thrombin stimulates smooth muscle cell differentiation from peripheral blood mononuclear cells via protease-activated receptor-1, RhoA, and myocardin. Circ Res. 2009;105(3):214–8. doi:10.1161/CIRCRESAHA.109.199984.
Andreeva ER, Pugach IM, Orekhov AN. Subendothelial smooth muscle cells of human aorta express macrophage antigen in situ and in vitro. Atherosclerosis. 1997;135(1):19–27.
Rong JX, Shapiro M, Trogan E, Fisher EA. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A. 2003;100(23):13531–6. doi:10.1073/pnas.1735526100.
Gomez D, Owens GK. Smooth muscle cell phenotypic switching in atherosclerosis. Cardiovasc Res. 2012. doi:10.1093/cvr/cvs115.
Lee RT, Libby P. The unstable atheroma. Arterioscler Thromb Vasc Biol. 1997;17(10):1859–67.
Falk E. Why do plaques rupture? Circulation. 1992;86(6 Suppl):III30–42.
Owens GK. Molecular control of vascular smooth muscle cell differentiation. Acta Physiol Scand. 1998;164(4):623–35.
McDonald OG, Wamhoff BR, Hoofnagle MH, Owens GK. Control of SRF binding to CArG box chromatin regulates smooth muscle gene expression in vivo. J Clin Invest. 2006;116(1):36–48. doi:10.1172/JCI26505.
Sano H, Sudo T, Yokode M, Murayama T, Kataoka H, Takakura N, et al. Functional blockade of platelet-derived growth factor receptor-beta but not of receptor-alpha prevents vascular smooth muscle cell accumulation in fibrous cap lesions in apolipoprotein E-deficient mice. Circulation. 2001;103(24):2955–60.
Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992;89(2):507–11. doi:10.1172/JCI115613.
Amento EP, Ehsani N, Palmer H, Libby P. Cytokines and growth factors positively and negatively regulate interstitial collagen gene expression in human vascular smooth muscle cells. Arterioscler Thromb. 1991;11(5):1223–30.
Johnstone SR, Ross J, Rizzo MJ, Straub AC, Lampe PD, Leitinger N, et al. Oxidized phospholipid species promote in vivo differential cx43 phosphorylation and vascular smooth muscle cell proliferation. Am J Pathol. 2009;175(2):916–24. doi:10.2353/ajpath.2009.090160.
Couffinhal T, Duplaa C, Moreau C, Lamaziere JM, Bonnet J. Regulation of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 in human vascular smooth muscle cells. Circ Res. 1994;74(2):225–34.
Loppnow H, Libby P. Proliferating or interleukin 1-activated human vascular smooth muscle cells secrete copious interleukin 6. J Clin Invest. 1990;85(3):731–8. doi:10.1172/JCI114498.
Fabunmi RP, Baker AH, Murray EJ, Booth RF, Newby AC. Divergent regulation by growth factors and cytokines of 95 kDa and 72 kDa gelatinases and tissue inhibitors or metalloproteinases-1, −2, and −3 in rabbit aortic smooth muscle cells. Biochem J. 1996;315(Pt 1):335–42.
Speer MY, Yang HY, Brabb T, Leaf E, Look A, Lin WL, et al. Smooth muscle cells give rise to osteochondrogenic precursors and chondrocytes in calcifying arteries. Circ Res. 2009;104(6):733–41. doi:10.1161/CIRCRESAHA.108.183053.
Jiang J, Chan YS, Loh YH, Cai J, Tong GQ, Lim CA, et al. A core Klf circuitry regulates self-renewal of embryonic stem cells. Nat Cell Biol. 2008;10(3):353–60. doi:10.1038/ncb1698.
Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, et al. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448(7151):318–24. doi:10.1038/nature05944.
Yoshida T, Kaestner KH, Owens GK. Conditional deletion of Kruppel-like factor 4 delays downregulation of smooth muscle cell differentiation markers but accelerates neointimal formation following vascular injury. Circ Res. 2008;102(12):1548–57. doi:10.1161/CIRCRESAHA.108.176974.
Wamhoff BR, Hoofnagle MH, Burns A, Sinha S, McDonald OG, Owens GK. A G/C element mediates repression of the SM22alpha promoter within phenotypically modulated smooth muscle cells in experimental atherosclerosis. Circ Res. 2004;95(10):981–8. doi:10.1161/01.RES.0000147961.09840.fb.
Salmon M, Gomez D, Greene E, Shankman L, Owens GK. Cooperative binding of KLF4, pELK-1, and HDAC2 to a G/C repressor element in the SM22alpha promoter mediates transcriptional silencing during SMC phenotypic switching in vivo. Circ Res. 2012;111(6):685–96. doi:10.1161/CIRCRESAHA.112.269811.
Starke RM, Chalouhi N, Ali MS, Jabbour PM, Tjoumakaris SI, Gonzalez LF, et al. The role of oxidative stress in cerebral aneurysm formation and rupture. Curr Neurovasc Res. 2013;10(3):247–55.
Alfano JM, Kolega J, Natarajan SK, Xiang J, Paluch RA, Levy EI, et al. Intracranial aneurysms occur more frequently at bifurcation sites that typically experience higher hemodynamic stresses. Neurosurgery. 2013. doi:10.1227/NEU.0000000000000016.
Kolega J, Gao L, Mandelbaum M, Mocco J, Siddiqui AH, Natarajan SK, et al. Cellular and molecular responses of the basilar terminus to hemodynamics during intracranial aneurysm initiation in a rabbit model. J Vasc Res. 2011;48(5):429–42. doi:10.1159/000324840.
Aoki T, Kataoka H, Nishimura M, Ishibashi R, Morishita R, Miyamoto S. Ets-1 promotes the progression of cerebral aneurysm by inducing the expression of MCP-1 in vascular smooth muscle cells. Gene Ther. 2010;17(9):1117–23. doi:10.1038/gt.2010.60.
Bygglin H, Laaksamo E, Myllarniemi M, Tulamo R, Hernesniemi J, Niemela M, et al. Isolation, culture, and characterization of smooth muscle cells from human intracranial aneurysms. Acta Neurochir (Wien). 2011;153(2):311–8. doi:10.1007/s00701-010-0836-x.
Chalouhi N, Ali MS, Starke RM, Jabbour PM, Tjoumakaris SI, Gonzalez LF, et al. Cigarette smoke and inflammation: role in cerebral aneurysm formation and rupture. Mediators Inflamm. 2012;2012:271582. doi:10.1155/2012/271582.
Kosierkiewicz TA, Factor SM, Dickson DW. Immunocytochemical studies of atherosclerotic lesions of cerebral berry aneurysms. J Neuropathol Exp Neurol. 1994;53(4):399–406.
Frosen J, Marjamaa J, Myllarniemi M, Abo-Ramadan U, Tulamo R, Niemela M, et al. Contribution of mural and bone marrow-derived neointimal cells to thrombus organization and wall remodeling in a microsurgical murine saccular aneurysm model. Neurosurgery. 2006;58(5):936–44. doi:10.1227/01.NEU.0000210260.55124.A4. discussion −44.
Starke RM, Ali M, Jabbour P, Tjoumakaris SI, Gonzalez LF, Hasan D, et al. Cigarette smoke modulates vascular smooth muscle phenotype: implications for carotid and cerebrovascular disease. Plos One. 2013;8(8):e71954. doi:10.1371/journal.pone.0071954.
Nakajima N, Nagahiro S, Sano T, Satomi J, Satoh K. Phenotypic modulation of smooth muscle cells in human cerebral aneurysmal walls. Acta Neuropathol. 2000;100(5):475–80.
Kilic T, Sohrabifar M, Kurtkaya O, Yildirim O, Elmaci I, Gunel M, et al. Expression of structural proteins and angiogenic factors in normal arterial and unruptured and ruptured aneurysm walls. Neurosurgery. 2005;57(5):997–1007. discussion 997.
Pera J, Korostynski M, Krzyszkowski T, Czopek J, Slowik A, Dziedzic T, et al. Gene expression profiles in human ruptured and unruptured intracranial aneurysms: what is the role of inflammation? Stroke. 2010;41(2):224–31. doi:10.1161/STROKEAHA.109.562009.
Sibon I, Mercier N, Darret D, Lacolley P, Lamaziere JM. Association between semicarbazide-sensitive amine oxidase, a regulator of the glucose transporter, and elastic lamellae thinning during experimental cerebral aneurysm development: laboratory investigation. J Neurosurg. 2008;108(3):558–66. doi:10.3171/jns/2008/108/3/0558.
Merei FT, Gallyas F. Role of the structural elements of the arterial wall in the formation and growth of intracranial saccular aneurysms. Neurol Res. 1980;2(3–4):283–303.
Coucke PJ, Willaert A, Wessels MW, Callewaert B, Zoppi N, De Backer J, et al. Mutations in the facilitative glucose transporter GLUT10 alter angiogenesis and cause arterial tortuosity syndrome. Nat Genet. 2006;38(4):452–7. doi:10.1038/ng1764.
Ali MS, Starke RM, Jabbour P, Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, et al. 184 Infliximab suppresses TNF-alpha induced inflammatory phenotype in cerebral vascular smooth muscle cells: implications for cerebral aneurysm formation. Neurosurgery. 2013;60 Suppl 1:181. doi:10.1227/01.neu.0000432774.35977.11.
Chalouhi N, Points L, Pierce GL, Ballas Z, Jabbour P, Hasan D. Localized increase of chemokines in the lumen of human cerebral aneurysms. Stroke. 2013. doi:10.1161/STROKEAHA.113.002361.
Aoki T, Kataoka H, Ishibashi R, Nozaki K, Egashira K, Hashimoto N. Impact of monocyte chemoattractant protein-1 deficiency on cerebral aneurysm formation. Stroke. 2009;40(3):942–51. doi:10.1161/STROKEAHA.108.532556.
Aoki T, Kataoka H, Ishibashi R, Nozaki K, Morishita R, Hashimoto N. Reduced collagen biosynthesis is the hallmark of cerebral aneurysm: contribution of interleukin-1beta and nuclear factor-kappaB. Arterioscler Thromb Vasc Biol. 2009;29(7):1080–6. doi:10.1161/ATVBAHA.108.180760.
Aoki T, Kataoka H, Shimamura M, Nakagami H, Wakayama K, Moriwaki T, et al. NF-kappaB is a key mediator of cerebral aneurysm formation. Circulation. 2007;116(24):2830–40. doi:10.1161/CIRCULATIONAHA.107.728303.
Kim SC, Singh M, Huang J, Prestigiacomo CJ, Winfree CJ, Solomon RA, et al. Matrix metalloproteinase-9 in cerebral aneurysms. Neurosurgery. 1997;41(3):642–66. discussion 6–7.
Aoki T, Kataoka H, Morimoto M, Nozaki K, Hashimoto N. Macrophage-derived matrix metalloproteinase-2 and -9 promote the progression of cerebral aneurysms in rats. Stroke. 2007;38(1):162–9. doi:10.1161/01.STR.0000252129.18605.c8.
Moriwaki T, Takagi Y, Sadamasa N, Aoki T, Nozaki K, Hashimoto N. Impaired progression of cerebral aneurysms in interleukin-1beta-deficient mice. Stroke. 2006;37(3):900–5. doi:10.1161/01.STR.0000204028.39783.d9.
Fukuda S, Hashimoto N, Naritomi H, Nagata I, Nozaki K, Kondo S, et al. Prevention of rat cerebral aneurysm formation by inhibition of nitric oxide synthase. Circulation. 2000;101(21):2532–8.
Jayaraman T, Berenstein V, Li X, Mayer J, Silane M, Shin YS, et al. Tumor necrosis factor alpha is a key modulator of inflammation in cerebral aneurysms. Neurosurgery. 2005;57(3):558–64. discussion −64.
Sakaki T, Kohmura E, Kishiguchi T, Yuguchi T, Yamashita T, Hayakawa T. Loss and apoptosis of smooth muscle cells in intracranial aneurysms. Studies with in situ DNA end labeling and antibody against single-stranded DNA. Acta Neurochir (Wien). 1997;139(5):469–74.
Hara A, Yoshimi N, Mori H. Evidence for apoptosis in human intracranial aneurysms. Neurol Res. 1998;20(2):127–30.
Guo F, Li Z, Song L, Han T, Feng Q, Guo Y, et al. Increased apoptosis and cysteinyl aspartate specific protease-3 gene expression in human intracranial aneurysm. J Clin Neurosci. 2007;14(6):550–5. doi:10.1016/j.jocn.2005.11.018.
Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi:10.1016/j.cell.2007.11.019.
Frosen J, Tulamo R, Paetau A, Laaksamo E, Korja M, Laakso A, et al. Saccular intracranial aneurysm: pathology and mechanisms. Acta Neuropathol. 2012;123(6):773–86. doi:10.1007/s00401-011-0939-3.
Vlak MH, Algra A, Brandenburg R, Rinkel GJ. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. Lancet Neurol. 2011;10(7):626–36. doi:10.1016/S1474-4422(11)70109-0.
Vlak MH, Rinkel GJ, Greebe P, Algra A. Independent risk factors for intracranial aneurysms and their joint effect: a case–control study. Stroke. 2013;44(4):984–7. doi:10.1161/STROKEAHA.111.000329.
Gerzanich V, Zhang F, West GA, Simard JM. Chronic nicotine alters NO signaling of Ca(2+) channels in cerebral arterioles. Circ Res. 2001;88(3):359–65.
Starke RM, Chalouhi N, Ali MS, Jabbour P, Tjoumakaris SI, Gonzalez LF, et al. 190 critical role of TNF-a in cerebral aneurysm formation and rupture. Neurosurgery. 2013;60 Suppl 1:183. doi:10.1227/01.neu.0000432780.74094.e2.
Faustman D, Davis M. TNF receptor 2 pathway: drug target for autoimmune diseases. Nat Rev Drug Discov. 2010;9(6):482–93. doi:10.1038/nrd3030.
Bonilla-Hernan MG, Miranda-Carus ME, Martin-Mola E. New drugs beyond biologics in rheumatoid arthritis: the kinase inhibitors. Rheumatology (Oxford). 2013;50(9):1542–50. doi:10.1093/rheumatology/ker192.
Hasan D, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, et al. Early change in ferumoxytol-enhanced magnetic resonance imaging signal suggests unstable human cerebral aneurysm: a pilot study. Stroke. 2012;43(12):3258–65. doi:10.1161/STROKEAHA.112.673400.
Hasan DM, Chalouhi N, Jabbour P, Dumont AS, Kung DK, Magnotta VA, et al. Evidence that acetylsalicylic acid attenuates inflammation in the walls of human cerebral aneurysms: preliminary results. J Am Heart Assoc. 2013;2(1):e000019. doi:10.1161/JAHA.112.000019.
Hasan DM, Chalouhi N, Jabbour P, Magnotta VA, Kung DK, Young WL. Imaging aspirin effect on macrophages in the wall of human cerebral aneurysms using ferumoxytol-enhanced MRI: preliminary results. J Neuroradiol. 2013;40(3):187–91. doi:10.1016/j.neurad.2012.09.002.
Hasan DM, Mahaney KB, Magnotta VA, Kung DK, Lawton MT, Hashimoto T, et al. Macrophage imaging within human cerebral aneurysms wall using ferumoxytol-enhanced MRI: a pilot study. Arterioscler Thromb Vasc Biol. 2012;32(4):1032–8. doi:10.1161/ATVBAHA.111.239871.
Acknowledgments
This work was supported by the Joseph and Marie Field Cerebrovascular Research Laboratory Endowment and by the National Institute of Neurological Disorders and Stroke [1K08NS067072 to ASD].
Conflict of Interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Starke, R.M., Chalouhi, N., Ding, D. et al. Vascular Smooth Muscle Cells in Cerebral Aneurysm Pathogenesis. Transl. Stroke Res. 5, 338–346 (2014). https://doi.org/10.1007/s12975-013-0290-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12975-013-0290-1